Abstract
Converging research efforts suggest that nicotine and other drugs that act at nicotinic acetylcholine receptors (nAChRs) may be beneficial in the management of Parkinson’s disease. This idea initially stemmed from the results of epidemiological studies which demonstrate that smoking is associated with a decreased incidence of Parkinson’s disease. The subsequent finding that nicotine administration protected against nigrostriatal damage in parkinsonian animal models led to the idea that nicotine in tobacco products may contribute to this apparent protective action. Nicotine most likely exerts its effects by interacting at nAChRs. Accumulating research indicates that multiple subtypes, including α4β2, α6β2 and/or α7 containing nAChRs, may be involved. Stimulation of nAChRs initially activates various intracellular transduction pathways primarily via alterations in calcium signaling. Consequent adaptations in immune responsiveness and trophic factors may ultimately mediate nicotine’s ability to reduce/halt the neuronal damage that arises in Parkinson’s disease. In addition to a potential neuroprotective action, nicotine also has anti-depressant properties and improves attention/cognition. Altogether, these findings suggest that nicotine and nAChR drugs represent promising therapeutic agents for the management of Parkinson’s disease.
Keywords: Neuroprotection, Nicotine, Nicotinic, Nigrostriatal damage, Parkinson’s disease
Introduction
A critical unmet need in the management of Parkinson’s disease is the development of strategies to slow, stop, or preferably reverse the neurodegenerative process. Parkinson’s disease is a neurological disorder characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta that results in tremor, rigidity and bradykinesia 1–7. Although the nigrostriatal dopaminergic deficits are the most severe, there are also declines in numerous other CNS neurotransmitter systems. These most likely underlie the non-motor problems associated with Parkinson’s disease, including autonomic deficits, psychiatric symptoms, behavioral changes, dementia, sleep disorders and others 1–7. Dopamine replacement therapies provide effective control of the motor symptoms, particularly in the early stages of the disease. However, they do not adequately manage the non-motor deficits and, in addition, induce a variety of motor and psychiatric side effects. Moreover, they provide only symptomatic relief while the underlying disease continues to worsen. These shortcomings highlight the importance of identifying novel treatment strategies that delay or halt disease progression, or ideally restore function in Parkinson’s disease.
Development of neuroprotective agents for Parkinson’s disease
Although drug development has yielded numerous agents for the symptomatic control of motor impairments in Parkinson’s disease, there are as yet no approved drugs capable of reducing disease progression. One reason for this relates to uncertainty as to the cause of Parkinson’s disease (Table 1). Accumulating evidence indicates that exposure to environmental agents, such as fungicides, herbicides, pesticides and metals, is associated with an increased risk of Parkinson’s disease 8–12. In addition, specific gene defects have been linked to familial and sporadic forms of Parkinson’s disease including mutations in LRRK2, alpha-synuclein, parkin, DJ-1, PINK1 and others 13–15. However, it is unclear how these environmental insults and/or gene mutations contribute to the degenerative changes observed in Parkinson’s disease brain, for instance, mitochondrial dysfunction, oxidative stress, modifications in protein handling, adaptations in immune-modulators, as well as alterations in other molecular and cellular functions 1, 15. An understanding of the factors involved in the etiology of Parkinson’s disease and how they mediate subsequent pathological changes is essential for the development of rational neuroprotective strategies. Moreover, this knowledge may lead to the identification of an early biomarker for Parkinson’s disease. Symptoms only arise when there is already considerable neuronal degeneration; early detection would allow for the administration of protective treatments before the onset of disease symptoms.
TABLE 1.
Difficulties in developing neuroprotective strategies against Parkinson’s disease
|
Other factors (Table 1) that have hampered the identification of clinically effective neuroprotective agents for Parkinson’s disease include the lack of parkinsonian animal models that precisely mimic the pathogenesis of the disease with respect to its etiology, slow progressive nature and pattern of cell loss 16–20. Most of the neurotoxin-induced or genetic animal models lack one or more of these key features, although a more recent rotenone model may represent a better alternative 16–21. This shortcoming is exacerbated by difficulties in translating the animal data to the design of an effective clinical trial with respect to optimal drug dosage and timing. A drug treatment regimen in an animal model may not be suitable in Parkinson’s disease patients because of differences in drug metabolism, pharmacokinetics and pharmacodynamics. Another obstacle in the development of effective neuroprotective strategies is an inability to discriminate between the acute and long term effects of a drug. For instance, the drug of interest may acutely improve the same clinical symptoms that are also the endpoint of the neuroprotective trial, thus complicating data interpretation. Continued pre-clinical and clinical research is necessary to resolve these issues and identify targeted neuroprotective drugs.
