Abstract
Bevacizumab is a monoclonal antibody that binds and neutralizes vascular endothelial growth factor (VEGF)-A, a key player in the angiogenesis pathway. Despite benefits of bevacizumab in cancer therapy, it is clear that the VEGF pathway is complex, involving multiple isoforms, receptors, and alternative ligands such as VEGF-B, and placental growth factor, which could enable escape from VEGF-A-targeted angiogenesis inhibition. Recently developed therapies have targeted other ligands in the VEGF pathway (eg, aflibercept, known as ziv-aflibercept in the United States), VEGF receptors (eg, ramucirumab), and their tyrosine kinase signaling (ie, tyrosine kinase inhibitors). The goal of the current review was to identify comparative preclinical data for the currently available VEGF-targeted therapies. Sources were compiled using PubMed searches (2007 to 2012), using search terms including, but not limited to: “bevacizumab,” “aflibercept,” “ramucirumab,” and “IMC-18F1.” Two preclinical studies were identified that compared bevacizumab and the newer agent, aflibercept. These studies identified some important differences in binding and pharmacodynamic activity, although the potential clinical relevance of these findings is not known. Newer antiangiogenesis therapies should help further expand treatment options for colorectal and other cancers. Comparative preclinical data on these agents is currently lacking.
Keywords: aflibercept, antiangiogenesis, metastatic colorectal cancer (mCRC), tyrosine kinase inhibitor (TKI), vascular endothelial growth factor (VEGF)
Introduction: why target angiogenesis?
Angiogenesis, the generation of new blood vessels, is an essential physiological process that can be dysregulated in various pathological conditions, including cancer.1,2 The vascular endothelial growth factor (VEGF) pathway is considered the most important and is a well-characterized contributor to angiogenesis.3,4 VEGF-A and other members of the VEGF family such as placental growth factor (PlGF) are upregulated in pathological conditions.5–8 VEGF-A, the first VEGF characterized, has served as a paradigm for the development of antiangiogenesis as a therapeutic strategy, including the clinical development of bevacizumab, a humanized monoclonal antibody targeting VEGF-A.9 In a pivotal trial, the use of bevacizumab in combination with irinotecan, 5-fluorouracil (5-FU), and leucovorin (IFL) was shown to improve the survival of patients with metastatic colorectal cancer (mCRC),10 resulting in its approval as the first antiangiogenic therapy.9 Nonetheless, the overall impact of agents such as bevacizumab in prolonging survival has been limited.2,11 While 2-year survival has improved to the 24- to 28-month range, the overall prognosis of mCRC remains poor, with 5-year survival generally between 5% and 8%, despite the availability of such therapy.11
Evidence is emerging that PlGF and other members of the VEGF family such as VEGF-B, although less well studied and understood, may also play a role in pathological angiogenesis.8,12,13 For example, in genetically modified mice, expression of host and tumor PlGF was required for maximal tumor angiogenesis, whereas PlGF deficiency resulted in poorly vascularized tumors.13 The upregulation of PlGF and other potentially angiogenic factors such as platelet-derived growth factors (PDGFs) and fibroblast growth factor (FGF) may also underlie disease progression in patients receiving bevacizumab.14 There are multiple strategies for targeting angiogenesis, and in the present review, the currently available biological agents in Phase III or later development for mCRC that target the VEGF pathway are highlighted. Some of the biological anti-VEGF agents currently approved or in Phase III evaluation are shown in Table 1. In addition, in a systematic review of the more recent literature, comparative preclinical studies among these agents were identified.
Table 1.
Biological anti-VEGF agents: currently approved and/or under phase III evaluation
| Agent | What it is | What it binds/inhibits | Rationale for use as an antiangiogenic agent? | Approved indications (potential indications) |
|---|---|---|---|---|
| Bevacizumab | Humanized monoclonal antibody | VEGF-A | Inhibition of VEGF-A will prevent pathological angiogenesis by inhibiting its interaction with VEGFR-2 | mCRC, glioblastoma, NSCLC, RCC |
| Aflibercept | Soluble decoy receptor | VEGF-A VEGF-B PlGF |
Targeting multiple VEGF ligands will allow for a broader inhibition of proangiogenic processes and inhibit possible resistance mechanisms | mCRC (melanoma, prostate cancer, glioblastoma, pancreatic cancer, NSCLC) |
| Regorafenib | Tyrosine kinase inhibitor | VEGFR-1, -2, -3 PDGFR c-kit FGFR |
Inhibition of VEGFR tyrosine kinase activity to prevent pathological angiogenesis in tumors | mCRC (RCC, breast cancer) |
| Ramucirumab | Fully human monoclonal antibody | VEGFR-2 extracellular domain | Inhibition of signaling by VEGFR-2 (receptor for VEGF-A) will prevent pathological angiogenesis by inhibiting VEGF-A activity | Currently investigational (mCRC, breast cancer, NSCLC) |
Abbreviations: FGFR, fibroblast growth factor receptor; mCRC, metastatic colorectal cancer; NSCLC, non-small-cell lung cancer; PDGFR, platelet-derived growth factor receptor; PlGF, placental growth factor; RCC, renal cell carcinoma; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Angiogenesis, the VEGF network: ligands and receptors
VEGF ligands
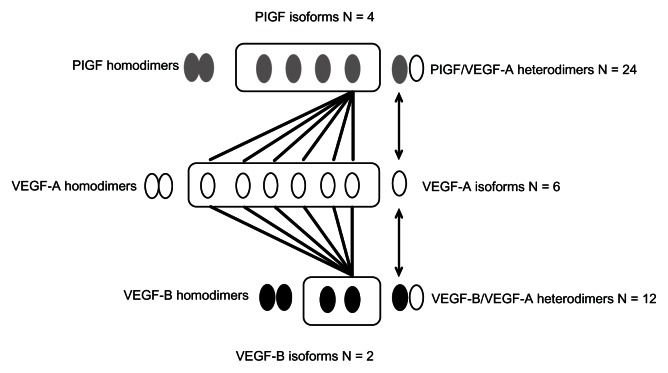
The VEGF family consists of five structurally related ligands, VEGF-A, -B, -C, and -D, and PlGF.15 VEGF-A interacts with VEGF receptor-1 (VEGFR-1) and VEGFR-2 and has potent proangiogenic and vascular permeability increasing effects. VEGF-B, by comparison, interacts with VEGFR-1 only, and its function has not been well characterized.16 While it does not appear to play a directly angiogenic role, there is evidence that VEGF-B may function as an angiogenesis survival factor.12 VEGF-C and VEGF-D both interact with VEGFR-2 and VEGFR-3, and these factors are believed to play a role in both angiogenesis and lymphangiogenesis; PlGF, like VEGF-B, also interacts with VEGFR-1 only and its function is incompletely understood, although there is accumulating evidence for a role of PlGF in pathological angiogenesis, as described below.17 The diversity in the VEGF ligand family is still further increased by the existence of multiple, alternatively spliced isoforms, each of which can have distinct and/or overlapping biological activities (Figure 1).5,6,18,19 These distinct VEGF isoforms also have the potential to homo- and heterodimerize, which can result in a very wide array of homo- and heterodimeric signaling molecules, the impetus of which is just beginning to be explored (Figure 1). For example, among ligands, which can interact with VEGFR-1, VEGF-A is known to exist in at least six isoforms, VEGF-B in at least two isoforms,16,20 and PlGF in at least four isoforms, resulting in at least some 36 potentially diverse signaling molecules arising from the heterodimeric combination of these isoforms (Figure 1).17 Although it has not been carefully studied, differences in properties such as heparin binding are observed among the different isoforms, and there could potentially be diverse biological functions of these molecules, and the implications of this for angiogenesis are not yet understood.5,6
Figure 1.