Putative neuroprotective strategies for Parkinson’s disease
Despite the above limitations, there is optimism in the development of disease modifying strategies for Parkinson’s disease. An expanding pre-clinical effort provides support for a growing number of agents that may be useful for neuroprotection against nigrostriatal damage. Results from in vitro and in vivo work have led to the design of a number of trials investigating neuroprotection in Parkinson’s disease patients (Table 2). Drugs under study include compounds that modulate mitochondrial function like creatine and coenzyme Q 22–24 and the antioxidant glutathione 25. Trophic factors 26, 27, immune-modulators 28–31, and the calcium channel blocker isradipine 32 have or are being tested for their ability to delay disease progression. The diversity of agents initially appears somewhat daunting but may simply reflect the numerous interactive mechanisms that play a role in neurodegeneration under different conditions.
TABLE 2.
Neuroprotection trials for Parkinson’s disease
| Drug/compound | ClinicalTrials. gov # | Purpose | Mechanisms of action | Status | Outcome |
|---|---|---|---|---|---|
| Green Tea Polyphenol | NCT00461942 | Delay disease progression | Antioxidant | Completed March 2009 | No Results Posted on ClinicalTrials.gov |
| Minocycline | NCT00063193 | Delay disease progression | Anti-inflammatory | Completed July 2005 | No Results Posted on ClinicalTrials.gov |
| Coenzyme Q10 | NCT00740714 | Neuroprotection | Modulates mitochondrial function | Completed Aug 2011 | No efficacy over placebo |
| Isradipine | NCT00753636 | Delay disease progression | Calcium channel blocker | Completed Feb 2010 | No significant change in UPDRS |
| GPI 1485 | NCT00076492 | Neuroprotection | Immunophilin compound | Completed | No Results Posted on ClinicalTrials.gov |
| Erythropoietin | NCT01010802 | Safety and tolerability for neuroprotection | Trophic factor | Completed May 2009 | No Results Posted on ClinicalTrials.gov |
| Rasagiline | NCT00256204 | Delay disease progression | Monoamine oxidase inhibitor | Completed June 2009 | Delayed need for symptomatic antiparkinsonian drugs 57 |
| MitoQ | NCT00329056 | Delay disease progression | Antioxidant | Completed Nov 2007 | No Results Posted on ClinicalTrials.gov |
| Folic acid and L-methylfolate | NCT00853879 | Delay disease progression | Decrease homocysteine levels | Completed | No Results Posted on ClinicalTrials.gov |
| GM1 Ganglioside | NCT00037830 | Neuroprotection | Improves lipid function | Ongoing | |
| Creatine | NCT00449865 | Neuroprotection | Modulates mitochondrial function | Ongoing | |
| Inosine | NCT00833690 | Safety and tolerability for neuroprotection | Urate elevation | Ongoing/recruiting | |
| Bee venom | NCT01341431 | Symptomatic & neuroprotective | Not known | Ongoing | |
| Deep brain stimulation | NCT00282152 | Safety and tolerability | Not known | Ongoing | |
| VIUSID/ALZER | NCT01016470 | Delay disease progression | Antiviral/anti-oxidant | Ongoing | |
| Nicotine | NCT00873392 | Neuroprotection | Acts at nicotinic receptors | Recruiting | |
| Nicotine | MJFF clinical trial | Neuroprotection | Acts at nicotinic receptors | Recruiting | |
| Deferiprone | NCT00943748 | Neuroprotection | Iron chelator | Recruiting | |
| Exendin-4 | NCT01174810 | Disease-modifying | Antidiabetic | Recruiting | |
| Granulocyte-colony Stimulating Factor | NCT01227681 | Neuroprotection | Trophic factor | Recruiting | |
| Pioglitazone | NCT01280123 | Neuroprotection | Antidiabetic | Recruiting | |
| Glutathione | NCT01398748 | Safety and tolerability for subsequent neuroprotection | Modulates mitochondrial function | Recruiting | |
| Preladenant | NCT01155479 | Safety and efficacy | Adenosine A2a antagonist | Recruiting | |
| Autologous Adipose-derived stromal cells | NCT01453803 | Improve disease pathology | Cell protection, repair and restoration | Recruiting | |
| Safinamide | NCT01028586 | Delay disease progression | MAO-B and glutamate release inhibitor | Recruiting | |
| N-Acetylcysteine | NCT01470027 | Neuroprotection | Antioxidant | Not yet recruiting |
Epidemiological work has also been instrumental in identifying agents that may protect against Parkinson’s disease 10, 11, 33, 34. The most consistent and notable of these findings are the inverse associations between Parkinson’s disease and elevated uric acid levels, coffee drinking and smoking. Uric acid, an antioxidant found in high concentrations in serum and brain, had been hypothesized to protect against oxidative damage and cell death as occurs in Parkinson’s disease. Indeed, subsequent studies showed an inverse correlation between elevated uric acid and Parkinson’s disease 35–38. These combined findings formed the basis for a clinical trial to test inosine, which elevates urate levels, for its potential to modify Parkinson’s disease progression (Table 2). An environmental factor that has been associated with a decreased incidence of Parkinson’s disease is coffee drinking. Coffee may be beneficial via an antagonistic action of caffeine at adenosine A2a receptors 34, 39, 40. A clinical trial to test the adenosine A2a antagonist preladenant is currently in progress (Table 2). Another lifestyle factor inversely correlated to the development of Parkinson’s disease is smoking. The epidemiological evidence for this association and the components in tobacco smoke that may be responsible for smoking’s apparent protective effect is the focus of the remainder of this review.