Diversity of VEGF and PlGF isoforms: homo- and heterodimers.
Notes: The VEGF ligands, VEGF-A, VEGF-B, and PlGF can all interact with VEGFR-1, and the illustration provides an example of how diversity in isoforms can result in a wide array of signaling molecules that can interact with the receptor. The four PlGF isoforms can homo- and heterodimerize with any of six distinct isoforms of VEGF-A, as can the two isoforms of VEGF-B, resulting in many combinations that can potentially exhibit different biological activity.5,6,17
Abbreviations: PlGF, placental growth factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
In terms of amount and biological activity, VEGF-A 165 appears to be the dominant isoform;4 however, it is also important to note that more recently, antiangiogenic isoforms of VEGF-A, of which there are at least five subtypes, have been identified.18 These findings have brought into question some of the original thinking in terms of the design of antiangiogenesis therapy, in as much as it is no longer clear that selective targeting of a single factor and/or isoform will necessarily achieve the desired result of angiogenesis inhibition in the tumor microenvironment. It is clear that multiple alternative and/or overlapping angiogenesis pathways exist; the potential for these mechanisms to be exploited by tumors in the face of targeted inhibitory molecules must be recognized.
VEGF receptors
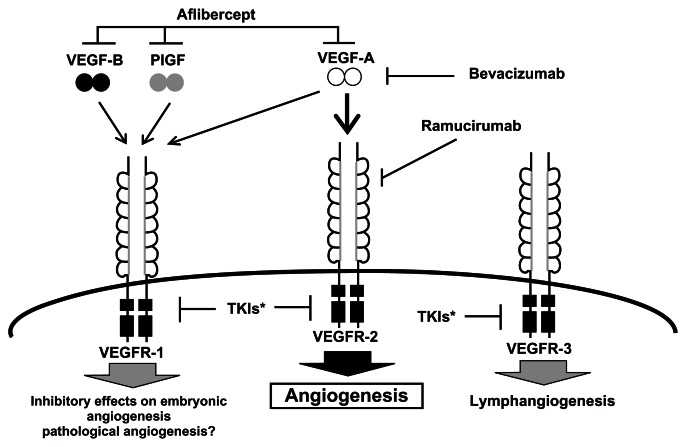
All three VEGFRs have intrinsic tyrosine kinase activity, and the neuropilins (NPs) also appear to serve as coreceptors and modulators of VEGFRs.6,15 Figure 2 shows the different VEGFRs and the ligands to which they bind.4 The function of VEGFR-1 is less well characterized but it is believed that VEGFR-1 has both vascular and nonvascular functions, and it is expressed in tumor cells, as well as monocytes and macrophages,4,6,17,21 whereas VEGFR-2 is believed to be the primary mediator of VEGF-A action on angiogenesis and increased vascular permeability. This receptor was also expressed on tumor cells and has been implicated in the activation of autocrine oncogenic pathways.6,22 The expression of VEGFR-3 is largely limited to lymphatic epithelial cells and is believed to mediate a lymphangiogenic function.4,6
Figure 2.
VEGF ligands, receptors, and inhibitors.4,5,15,19
Adapted with permission from Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785–1788.4
Abbreviations: PlGF, placental growth factor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Role of VEGF-B and PlGF
As noted above, the role of VEGFR-1 and its ligands PlGF and VEGF-B is incompletely understood.12,17,21 Gene targeting experiments have revealed no apparent impact of loss of PlGF on embryonic angiogenesis, even in combination with VEGF-B inactivation.13 Loss of PlGF, however, did lead to reduced angiogenesis in pathological conditions, including ischemia, inflammation, and cancer,13 and there is evidence for a benefit of anti-PlGF therapy in cancer cell models and inhibiting ocular neovascularization.23 Some evidence suggests that anti-PlGF antibodies are effective in inhibiting pathological angiogenesis across multiple tumor models.24 Notably, unlike anti-VEGFR-2 antibodies, anti-PlGF did not induce an angiogenic escape program.24 Other data, however, are conflicting and have shown no effect of anti-PlGF antibodies on tumor angiogenesis in multiple cell lines, including those resistant to VEGF-A antibodies.25 Significantly increased expression of PlGF isoforms has been observed in colorectal cancers, compared with normal tissue, and in those with poor outcomes.7 There is also increasing evidence for a role of PlGF and other factors as possible escape mechanisms during antiangiogenesis therapy with bevacizumab.14,26 Increased expression of PlGF in particular was observed prior to progressive disease in patients receiving bevacizumab and chemotherapy.14 Additional findings suggest that VEGFR-1, while apparently not promoting angiogenesis, may nonetheless participate in an autocrine/paracrine growth pathway, particularly in cells susceptible to anti-PlGF antibody.27 In addition, while it is apparently dispensable for blood vessel growth, VEGF-B was necessary for blood vessel survival, and targeting of VEGF-B could inhibit pathological angiogenesis.28 Taken together, these findings suggest that VEGF-B and PlGF, while perhaps not directly proangiogenic themselves, may nonetheless play an important role in activating VEGFR-1 under certain pathological conditions.
Bevacizumab: the first antiangiogenesis therapy in mCRC
Bevacizumab, the first antiangiogenesis therapy to be approved for use in mCRC, is a humanized monoclonal antibody that binds to all isoforms of VEGF-A.9 Evidence for the clinical efficacy of bevacizumab in cancer, notably in the treatment of mCRC, has been reviewed elsewhere.29 As noted earlier, the recent identification of alternatively spliced variants of VEGF-A, some of which may be antiangiogenic, complicates the initial rationale for inhibiting VEGF-A.18 In the United States, bevacizumab is currently indicated in combination with intravenous 5-FU-based chemotherapy for the first- or second-line treatment of mCRC.30
The mechanisms underlying the apparent benefit of bevacizumab in combination with chemotherapy are not well understood. It was previously thought that inhibition of VEGF would lead to vessel “normalization” and increase delivery of chemotherapies to the tumor.31 Recent studies, however, have suggested that bevacizumab actually reduced tumor perfusion, with no evidence for improved drug delivery.32 Notably, as a single agent, bevacizumab is also indicated for the treatment of glioblastoma in patients who have progressed on other therapies.33 The efficacy of bevacizumab in glioblastoma, a highly vascularized tumor, could imply a greater dependence on angiogenesis in this tumor type and a greater susceptibility for inhibition.34,35 Indeed, recent data have shown that bevacizumab therapy reduces blood vessel number, tumor perfusion, and oxygenation in experimental glioblastoma models.35
New biological agents targeting the VEGF pathway: mechanisms
Aflibercept (known in the United States as ziv-aflibercept)
Aflibercept is a recombinant fusion protein consisting of the second immunoglobulin (Ig) domain of VEGFR-1 and the third Ig domain of VEGFR-2, fused to human IgG1. It exhibits affinity for VEGF-A, VEGF-B, and PlGF.36–39 Aflibercept exhibits potent inhibition of human and mouse tumor xenografts in preclinical studies.19 The biology of aflibercept and its antitumor effects in preclinical model systems has been reviewed in detail elsewhere.39 In Phase I studies of patients with advanced solid tumors, aflibercept has displayed a manageable safety profile.37 The recently reported Phase III VELOUR study investigated the efficacy of aflibercept in combination with irinotecan and 5-FU (FOLFIRI) in patients with mCRC who had progressed on a prior oxaliplatin-based regimen.40 It is important to recognize that 30.4% of patients in this study had received prior bevacizumab treatment. Results of VELOUR showed significant improvements in the primary endpoint of overall survival (OS), as well as secondary endpoints of progression-free survival (PFS) and overall response rate (ORR) with aflibercept and FOLFIRI.40 Median OS in the aflibercept arm was 13.50 months compared with 12.06 months with placebo (hazard ratio [HR] = 0.817; P = 0.0032); similarly, median PFS (6.90 months versus 4.67 months, respectively, HR = 0.758; P = 0.00007) and ORR (19.8% versus 11.1%; P = 0.0001) were also significantly improved with aflibercept relative to placebo.40 Grade 3 or higher adverse events (AEs) with a 2% or higher incidence with aflibercept relative to placebo included proteinuria and hypertension, as well as diarrhea, asthenia/fatigue, and stomatitis/ulceration, infections, abdominal pain, and neutropenia.40 The results of VELOUR suggest that targeting multiple ligands may be a viable option to inhibit angiogenesis in cancer, even in patients who have progressed after prior bevacizumab treatment. On the basis of VELOUR, aflibercept has now been approved by the US Food and Drug Administration (FDA) with the US generic name of ziv-aflibercept (ZALTRAP®) for use in combination with FOLFIRI in the treatment of mCRC that is resistant to or that has progressed following an oxaliplatin-containing regimen.41