Smoking is linked to a reduced incidence of Parkinson’s disease
An extensive epidemiological literature quite unexpectedly showed that tobacco use is associated with a lower incidence of Parkinson’s disease 10, 11, 33. Over 50 studies done over the last half century consistently demonstrate a reduced prevalence of Parkinson’s disease among smokers compared to never-smokers 12, 41–43. This inverse association between Parkinson’s disease and smoking is correlated with increased intensity and duration of smoking, is more pronounced in current compared with former smokers, decreases with years after quitting smoking and was observed with different types of tobacco products. Importantly, it did not appear to be due to selective survival of Parkinson’s disease cases or reporting bias 42–51. These combined findings provide strong evidence for a negative association between smoking and Parkinson’s disease.
Nicotine protects against nigrostriatal damage in parkinsonian animal models
Such compelling evidence for a decreased incidence of Parkinson’s disease with smoking prompted studies to identify the active component(s) as such work may yield insight about potential neuroprotective strategies. A drawback is that tobacco and its combustion products contain thousands of chemicals any of which may improve neuronal integrity. However, despite the extensive number of reagents, tobacco constituents have been identified that protect against nigrostriatal damage in animals models.
One of these is 2,3,6-trimethyl-1,4-naphthoquinone (TMN) an inhibitor of monoamine oxidase (MAO) A and B activity 52–54. TMN partially protects against MPTP-induced neurodegeneration in mice by reducing endogenous dopamine metabolism and consequently decreasing oxidative stress. It may also protect by blocking MAO-mediated activation of exogenous neurotoxins 55, 56. An example of a synthetic MAO B inhibitor currently used in the treatment of Parkinson’s disease is rasagiline. This drug appears to provide symptomatic relief and may also protect against nigrostriatal damage because of its ability to decrease dopamine metabolism and prolong the action of dopamine 56. In fact, rasagiline delayed the need for antiparkinsonian drugs in a recent clinical trial 57 (Table 2).
In addition to MAO inhibitors, another chemical in tobacco that has been the focus of intense research is nicotine. The rationale for investigating a role for nicotine is based on results demonstrating a close anatomical relationship between the nicotinic cholinergic and dopaminergic neurotransmitter systems in the striatum 58. Moreover, nicotine influences dopaminergic activity by acting at nicotinic receptors (nAChRs) on dopaminergic terminals and modulating dopamine release 59, 60. Such actions of nicotine may ultimately result in its overall functional effects including protection against nigrostriatal damage 61–63.