As in patients with mCRC, the use of aflibercept in patients with advanced ovarian cancer, advanced melanoma, metastatic pancreatic cancer,42 and androgen independent prostate cancer43 has been under investigation. It is worth noting that some of these studies have failed to reach their predetermined primary endpoint, in contrast to the results of VELOUR.40 For example, in the VITAL study,44 which examined the use of aflibercept in combination with docetaxel for second-line treatment of non-small-cell lung cancer (NSCLC), a significant improvement in OS was not observed (HR = 1.01); however, a benefit in PFS (HR = 0.82) and response rate (RR) (23.3% versus 8.9%) was seen with aflibercept.45 AEs associated with the use of aflibercept are consistent with those typically seen with agents that inhibit VEGF and include hypertension, proteinuria, thrombosis, and hemorrhage.46 As such, a pretreatment screening and management plan for hypertension and proteinuria should be in place, and patients receiving aflibercept should be educated about, and monitored for, the signs and symptoms of bleeding.46
Ramucirumab
Ramucirumab (also known as IMC-1121C) is a fully human monoclonal antibody that binds to the extracellular domain of VEGFR-2.47–49 Anti-VEGFR-2 antibodies have shown antitumor activity in a range of tumor model systems.50,51 In a Phase I study of patients with advanced solid tumors, tumor perfusion and vascularity were decreased with ramucirumab therapy in 69% of the patients.47 Ramucirumab is currently under investigation (in combination with chemotherapy) in a number of Phase III studies, including those of breast cancer, NSCLC, and as a second-line therapy for mCRC.48,49 Studies of ramucirumab currently underway in mCRC include a Phase III study of ramucirumab in combination with FOLFIRI chemotherapy in patients with progression following first-line combination therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine,52 and a Phase II study of ramucirumab, cetuximab, and irinotecan versus cetuximab and irinotecan in patients with mCRC and progression following a bevacizumab-based regimen.53 Toxicities associated with inhibition of the VEGF axis with ramucirumab (in Phase I studies) included hypertension, vascular thrombotic events, and proteinuria.47 Ramucirumab is also under investigation in combination with mFOLFOX6 in patients with mCRC, in an open-label, randomized, Phase II study (estimated enrollment = 150), which is currently recruiting patients.54 Patients in the trial had disease progression on an irinotecan-based, first-line chemotherapy regimen (FOLFIRI or CAPIRI), and the primary endpoint was PFS.54 Also recently described at the American Society of Clinical Oncology (ASCO) 2012 Annual Meeting is the RAISE trial;52 this is an ongoing, randomized, double-blinded, placebo-controlled Phase III trial of ramucirumab or placebo in combination with FOLFIRI in patients with mCRC who failed a first-line bevacizumab-, oxaliplatin-, or fluoropyrimidine-based regimen.55 The primary endpoint of the trial will be OS, with secondary endpoints of PFS, RR, safety, and biomarker analysis.55
IMC-18F1
Although it has received less attention than VEGFR-2, there is emerging evidence for a potential role of VEGFR-1 in human cancers, including mCRC, and the inhibition of VEGFR-1 signaling as a potential antiangiogenesis target is just beginning to be explored.56 IMC-18F1 is a high-affinity human VEGFR-1 neutralizing antibody that specifically binds the extracellular domain of VEGFR-1 and prevents its interaction with all of its known ligands (VEGF-A, VEGF-B, and PlGF); as such, it effectively blocks its biological activity in multiple preclinical models, and exhibits antiangiogenic and antiproliferative activity.57 There is also evidence from preclinical models that, similar to bevacizumab and aflibercept, this agent can potentiate the antitumor activity of cytotoxic chemotherapies.57 In the same open-label, randomized, Phase II study as that described above for ramucirumab,54 the combination of IMC 18F1 with mFOLFOX6 is under investigation in mCRC patients with disease progression on an irinotecan-based first-line chemotherapy regimen (FOLFIRI or CAPIRI), and results of this study should determine whether additional Phase III trials are warranted.
Other strategies – tyrosine kinase inhibitors (TKIs)
Because all of the known VEGFRs share an intrinsic tyrosine kinase activity,4,6 another means of targeting the VEGF pathway is through the use of TKIs.58 Many other cellular receptors utilize tyrosine kinases as a component in their signaling pathways; thus, many of these agents have unwanted “off-target” AEs associated with their inhibition of non-VEGFR kinases.58,59 The utility of TKIs as anti-VEGF agents can therefore be limited by their specificity for the various VEGFRs in relation to other receptor tyrosine kinases (ie, on-target and off-target effects).59 In a published review of these small molecule TKIs, although no inter-agent comparisons were done, the on-target effects appeared to be related to VEGFR inhibition. The data were compiled from a review of studies involving approximately 3000 patients treated with various small-molecule, VEGF-targeted TKIs.57 On-target AEs included VEGF inhibition-related events such as hypertension, proteinuria, and hemorrhage. Off-target AEs included events more likely related to the inhibition of other non-VEGF tyrosine kinases, such as fatigue, diarrhea, nausea, anorexia, and hand-foot reaction.59 A number of kinase inhibitors are under investigation as single agents or in combination with chemotherapies for the treatment of mCRC, with many in Phase II or III development. A summary of these agents and the combinations under investigation (not meant to be exhaustive) is shown in Table 2.60–70 One of these agents, regorafenib, is discussed below; it was recently approved by the FDA for treatment in patients with mCRC in the third or fourth line, ie, for those who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.71
Table 2.
Selected anti-VEGF kinase inhibitor agents currently under evaluation in mCRCa
| Agent | Combination | ClinicalTrials.gov (NCT#) | Phase | Trial completed? |
|---|---|---|---|---|
| Axitinib | Axitinib + FOLFOX | NCT0061505660 | 2 | Yes |
| Axitinib + FOLFIRI | ||||
| Single agent | NCT0149086661 | 2 | No | |
| BIBF1120 | BIBF1120 + mFOLFOX6 | NCT0090483962 | 2 | Yes |
| Bevacizumab + mFOLFOX6 | ||||
| Brivanib | Brivanib + Cetuximab | NCT0064047163 | 3 | Yes |
| Brivanib + Placebo | ||||
| Cediranib | Cediranib + FOLFOX | NCT0038417664 (HORIZON III) | 3 | No |
| Bevacizumab + FOLFOX | ||||
| Sunitinib | Sunitinib + FOLFIRI | NCT0045769165 | 3 | Yes |
| Placebo + FOLFIRI | ||||
| Sorafenib | Sorafenib + FOLFOX or FOLFIRI | NCT0088934366 | 2 | Terminated |
| Placebo + FOLFOX or FOLFIRI | ||||
| Regorafenib | Regorafenib | NCT0110332367 | 3 | No |
| Placebo | 3 | No | ||
| Regorafenib | NCT01538680 (CONSIGN)68 | |||
| Vandetanib | Vandetanib + FOLFOX | NCT0050029269 | 2 | No |
| Placebo + FOLFOX | 2 | Yes | ||
| Vandetanib + FOLFIRI | NCT0045411670 | |||
| Placebo + FOLFIRI |
Note:
List is not meant to be exhaustive.