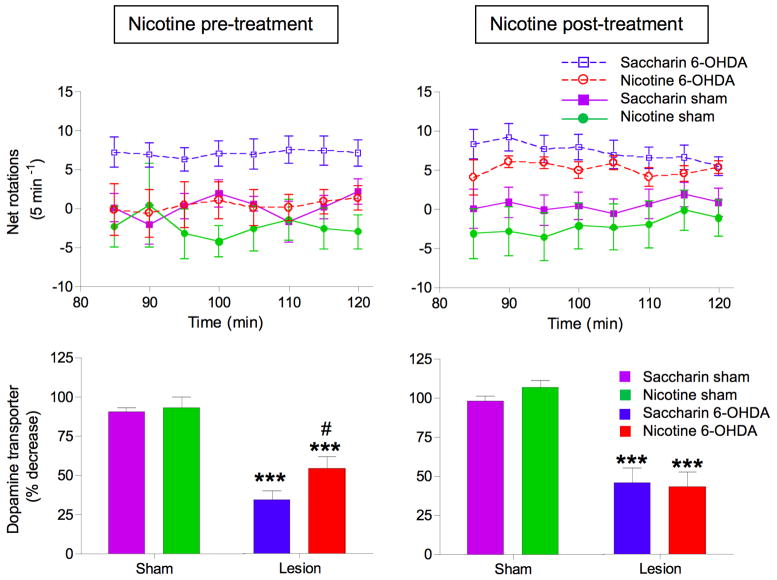
Numerous experimental studies have shown that nicotine administration enhances dopaminergic integrity in the striatum of parkinsonian rodents and monkeys 60–62. This includes protection against MPTP-, 6-hydroxydopamine- or paraquat-induced toxicity in rats and mice 64–70. Chronic nicotine administration also reduced MPTP-induced striatal damage in nonhuman primates, a model that shares many resemblances with the human disease 71, 72. Several months of nicotine exposure improved striatal tyrosine hydroxylase, the dopamine and vesicular monoamine transporters, dopamine levels, nAChR expression and normalized lesion-induced over activity of the nigrostriatal pathway. This effect of nicotine appears to be due to protection against ongoing degeneration, as nicotine treatment did not enhance dopaminergic measures when administered to animals with pre-existing nigrostriatal damage (Fig. 1) 66. These latter observations suggest that early treatment would yield optimal therapeutic benefit in Parkinson’s disease patients.
FIG. 1.
Nicotine is neuroprotective when administered before/during but not after nigrostriatal damage. For the pre-treatment studies, rats were first given nicotine in drinking water (50 μg/ml) for 2 wk after which they were lesioned with 6-hydroxydopamine, with nicotine maintained. Amphetamine-induced rotations were determined 2–3 wk later as an index of motor disability. The rats were then killed 2–3 wk later and the dopamine transporter measured. In the nicotine post-treatment study, rats were first lesioned and amphetamine-induced rotation measured 2 wk later. Immediately after behavioral assessment, nicotine treatment was initiated and maintained throughout. Rotational behavior was re-evaluated 3–4 wk after the start of nicotine dosing and the rats killed 3–4 wk later, such that the total number of wk on nicotine treatment was similar in the two paradigms. Top panels: Parkinsonism assessed by amphetamine-induced ipsilateral turning. Three-way ANOVA analyses showed a significant (p < 0.001) main effect of 6-OHDA lesioning and a significant (p < 0.05) interaction between nicotine treatment and 6-OHDA lesioning in rats treated with nicotine prior to the onset of nigrostriatal lesion. By contrast, nicotine treatment after completion of nigrostriatal damage yielded a significant main effect of 6-OHDA lesioning (p < 0.001) but no interaction. Bottom panels: Effects of nicotine pre- and post-treatment on neuronal damage. Dopamine transporter expression was significantly elevated in lesioned rats with nicotine pre- but not post-treatment. Significance of difference by two-way ANOVA followed by a Bonferroni post hoc test from the saccharin-sham group, ***p < 0.001; from the saccharin-lesioned group, #p < 0.05. Values represent the mean ± SEM of 6–9 rats per group. Taken with permission 66.
Altogether, these data form the basis for the idea that nicotine may contribute, at least in part, to the apparent neuroprotective effect of tobacco use in Parkinson’s disease.
Nicotine acts at nicotinic receptors
An important question is by what mechanisms nicotine protects against neuronal damage as such knowledge may allow for the development of drugs that selectively target the relevant molecular deficits. Considerable evidence suggests that nicotine primarily exerts its effects by acting at nAChRs. These are pentameric ligand-gated cation channels composed of varying combinations of different α and β subunits. The naturally occurring neurotransmitter for this receptor is acetylcholine which binds to the α or ligand binding subunit, of which there are 5 types in mammalian brain (α2, α3, α4, α6 and α7). In addition, the receptor may contain subunits which do not bind acetylcholine including the β2, β3, β4 and also the α5 subunit 73, 74.