Abbreviations: mCRC, metastatic colorectal cancer; NCT, National Clinical Trial; VEGF, vascular endothelial growth factor.
Regorafenib (BAY 73-4506)
Regorafenib is an inhibitor of PDGF receptors, c-kit, FGF receptor, and all three VEGFRs.58,72 In preclinical studies, regorafenib inhibited tumor growth and microvascular density in glioblastoma xenograft models.72 Regorafenib also inhibited tumor growth in breast and renal carcinoma xenograft models.72 There are currently several Phase III trials of regorafenib underway or finished in patients with mCRC, including the completed CORRECT study.54,55,72 Results of CORRECT, a randomized, Phase III study of regorafenib or placebo in patients with mCRC who have progressed after all approved drugs67 have shown that the study met its primary objective of improvement in OS, with no new safety concerns.73 The most common grade 3 or higher toxicities associated with regorafenib in this study included hand-foot skin reaction, fatigue, diarrhea, hyperbilirubinemia, and hypertension.73 On the basis of this trial, regorafenib was approved by the FDA for third- or fourth-line treatment in patients with mCRC. A Phase III interventional, open-arm study of regorafenib in patients with mCRC who have progressed after all standard therapies (CONSIGN) is currently underway.68
Comparing the Anti-VEGF agents: are there differences?
In our search, two studies that compared preclinical binding characteristics among the available anti-VEGF biological agents were identified.38,74 No direct comparative data comparing kinase inhibition or preclinical efficacy among the different TKIs under investigation in mCRC were identified.
Differences in VEGF-A–inhibitor complex formation
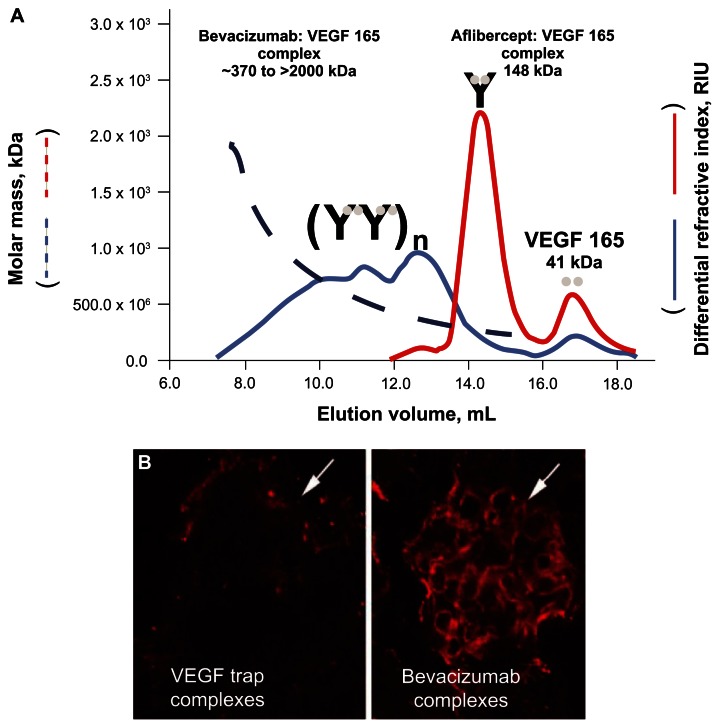
One study found in the search compared the VEGF-A binding characteristics of bevacizumab with those of aflibercept.74 These investigators found that unlike bevacizumab, aflibercept formed stable complexes in the circulation that remained bound to VEGF-A. In addition, although aflibercept formed inert 1:1 complexes with VEGF-A, bevacizumab formed heterogeneous multimeric immune complexes that were rapidly cleared from the circulation (Figure 3).74 These differences in binding and complex formation between bevacizumab and aflibercept could have important implications in terms of the AE profile; for example, in terms of renal damage and proteinuria resulting from the deposition of VEGF-A– bevacizumab complexes in the kidney.19,74
Figure 3.
Molecular masses of aflibercept-VEGF-A and bevacizumab-VEGF-A complexes. (A) Using a 1:2 molar ratio of aflibercept to VEGF 165, discrete peaks were observed at 17 mL and 14.5 mL. In contrast, a 1:2 molar ratio of bevacizumab to VEGF 165 revealed a heterogeneous multimeric complex that ranged in molar mass from 370 kDa to 2000 kDa.74 (B) One milligram of a preformed complex of aflibercept (VEGF Trap) and VEGF 165 or bevacizumab and VEGF 165 were injected into the left ventricle of 2- to 3-month-old C57bl6 mice. After 10 minutes, the mice were killed, and their kidneys were processed for immunocytochemistry, using an antihuman Fc reporter antibody to the human Fc moiety present on both aflibercept and bevacizumab. Significant staining was observed in the glomeruli of bevacizumab/VEGF-treated mice but not in the glomeruli of aflibercept/VEGF-treated mice (white arrows).74 Reprinted with permission from Rudge JS, Holash J, Hylton D, et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A. 2007;104(47):18363–18370.74 Copyright 2007 National Academy of Sciences, USA.
Abbreviations: RIU, refractive index units; VEGF, vascular endothelial growth factor.
Differences in VEGF binding
In another, more recent study, the binding characteristics of bevacizumab and aflibercept were compared using a variety of preclinical assessments.38 In this study, aflibercept showed tight binding to VEGF-A 165, and the dissociation constant (KD) was significantly lower with aflibercept compared with dimerized VEGFR-1 or VEGFR-2. The KD of aflibercept (0.490 pM) was approximately 100-fold lower compared with bevacizumab (58 pM) (Table 3).38 Notably, this suggests a 100-fold tighter binding to VEGF-A 165 by aflibercept. A lower KD for aflibercept in binding to VEGF-A 165 was predominantly attributable to its faster association rate (KA), which was 77-fold faster than that seen for bevacizumab (Table 3).38 Consistent with its design, aflibercept was also shown to bind to VEGF-B, PlGF-2, and PlGF-1, whereas bevacizumab did not (Table 3).38
Table 3.
| Binding activity | KD (pM) | Difference | KA (M−1S−1) | Difference in binding activity |
|---|---|---|---|---|
| Binding to VEGF-A 165 | ||||
| Aflibercept | 0.490 | 118-fold | 410.0 | ←77-fold |
| Bevacizumab | 58 | 5.3 | ||
| Binding to PlGF-2 | ||||
| Aflibercept | 38.9 | NA | 17.5 | NA |
| Bevacizumab | NB | NB | ||
| Inhibition of: | IC 50 (pM) | Difference in inhibitory activity | ||
|
| ||||
| Activation of VEGFR-2 by human VEGF-A 165 | ||||
| Aflibercept | 26 | ←51-fold | ||
| Bevacizumab | 1323 | |||
| Activation of VEGFR-1 by human PlGF-2 | ||||
| Aflibercept | 2890 | NA | ||
| Bevacizumab | NI | |||
| Activation of VEGFR-1 by human VEGF-A 165 | ||||
| Aflibercept | 16 | ←92-fold | ||
| Bevacizumab | 1476 | |||
| Ca2+ mobilization in HUVE cells by VEGF-A 165 | ||||
| Aflibercept | 2.6 | ←27-fold | ||
| Bevacizumab | 70.8 | |||
Note:
Data are derived from preclinical modeling and may not be reflective of clinical differentiation.
Abbreviations: HUVE, human umbilical vein endothelial; IC, inhibitory concentration; KA, association rate; KD, equilibrium dissociation constant; NA, not applicable; NB, no binding under assay conditions; NI, not inhibited; PlGF, placental growth factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Differences in biological activity
In terms of their biological activity as assessed via inhibition of VEGF-A or PlGF-2-induced activation of VEGFR-1, both bevacizumab and aflibercept inhibited VEGFR-1 activation induced by VEGF-A 165 or VEGF-A 121. Aflibercept, however, demonstrated 92-fold greater potency than bevacizumab when evaluated in this assay (Table 3).38 In line with its binding profile, aflibercept also inhibited VEGFR-1 activation by PlGF-2, whereas bevacizumab did not show any inhibitory activity (Table 3).38 When the ability to inhibit VEGF-A-induced activation of VEGFR-2 was examined in this study, aflibercept also inhibited activation of VEGFR-2 induced by VEGF-A 165 and was 51-fold more potent than bevacizumab (Table 3).38 As expected, PlGF-2 did not activate VEGFR-2 in this assay system.
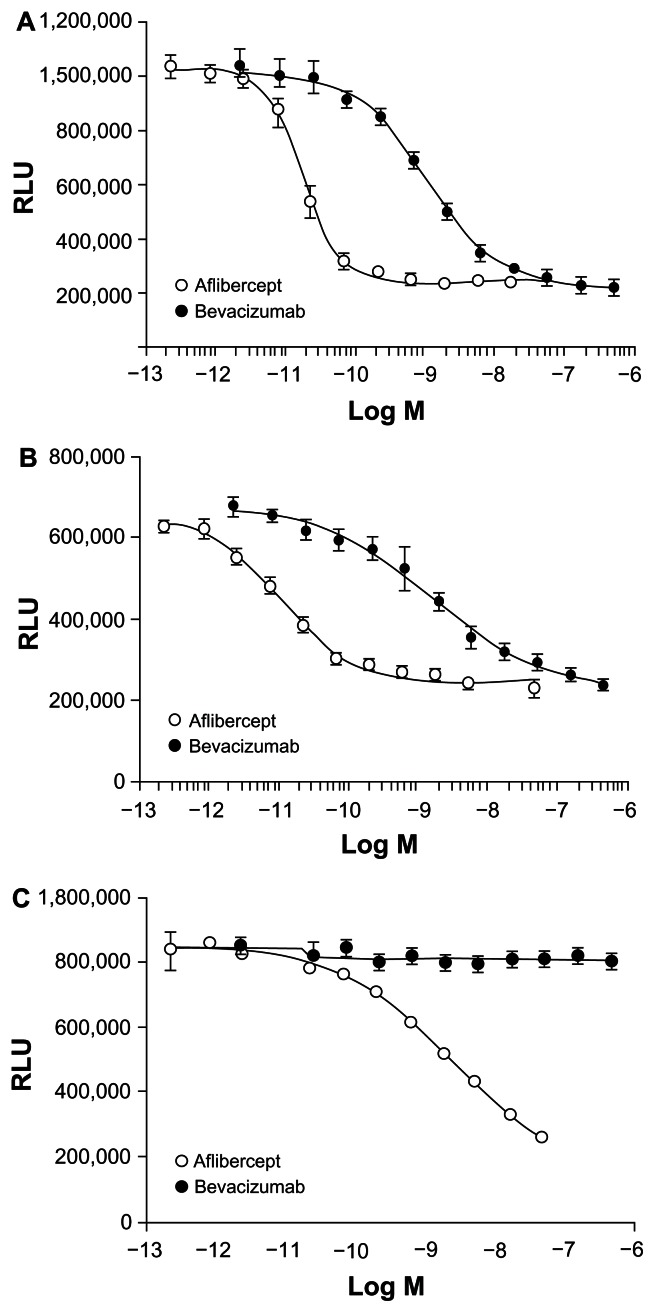
In cells expressing VEGFR-1, aflibercept was also more potent than bevacizumab in inhibiting luciferase activity as stimulated by either VEGF-A 121 or VEGF-A 165 (Figure 4A and B).38 When the ability to inhibit calcium mobilization in endothelial cells was examined in this study, bevacizumab effectively blocked Ca2+ mobilization in endothelial cells (which express VEGFR-1 and VEGFR-2) when induced by VEGF-A 165 (Table 3).38 The inhibitory concentration (IC) 50 for aflibercept was 27-fold lower than bevacizumab in this assay. Similarly, when the ability to inhibit human umbilical vein endothelial cell (HUVEC) migration induced by VEGF-A 165 or PlGF-2 was examined, aflibercept dose-dependently reduced HUVEC migration induced by VEGF-A 165 or PlGF-2.38 Bevacizumab also inhibited HUVEC migration induced by VEGF-A 165, but required greater molar concentrations than aflibercept to produce the same level of inhibition. As expected, bevacizumab did not inhibit HUVEC migration induced by PlGF-2.38
Figure 4.
(A) Effect of aflibercept and bevacizumab on luciferase activity in cells expressing VEGFR-1 stimulated by VEGF-A 121.38 (B) Effect of aflibercept and bevacizumab on luciferase activity in cells expressing VEGFR-1 stimulated by VEGF-A 165.38 (C) Effect of aflibercept and bevacizumab on luciferase activity in cells expressing VEGFR-1 stimulated by PlGF.38
Adapted from Papadopoulos N. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. The article/figure is published under Creative Commons License 2.0 CC-BY.38
Abbreviations: RLU, relative luciferase units; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
As a caveat to these findings, it must be noted that preclinical studies are not predictive of clinical efficacy in the treatment of cancer or other pathological conditions. It should also be mentioned that infusion and/or hypersensitivity reactions could distinguish agents such as bevacizumab and aflibercept from the TKIs, which are less likely to cause such AEs.30
AEs
Safety considerations are of course a key aspect of antiangiogenesis therapy, but comparative data can only be gleaned in the context of randomized controlled trials, and the approved labeling of these agents when used in mCRC. Because AEs are also impacted by the concomitant regimen and patient population studied, these factors must also be considered, and as such no directly comparative data exist among the approved biologic agents, bevacizumab and aflibercept. Leukopenia, diarrhea, neutropenia, proteinuria, increased aspartate transaminase, stomatitis, fatigue, throm- bocytopenia, increased alanine transaminase, hypertension, decreased weight, decreased appetite, epistaxis, abdominal pain, dysphonia, increased serum creatinine, and headache were the most common AEs of 20% or greater incidence occurring at higher incidence (2% or more) with aflibercept when used in combination with FOLFIRI (VELOUR trial).41 Neutropenia (37%), diarrhea (19%), hypertension (19%), leukopenia (16%), stomatitis (13%), fatigue (13%), proteinuria (8%), and asthenia (5%) were the most common Grade 3 to 4 adverse reactions of 5% or higher incidence reported at a higher incidence (2% or greater) in the aflibercept/FOLFIRI arm.41 The prescribing information for bevacizumab lists the grade 3 and 4 toxicities (5% or higher incidence) of bevacizumab in combination with IFL as leukopenia (37%), diarrhea (34%), neutropenia (21%), hypertension (12%), asthenia (10%), deep vein thrombosis (9%), abdominal pain (8%), and pain (8%).30 AEs associated with the use of bevacizumab in combination with FOLFIRI (ie, those most comparable to aflibercept) are not available from the prescribing information.