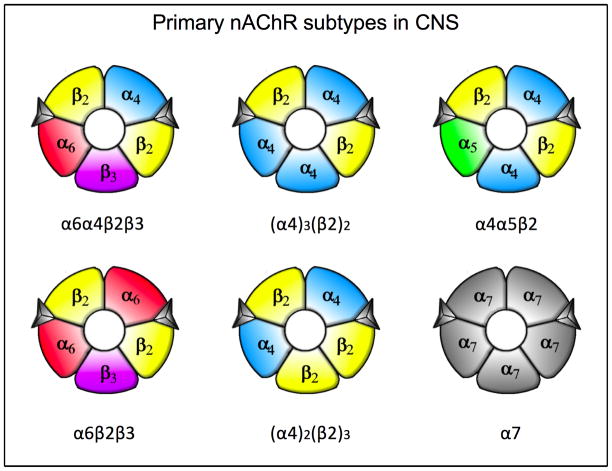
These receptor subunits co-assemble to form a diverse family of nAChRs, the most abundant of which are homomeric α7 nAChRs and heteromeric β2 containing nAChRs (Fig. 2). These latter subtypes generally also contain α4 or α6 subunits to form two primary subpopulations, the α4β2* and α6β2* nAChRs (the asterisk denoting the possible presence of other subunits in the receptor complex). The α4β2* nAChRs are widely distributed throughout the brain, including the nigrostriatal pathway, while α6β2* nAChRs exhibit a more restricted CNS distribution that includes the nigrostriatal system 59, 74, 75. Homomeric α7 nAChRs, like the α4β2* nAChRs, are also extensively localized throughout the brain although α7 receptors are expressed at a very low density in the nigrostriatal system of rats and monkeys. These findings suggest that, if α7 receptors influence nigrostriatal function, it would be through secondary effects on other brain regions.
FIG. 2.
Primary nAChR subtypes in the mammalian CNS. The α6α4β2β3 and α6β2β3 nAChRs have a relatively restricted distribution in the CNS, including the nigrostriatal system. By contrast the α4β2 nAChRs, which exists in two unique conformations, and the α4α5β2 nAChR are widely present throughout the brain, including the nigrostriatal pathway. The homomeric α7 nAChR also exhibits an extensive distribution in the mammalian CNS, although this subtype is not densely expressed in the rat and monkey nigrostriatal system.
Evidence derived from studies using multiple experimental strategies have further helped define the composition of the α4β2* and α6β2* nAChR populations (Fig. 2). This includes immunoprecipitation experiments with nAChR subtype selective antibodies, lesions of specific neuronal pathways and the use of genetically modified nAChR mice. These combined approaches indicate that the primary populations in the nigrostriatal system are composed of α4β2, α4α5β2, α6α4β2β3 and α6β2β3 subunits 60, 76
Nicotinic receptor subtypes that mediate neuroprotection
Our understanding of the specific nAChR subtypes involved in nicotine-mediated protection against neurotoxic insults is primarily derived from studies with cells in culture 61, 62, 77–79. Experiments using neuronal cell lines or primary cultures from striatal, nigral, cortical, cerebellar and other brain regions show that nicotine pre-treatment can reduce damage from toxic insults by acting at α4β2* or α7 nAChRs 61, 62, 77–79. This includes nicotine-mediated protection against glutamate-, β-amyloid- and ethanol-induced toxicity, as well as against nerve growth factor deprivation. The diversity of nicotine’s action against varying toxic insults in cultures from different brain regions suggests that nicotine has the capacity to exert a widespread protective action. This could be important for Parkinson’s disease since the neuronal deficits in this disorder are known to extend throughout the peripheral and central nervous system 80–82.
Knowledge concerning the specific nAChR subtypes through which nicotine protects nigrostriatal damage in parkinsonian animal models is much more limited because of the scarcity of subtype selective nAChR drugs currently available. However, the use of nonselective nAChR antagonists demonstrates that the effect of nicotine is mediated through nAChR 65. Furthermore, work with α4 nAChR null mutant mice indicate that protection is reduced in striatum of such animals suggesting that the α4β2* nAChR subtype is important 68. Other studies using rats with nigrostriatal lesions show that nicotine-mediated protection is not observed when the α6α4β2β3 nAChR subtype is lost, providing indirect evidence for an involvement of this receptor subtype66.
The combined results of the in vitro and in vivo studies suggest that both β2* and α7 nAChR drugs may be useful for protection against the motor and non-motor deficits associated with Parkinson’s disease pathology.