Although assessed in an open-label trial without a placebo control, when used as a first-line therapy in patients (n = 209) with mCRC, bevacizumab in combination with FOLFIRI was associated with grade 3 or 4 AEs (5% or more of patients) of neutropenia (29%), venous thromboembolic events ([VTE], 18%), diarrhea (12%), fatigue (10%), vomiting (7%), deep vein thrombosis (7%), pulmonary embolism (PE, 7%), nausea (6%), febrile neutropenia (6%), and hypertension (5%), with the VTE, PE, and febrile neutropenia events each including at least one event leading to death.75 In addition, at least one grade 3 or 4 targeted AE (ie, those historically associated with the use of bevacizumab), including VTE, hypertension, bleeding, proteinuria, gastrointestinal perforation, and wound healing complications occurred in 35% of patients in this trial overall.75 Taken together, although no directly comparative data are available, despite its broader binding specificity, aflibercept appears to have an AE profile related to inhibition of the VEGF axis, with no major distinguishing event from bevacizumab emerging as yet. Adverse reactions associated with the use of ramucirumab and IMC-18F1 will await results from randomized controlled clinical trials of these agents in mCRC.
Beyond colorectal cancer
Although antiangiogenesis therapy has been most successful in colorectal cancer, and in combination with chemotherapy, increasing diversity of biological agents may allow for a broader use across certain cancers, including glioblastoma76,77 and prostate cancer.78,79 The AVAglio trial is designed to assess the efficacy and safety of bevacizumab in combination with temozolomide in newly diagnosed glioblastoma patients,77 and aflibercept is also currently under investigation in a Phase II study of patients with recurrent malignant glioma that did not respond to temozolomide.80 Bevacizumab has been used in combination with docetaxel and prednisone (DP) in men with metastatic castration-resistant prostate cancer in the CALGB 90401 trial.78 Recently reported results showed no significant improvement in OS with DP plus bevacizumab compared with placebo (HR = 0.91; P = 0.181), although PFS (9.9 versus 7.5 months; P < 0.001) and objective response (49.4% versus 35.5%; P = 0.0013) were significantly improved.78 Aflibercept was also being studied (in combination with DP) in the Phase III VENICE trial of patients with metastatic androgen-independent prostate cancer. It was announced in early 2012, however, that the trial did not meet the prespecified endpoint of improvement in OS.79 Ramucirumab is also currently under investigation in patients with recurrent glioblastoma multiforme,81 and in patients with advanced androgen-independent prostate cancer.82
Conclusion
Angiogenesis continues to be a viable therapeutic target for pathological conditions, including cancer. The development and investigation of bevacizumab as a therapeutic agent has provided a basis for understanding the clinical potential for biological therapies that target angiogenesis. Nonetheless, it is clear that VEGF family members other than VEGF-A (eg, VEGF-B, PlGF) may have roles as angiogenic mediators in these conditions that are currently less clearly defined. There is additional evidence that these and other factors could also contribute to disease progression in patients treated with mono-targeted therapies for VEGF-A such as bevacizumab.14 The development of alternative biological therapies targeting angiogenesis, including those that target multiple ligands (eg, aflibercept), those targeting the VEGFRs (eg, ramucirumab), and the TKIs, are certainly expanding the potential for antiangiogenesis therapy. As recently pointed out by Zoppoli et al83 however, important unanswered questions remain, including how to determine which patients and which tumors are the best candidates for these therapies; clearly, identifying antiangiogenesis biomarkers will be an essential component of the safer, more efficient, and cost-effective application of these therapies in mCRC and other cancers. The availability of such markers would favor a more personalized approach to the treatment of mCRC, which considers both patient- and tumor-specific factors; a good example of this is the negative predictive value of the KRAS gene with the use of epidermal growth factor receptor (EGFR) inhibitors, cetuximab and panitumumab, in mCRC.84 The availability of this marker prevents the unnecessary use of these costly therapies in patients who are unlikely to respond.84 In addition, with an increasing number of targeted antiangiogenesis therapies available and used in combination, it may be necessary to move beyond a solely biomarker-centered approach to a more comprehensive view of angiogenesis as a complex biologic system; a recent review considers this topic in detail in the context of the EGFR inhibitors.84
In a review of the available preclinical literature of the antiangiogenic agents,59 it was found that inter-agent and preclinical comparisons between the anti-VEGF TKIs are currently lacking. It will be of interest to determine whether such differences, if observed, can be related to efficacy.59 It also remains to be seen whether differences in kinase inhibitory activity among these agents (ie, on-target versus off-target effects) will be of predictive value in determining which patients and/or tumors will benefit from which TKIs. There is, however, some evidence for notable preclinical differences in binding, as well as assessable biological activity, among the available antiangiogenic biological therapies.38 Whether these differences will translate into improved efficacy and/or expanded indications for these agents remains to be further explored. Another important unresolved issue is how these agents can best be integrated into a sequential treatment plan for mCRC patients. The recent results from the CORRECT trial have, for example, established a role for the more broadly-targeted regorafenib in the third-line setting.73 In addition, results of the VELOUR trial have established the efficacy of aflibercept in a population of mCRC patients, approximately one third of whom had progressed on a regimen containing prior bevacizumab.40 There is a need to better understand whether this relates to the broader specificity of aflibercept as compared to single-targeted bevacizumab; this could form the basis for the logical use of these agents in sequence.
Acknowledgments
Medical editorial assistance was provided by Susan DePetris, PhD, of Phase Five Communications Inc, and supported by Sanofi-aventis US LLC, in collaboration with Regeneron Pharmaceuticals.
Footnotes
Disclosure
The author declares no conflicts of interest in this work.
References
- 1.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 2.Samant RS, Shevde LA. Recent advances in anti-angiogenic therapy of cancer. Oncotarget. 2011;2(3):122–134. doi: 10.18632/oncotarget.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9( Suppl 1):2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785–1788. doi: 10.1248/bpb.34.1785. [DOI] [PubMed] [Google Scholar]
- 5.Crawford Y, Ferrara N. VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res. 2009;335(1):261–269. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 7.Escudero-Esparza A, Martin TA, Davies ML, Jiang WG. PGF isoforms, PlGF-1 and PlGF-2, in colorectal cancer and the prognostic significance. Cancer Genomics Proteomics. 2009;6(4):239–246. [PubMed] [Google Scholar]
- 8.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333(2):328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11(1):1–13. doi: 10.1016/j.clcc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Lee C, Tang Z, et al. VEGF-B: a survival, or an angiogenic factor? Cell Adh Migr. 2009;3(4):322–327. doi: 10.4161/cam.3.4.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 14.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28(3):453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korpanty G, Sullivan LA, Smyth E, Carney DN, Brekken RA. Molecular and clinical aspects of targeting the VEGF pathway in tumors. J Oncol. 2010;2010:652320. doi: 10.1155/2010/652320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olofsson B, Korpelainen E, Pepper MS, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci U S A. 1998;95(20):11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: a historical review. Angiogenesis. 2008;11(3):215–221. doi: 10.1007/s10456-008-9114-4. [DOI] [PubMed] [Google Scholar]
- 18.Hilmi C, Guyot M, Pagès G. VEGF spliced variants: possible role of anti-angiogenesis therapy. J Nucleic Acids. 2012;2012:162692. doi: 10.1155/2012/162692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu QS. Aflibercept (AVE0005): an alternative strategy for inhibiting tumour angiogenesis by vascular endothelial growth factors. Expert Opin Biol Ther. 2009;9(2):263–271. doi: 10.1517/14712590802666397. [DOI] [PubMed] [Google Scholar]
- 20.Olofsson B, Pajusola K, von Euler G, Chilov D, Alitalo K, Eriksson U. Genomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoform. J Biol Chem. 1996;271(32):19310–19317. doi: 10.1074/jbc.271.32.19310. [DOI] [PubMed] [Google Scholar]
- 21.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8(12):942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 22.Hamerlik P, Lathia JD, Rasmussen R, et al. Autocrine VEGF-VEGFR2- Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209(3):507–520. doi: 10.1084/jem.20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van de Veire S, Stalmans I, Heindryckx F, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141(1):178–190. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Bais C, Wu X, Yao J, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141(1):166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Loges S, Schmidt T, Carmeliet P. Antimyeloangiogenic therapy for cancer by inhibiting PlGF. Clin Cancer Res. 2009;15(11):3648–3653. doi: 10.1158/1078-0432.CCR-08-2276. [DOI] [PubMed] [Google Scholar]
- 27.Yao J, Wu X, Zhuang G, et al. Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. Proc Natl Acad Sci U S A. 2011;108(28):11590–11595. doi: 10.1073/pnas.1109029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Tang Z, Hou X, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A. 2009;106(15):6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tol J, Punk CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther. 2010;32(3):437–453. doi: 10.1016/j.clinthera.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Avastin®(bevacizumab) [prescribing information] South San Francisco, CA: Genentech, Inc; 2013. [Google Scholar]
- 31.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 32.Van der Veldt AA, Lubberink M, Bahce I, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21(1):82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Maitland ML, Lou XJ, Ramirez J, et al. Vascular endothelial growth factor pathway. Pharmacogenet Genomics. 2010;20(5):346–349. doi: 10.1097/FPC.0b013e3283364ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 35.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108(9):3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tew WP, Gordon M, Murren J, et al. Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin Cancer Res. 2010;16(1):358–366. doi: 10.1158/1078-0432.CCR-09-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teng LS, Jin KT, He KF, Zhang J, Wang HH, Cao J. Clinical applications of VEGF-trap (aflibercept) in cancer treatment. J Chin Med Assoc. 2010;73(9):449–456. doi: 10.1016/S1726-4901(10)70097-6. [DOI] [PubMed] [Google Scholar]
- 40.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 41.ZALTRAP®(ziv-aflibercept) [prescribing information] Bridgewater, NJ: Regeneron Pharmaceuticals, Inc/sanofi-aventis US LLC; 2012. [Google Scholar]
- 42.Sanofi. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed April 26, 2013]. A Multinational, randomized, double-blind study, comparing the efficacy of aflibercept once every 2 weeks versus placebo in patients treated with gemcitabine for metastatic pancreatic cancer (VANILLA) Available from: http://www.clinicaltrials.gov/ct2/show/NCT00574275. NLM identifier: NCT00574275. [Google Scholar]
- 43.Sanofi. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed April 26, 2013]. A multicenter, randomized, double blind study comparing the efficacy and safety of aflibercept versus placebo administered every 3 weeks in patients treated with docetaxel/prednisone for metastatic androgen-independent prostate cancer (VENICE) Available from: http://clinicaltrials.gov/show/NCT00519285. NLM identifier: NCT00519285. [Google Scholar]
- 44.Sanofi. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed April 26, 2013]. A multinational, randomized, double-blind study comparing aflibercept versus placebo in patients treated with second-line docetaxel after failure of one platinum based therapy for locally advanced or metastatic non-small-cell lung cancer (VITAL) Available from: http://clinicaltrials.gov/show/NCT00532155. NLM identifier: NCT00532155. [Google Scholar]
- 45.Sanofi-aventis and Regeneron Report Top-line Results from Phase III Study with aflibercept (VEGF Trap) in Second-Line Non-Small Cell Lung Cancer [webpage on the Internet] PR Newswire. Mar 10, 2011. [Accessed November 30, 2012]. Available from: http://www.prnewswire.com/news-releases/sanofi-aventis-and-regeneron-report-top-line-results-from-phase-iii-study-with-aflibercept-vegf-trap-in-second-line-non-small-cell-lung-cancer-117757228.html.
- 46.Jin K, Shen Y, He K, Xu Z, Li G, Teng L. Aflibercept (VEGF Trap): one more double edged sword of anti-VEGF therapy for cancer? Clin Transl Oncol. 2010;12(8):526–532. doi: 10.1007/s12094-010-0550-4. [DOI] [PubMed] [Google Scholar]
- 47.Spratlin J. Ramucirumab (IMC-1121B): monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr Oncol Rep. 2011;13(2):97–102. doi: 10.1007/s11912-010-0149-5. [DOI] [PubMed] [Google Scholar]
- 48.Krupitskaya Y, Wakelee HA. Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr Opin Investig Drugs. 2009;10(6):597–605. [PubMed] [Google Scholar]
- 49.Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6(9):507–518. doi: 10.1038/nrclinonc.2009.110. [DOI] [PubMed] [Google Scholar]
- 50.Bruns CJ, Liu W, Davis DW, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89(3):488–499. [PubMed] [Google Scholar]
- 51.Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59(20):5209–5218. [PubMed] [Google Scholar]
- 52.Eli Lilly and Company/ImClone LLC. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed April 26, 2013]. A randomized, double-blind, multicenter phase 3 study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progressive during or following first-line combination therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine. Available from: http://clinicaltrials.gov/show/NCT01183780. NLM identifier: NCT01183780. [Google Scholar]
- 53.National Cancer Institute (NCI) ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed April 26, 2013]. A randomized phase II study of irinotecan and cetuximab with or without the anti-angiogenic antibody, ramucirumab (IMC-1121B), in advanced, K-ras wild-type colorectal cancer following progression on bevacizumab-containing chemotherapy ) Available from: http://clinicaltrials.gov/show/NCT01079780. NLM identifier: NCT01079780. [Google Scholar]
- 54.ImClone LLC. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed April 26, 2013]. An open-label, multicenter, randomized phase 2 study evaluating the safety and efficacy of 5 FU/FA and oxaliplatin (modified FOLFOX 6) in combination with IMC-1121B or IMC-18F1 or without investigational therapy as second line therapy in patients with metastatic colorectal cancer following disease progression on first line irinotecan-based therapy. Available from: http://clinicaltrials.gov/show/NCT01111604. NLM identifier: NCT01111604. [Google Scholar]