Molecular signaling mechanisms that mediate effects of nicotine
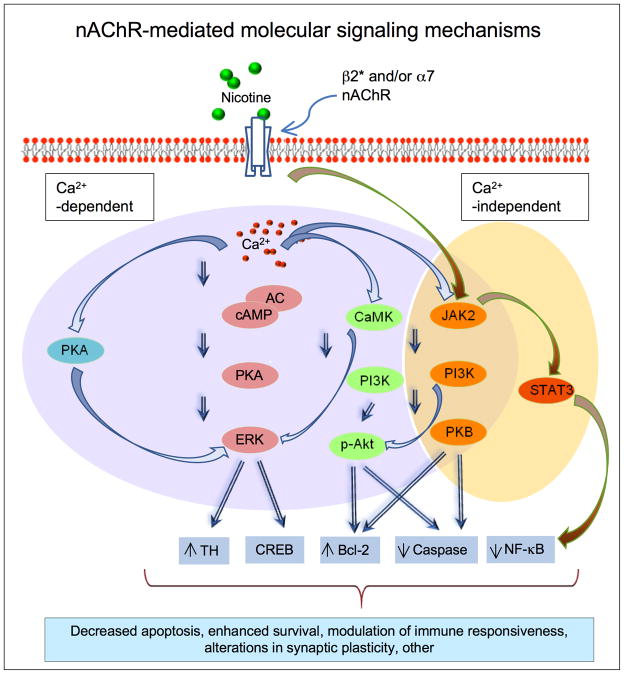
The next question is how an interaction at nAChRs leads to overall functional effects such as protection against neuronal damage. Although the intracellular mechanisms whereby nicotine mediates neuroprotection are only beginning to be understood, an important first step most likely involves alterations in calcium signaling, although calcium independent nAChR-mediated mechanisms have also been reported (Fig. 3) 62, 77, 79, 83–87. Increased intracellular calcium may occur via an influx of calcium through nAChRs, secondarily via other membrane channels and/or through local increases in cellular calcium.
FIG. 3.
Molecular mechanisms through which nicotine mediates its effects in the nervous system.
nAChR-mediated increases in calcium then trigger diverse downstream signaling molecules to ultimately modify neuronal function (Fig. 3) 62, 77, 79, 83–87. Cellular molecules activated in response to nAChR-mediated changes in calcium include kinases such as protein kinase A (PKA) and extracellular signal-regulated mitogen-activated protein kinase (ERK/MAPK). Another signal transduction pathway activated by nicotine is one involving the calcium effector protein calmodulin (CaM) and phosphatidylinositol 3-kinase (PI3K)/Akt-or protein kinase B-dependent signaling 69. There may also be modifications in the JAK2 (Janus kinase 2)/PI3K and/or JAK2/STAT3 (signal transducer and activator of transcription 3) pathways, with the latter possibly being calcium independent 83. Activation of these diverse signalling cascades has been reported to modulate caspase activity (3, 8 and 9), cell survival proteins such as Bcl-2 (B-cell lymphoma 2) and Bcl-x, NFκB (nuclear factor-kappaB), CREB (cAMP response element-binding), tyrosine hydroxylase and other molecular components 62, 77, 79, 83–87. These in turn may lead to decreased apoptosis, enhanced neuronal survival, modified immune responsiveness and alterations in synaptic plasticity. Of specific relevance to neuroprotection are nicotine-induced changes in basic fibroblast growth factor-2 (FGF-2), brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain dopaminergic and other regions, which could attenuate neuronal damage 88–91. Nicotine may also act by modulating immune function, as cytokine production has been shown to protect against toxic insults and promote neuronal repair 83, 92–94.
Although nicotine mediates its effects primarily by interacting at nAChRs, receptor-independent mechanisms may also contribute to nicotine’s neuroprotective potential. These include a reduction in mitochondrial complex 1 activity, inhibition of reactive oxygen species generation, oxidative or anti-oxidative potential and radical scavenging properties 95–99.
Overall, current evidence suggests that multiple molecular transduction mechanisms may be involved in nicotine-mediated adaptive changes, such as neuroprotection against neuronal injury. This finding may reflect the interactive nature of these processes or suggest that distinct signaling events are involved under various pathological conditions.
Usefulness of nicotine for Parkinson’s disease therapeutics
In addition to a role for nicotine as a protectant against nigrostriatal damage, it may also be useful in reducing the dyskinesias that arise with long term L-dopa use in Parkinson’s disease. Evidence for this idea stems from data from parkinsonian animal models which show that nicotine decreases L-dopa-induced dyskinesias in MPTP-lesioned monkeys, when administered before the onset of dyskinesias or once they are established 63. There was also an improvement in L-dopa-induced abnormal involuntary movements in parkinsonian rodents treated with nicotine via several routes including drinking water, minipump or injection 100–102. The mechanism whereby nicotine reduces L-dopa-induced dyskinesias is currently uncertain, but may involve an interaction at nAChRs, specifically β2* subtypes 102. This basic work in parkinsonian animal models has led to a clinical trial to test nicotine against L-dopa-induced dyskinesias in Parkinson’s disease patients; the results suggest that nicotine (designated NP002) may be beneficial (http://www.neuraltus.com).
An important question is whether nicotine directly affects Parkinson’s disease motor symptoms. Our research studies indicate that acute nicotine administration did not modify parkinsonism in monkeys, rats or mice either ON or OFF L-dopa 63, 100, 102. On the other hand, it did enhance the effects of L-dopa in other reports 103, 104, leaving its effects on parkinsonism in experimental animal models unclear. The role of nicotine for motor symptoms in Parkinson’s disease patients are also uncertain. The results of clinical trials and case studies showed that nicotine treatment improved symptoms in five of ten published studies, with no effect in four and a worsening in one 105–113. The reason for these differential outcomes may relate to variations in the mode of administration of nicotine (patch, gum, intravenous), inadequate dosing, timing or duration (days to weeks) of treatment, as well as differences in the degree of parkinsonism and type of trial (open-label versus double-blinded). In summary, results from both animal and clinical studies shed doubt on a direct beneficial effect of nicotine on motor symptoms 114, 115. By contrast, current findings do yield compelling evidence that nicotine may be useful for the treatment of L-dopa-induced dyskinesias and for neuroprotection against ongoing disease progression.
An important issue with respect to therapeutic management is what would be the most effective nicotine delivery system for Parkinson’s disease patients. Tobacco use is not an option since it leads to major health problems worldwide and decreases life expectancy due to tobacco-related cancers, cardiovascular disease, pulmonary disease and other adverse health conditions 116–120. However, nicotine itself exhibits a favorable safety profile and is widely available over-the-counter as a smoking cessation aid, with several nicotine formulations readily accessible at relatively low cost, including the transdermal nicotine patch, gum, lozenge, inhaler and spray 116–120. With respect to optimal protective potential, it should be noted that nicotine appears to reduce ongoing neuronal damage in parkinsonian animal models but is not neurorestorative. These findings suggest that therapeutic intervention would be most effective in early stage Parkinson’s disease. A double-blinded, placebo-controlled clinical trial currently in progress to test the transdermal nicotine patch in newly diagnosed patients (Table 2) should help evaluate nicotine’s neuroprotective potential for Parkinson’s disease.
Concluding Remarks
Extensive evidence from epidemiological and basic research studies indicates that nicotine may represent a drug with potential for protection against Parkinson’s disease. Since nicotine acts at nAChRs, these data suggest that administration of nicotine and/or nAChR agonists in early Parkinson’s disease may slow down and/or halt disease progression. This would help retard declines in motor function and also in non-motor deficits, including olfactory and autonomic problems, sleep disorders, cognitive declines, depression and pain 4, 7, 121, 122. In addition to a neuroprotective role, nicotine treatment may directly improve some of these non-motor complications as an extensive literature shows that acute nicotine and/or nAChR drugs facilitate cognitive performance, reduce pain and alleviate depression in experimental animal models 123–130.
Acknowledgments
The authors thank Maya Hrachova for assistance with the figures. This work was supported by NIH grants NS59910 and NS65851, and the California Tobacco Related Disease Research Program 17RT-0119.
Footnotes
Relevant conflict of interest: M. Quik is on a patent for the use of nicotine for L-dopa-induced dyskinesias. There are no other conflicts of interest.
Author roles
Manuscript Preparation:
A. Writing of the first draft, M. Quik
B. Review and Critique; M. Quik, X.A. Perez, T. Bordia
References
- 1.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26(6):1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 2.Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson’s disease research. Nat Rev Drug Discov. 2011;10(5):377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 3.Rascol O, Lozano A, Stern M, Poewe W. Milestones in Parkinson’s disease therapeutics. Mov Disord. 2011;26(6):1072–1082. doi: 10.1002/mds.23714. [DOI] [PubMed] [Google Scholar]
- 4.Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci. 2009;30(1):41–47. doi: 10.1016/j.tips.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Wichmann T, DeLong MR, Guridi J, Obeso JA. Milestones in research on the pathophysiology of Parkinson’s disease. Mov Disord. 2011;26(6):1032–1041. doi: 10.1002/mds.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halliday G, Lees A, Stern M. Milestones in Parkinson’s disease--clinical and pathologic features. Mov Disord. 2011;26(6):1015–1021. doi: 10.1002/mds.23669. [DOI] [PubMed] [Google Scholar]
- 7.Obeso JA, Rodriguez-Oroz MC, Goetz CG, et al. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16(6):653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 8.Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26(7):547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanyal J, Chakraborty DP, Sarkar B, et al. Environmental and familial risk factors of Parkinsons disease: case-control study. Can J Neurol Sci. 2010;37(5):637–642. doi: 10.1017/s0317167100010829. [DOI] [PubMed] [Google Scholar]