- 55.Grothey A, Tabernero J, Rougier P, et al. A randomized, double-blind, phase (Ph) III study of the irinotecan-based chemotherapy FOLFIRI plus ramucirumab (RAM) or placebo (PL) in patients (pts) with metastatic colorectal carcinoma (mCRC) progressive during or following first-line therapy with bevacizumab (BEV), oxaliplatin (OXALI), and a fluoropyrimidine (FP) (RAISE) ( NCT01183780) J Clin Oncol. 2012;30(Suppl) Abstract TPS3634. [Google Scholar]
- 56.Schwartz JD, Rowinsky EK, Youssoufian H, Pytowski B, Wu Y. Vascular endothelial growth factor receptor-1 in human cancer: concise review and rationale for development of IMC-18F1 (human antibody targeting vascular endothelial growth factor receptor-1) Cancer. 2010;116(Suppl 4):1027–1032. doi: 10.1002/cncr.24789. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y, Zhong Z, Huber J, et al. Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin Cancer Res. 2006;12(21):6573–6584. doi: 10.1158/1078-0432.CCR-06-0831. [DOI] [PubMed] [Google Scholar]
- 58.Bhargava P, Robinson MO. Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep. 2011;13(2):103–111. doi: 10.1007/s11912-011-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6(10):569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 60.Pfizer. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2011. [Accessed April 26, 2013]. A randomized, phase 2 study of FOLFOX Or FOLFIRI with AG-013736 or bevacizumab (Avastin) in patients with metastatic colorectal cancer after failure of an irinotecan or oxaliplatin-containing first-line regimen. Available from: http://clinicaltrials.gov/ct2/show/NCT00615056. NLM identifier: NCT00615056. [Google Scholar]
- 61.Pfizer. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2011. [Accessed April 26, 2013]. A phase II trial of single agent axitinib as maintenance therapy for patients with first line metastatic colorectal cancer (mCRC) Available from: http://www.clinicaltrials.gov/ct2/show/NCT01490866. NLM identifier: NCT01490866. [Google Scholar]
- 62.Boehringer Ingelheim Pharmaceuticals. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2011. [Accessed April 26, 2013]. A phase I-II study of BIBF 1120 and FOLFOX compared to bevacizumab and FOLFOX in first line metastatic colorectal cancer patients. Available from: http://clinicaltrials.gov/show/NCT00904839. NLM identifier: NCT00904839. [Google Scholar]
- 63.NCIC Clinical Trials Group. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2013. [Accessed April 26, 2013]. A phase III randomized study of Brivanib Alaninate (BMS-582664) in combination with cetuximab (Erbitux®) versus placebo in combination with cetuximab (Erbitux®) in patients with K-RAS wild type tumors previously treated with combination chemotherapy for metastatic colorectal carcinoma. Available from: http://clinicaltrials.gov/show/NCT00640471. NLM identifier: NCT00640471. [Google Scholar]
- 64.AstraZeneca. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2013. [Accessed April 26, 2013]. A randomised, double-blind, multicentre phase II/III study to compare the efficacy of Cediranib (RECENTIN™, AZD2171) in combination with 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX), to the efficacy of bevacizumab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (HORIZON III) Available from: http://clinicaltrials.gov/show/NCT00384176. NLM identifier: NCT00384176. [Google Scholar]
- 65.Pfizer. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2013. [Accessed April 26, 2013]. A multicenter, randomised, double-blind, phase 3 study of sunitinib in metastatic colorectal cancer patients receiving irinotecan, 5-fluorouracil and leucovorin (FOLFIRI) as first line treatment. Available from: http://clinicaltrials.gov/show/NCT00457691. NLM identifier: NCT00457691. [Google Scholar]
- 66.AIO-Studien-gGmbH. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2013. [Accessed April 26, 2013]. A controlled randomized double-blind multicenter phase II study of FOLFOX6 or FOLFIRI combined with sorafenib versus placebo in second-line metastatic colorectal carcinoma. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00889343. NLM identifier: NCT00889343. [Google Scholar]
- 67.Bayer. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2013. [Accessed April 26, 2013]. A randomized, double-blind, placebo-controlled phase III study of regorafenib plus BSC versus placebo plus BSC in patients with metastatic colorectal cancer (CRC) who have progressed after standard therapy. Available from: http://clinicaltrials.gov/show/NCT01103323. NLM identifier: NCT01103323. [Google Scholar]
- 68.Bayer. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2013. [Accessed April 26, 2013]. An open-label phase IIIb study of regorafenib in patients with metastatic colorectal cancer (CRC) who have progressed after standard therapy (CONSIGN) Available from: http://clinicaltrials.gov/show/NCT01538680. NLM identifier: NCT01538680. [Google Scholar]
- 69.AstraZeneca. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2013. [Accessed April 26, 2013]. A phase II, double-blind, placebo controlled, randomized study to assess the efficacy and safety of 2 doses of ZD6474 (Vandetanib) in combination with FOLFOX vs FOLFOX alone for the treatment of colorectal cancer in patients who have failed therapy with an irinotecan and fluoropyrimidine regimen. Available from: http://clinicaltrials.gov/show/NCT00500292. NLM identifier: NCT00500292. [Google Scholar]
- 70.AstraZeneca. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; 2011. [Accessed April 26, 2013]. A phase II, double blind, placebo controlled, randomised study to assess the efficacy and safety of 2 doses of ZACTIMA™(ZD6474) in combination with FOLFIRI vs FOLFIRI alone for the treatment of colorectal cancer in patients who have failed therapy with anoxaliplatin and fluoropyrimidine containing regimen. Available from: http://clinicaltrials.gov/show/NCT00454116. NLM identifier: NCT00454116. [Google Scholar]
- 71.Stivarga®(regorafenib) [prescribing information] Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; 2013. [Google Scholar]
- 72.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 73.Grothey A, Van Cutsem E, Sobrero A, et al. CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 74.Rudge JS, Holash J, Hylton D, et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A. 2007;104(47):18363–18370. doi: 10.1073/pnas.0708865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sobrero A, Ackland S, Clarke S, et al. AVIRI Trial investigators. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77(2):113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 76.Narayana A, Gruber D, Kunnakkat S, et al. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg. 2012;116(2):341–345. doi: 10.3171/2011.9.JNS11656. [DOI] [PubMed] [Google Scholar]
- 77.Chinot OL, de La Motte Rouge T, Moore N, et al. AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28(4):334–340. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 78.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo- controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30(13):1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanofi/Regeneron. Sanofi and Regeneron Announce Regulatory and Clinical Update for Zaltrap® (aflibercept) [press release] Paris: Sanofi/Tarrytown, NY: Regeneron; Apr 5, 2012. [Accessed November 30, 2012]. Available from: http://en.sanofi.com/Images/30138_20120405_ZALTRAP_BLA_VENICE_en.pdf. [Google Scholar]
- 80.National Cancer Institute (NCI) ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; [Accessed April 26, 2013]. Phase II single arm trial of VEGF trap in patients with recurrent temozolomide-resistant malignant gliomas. Available from: http://clinicaltrials.gov/show/NCT00369590. NLM identifier: NCT00369590. [Google Scholar]
- 81.National Cancer Institute (NCI)/ImClone LLC. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; [Accessed April 26, 2013]. An open label, phase 2 study evaluating the safety and efficacy of IMC-3G3 or IMC-1121B in patients with recurrent glioblastoma multiforme. Available from: http://clinicaltrials.gov/show/NCT00895180. NLM identifier: NCT00895180. [Google Scholar]
- 82.ImClone LLC. ClinicalTrials.gov [website on the internet] Bethesda, MD: US National Library of Medicine; [Accessed April 26, 2013]. A phase 2, multicenter, randomized study of IMC-A12 or IMC-1121B plus mitoxantrone and prednisone in metastatic androgen-independent prostate cancer (AIPC) following disease progression on docetaxel-based chemotherapy. Available from: http://clinicaltrials.gov/show/NCT00683475. NLM identifier: NCT00683475. [Google Scholar]
- 83.Zoppoli G, Ferrando V, Scabini S. On biomarkers and pathways in rectal cancer: What’s the target? World J Gastrointest Surg. 2012;4(12):275–277. doi: 10.4240/wjgs.v4.i12.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ballestrero A, Garuti A, Cirmena G, et al. Patient-tailored treatments with anti-EGFR monoclonal antibodies in advanced colorectal cancer: KRAS and beyond. Curr Cancer Drug Targets. 2012;12(4):316–328. doi: 10.2174/156800912800190956. [DOI] [PubMed] [Google Scholar]