- 10.Tanner CM. Advances in environmental epidemiology. Mov Disord. 2010;25 (Suppl 1):S58–62. doi: 10.1002/mds.22721. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S. Parkinson’s disease: 10 years of progress, 1997–2007. Mov Disord. 2010;25 (Suppl 1):S2–14. doi: 10.1002/mds.22796. [DOI] [PubMed] [Google Scholar]
- 12.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26 (Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 13.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66(5):646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng LR, Maguire-Zeiss KA. Gene therapy in Parkinson’s disease: rationale and current status. CNS Drugs. 2010;24(3):177–192. doi: 10.2165/11533740-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olanow CW, McNaught K. Parkinson’s disease, proteins, and prions: milestones. Mov Disord. 2011;26(6):1056–1071. doi: 10.1002/mds.23767. [DOI] [PubMed] [Google Scholar]
- 16.Cenci MA, Ohlin KE. Rodent models of treatment-induced motor complications in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15 (Suppl 4):S13–17. doi: 10.1016/S1353-8020(09)70828-4. [DOI] [PubMed] [Google Scholar]
- 17.Jenner P. Functional models of Parkinson’s disease: A valuable tool in the development of novel therapies. Annals of neurology. 2009;64(S2):S16–S29. doi: 10.1002/ana.21489. [DOI] [PubMed] [Google Scholar]
- 18.Chesselet MF, Richter F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011;10(12):1108–1118. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- 19.Magen I, Chesselet MF. Genetic mouse models of Parkinson’s disease The state of the art. Prog Brain Res. 2010;184:53–87. doi: 10.1016/S0079-6123(10)84004-X. [DOI] [PubMed] [Google Scholar]
- 20.Bezard E, Przedborski S. A tale on animal models of Parkinson’s disease. Mov Disord. 2011;26(6):993–1002. doi: 10.1002/mds.23696. [DOI] [PubMed] [Google Scholar]
- 21.Pan-Montojo F, Anichtchik O, Dening Y, et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5(1):e8762. doi: 10.1371/journal.pone.0008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schapira AH. Progress in neuroprotection in Parkinson’s disease. Eur J Neurol. 2008;15 (Suppl 1):5–13. doi: 10.1111/j.1468-1331.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Calingasan NY, Wille EJ, et al. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J Neurochem. 2009;109(5):1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeWitt PA. Neuroprotection for Parkinson’s disease. J Neural Transm Suppl. 2006;(71):113–122. [PubMed] [Google Scholar]
- 25.Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson’s disease. Exp Neurol. 2007;203(2):512–520. doi: 10.1016/j.expneurol.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reglodi D, Kiss P, Lubics A, Tamas A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17(10):962–972. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- 27.Lindvall O, Wahlberg LU. Encapsulated cell biodelivery of GDNF: a novel clinical strategy for neuroprotection and neuroregeneration in Parkinson’s disease? Exp Neurol. 2008;209(1):82–88. doi: 10.1016/j.expneurol.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 28.L’ Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Glia as a turning point in the therapeutic strategy of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2010;9(3):349–372. doi: 10.2174/187152710791292639. [DOI] [PubMed] [Google Scholar]
- 29.von Bernhardi R, Tichauer JE, Eugenin J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112(5):1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- 30.Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 2010;31(1):7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 32.Chan CS, Guzman JN, Ilijic E, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447(7148):1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti A, Pugliese P, Nicoletti G, et al. Voluptuary habits and clinical subtypes of Parkinson’s disease: the FRAGAMP case-control study. Mov Disord. 2010;25(14):2387–2394. doi: 10.1002/mds.23297. [DOI] [PubMed] [Google Scholar]
- 34.Morelli M, Carta AR, Kachroo A, Schwarzschild MA. Pathophysiological roles for purines: adenosine, caffeine and urate. Prog Brain Res. 2010;183:183–208. doi: 10.1016/S0079-6123(10)83010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol. 1996;144(5):480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 36.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Annals of neurology. 2005;58(5):797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65(6):716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166(5):561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo N, Kincses ZT, Vecsei L. Novel therapy in Parkinson’s disease: adenosine A(2A) receptor antagonists. Expert Opin Drug Metab Toxicol. 2011;7(4):441–455. doi: 10.1517/17425255.2011.557066. [DOI] [PubMed] [Google Scholar]
- 40.Prediger RD. Effects of caffeine in Parkinson’s disease: from neuroprotection to the management of motor and non-motor symptoms. J Alzheimers Dis. 2010;20 (Suppl 1):S205–220. doi: 10.3233/JAD-2010-091459. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morens DM, Grandinetti A, Reed D, White LR, Ross GW. Cigarette smoking and protection from Parkinson’s disease: false association or etiologic clue? Neurology. 1995;45(6):1041–1051. doi: 10.1212/wnl.45.6.1041. [DOI] [PubMed] [Google Scholar]
- 43.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64(7):990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- 44.Thacker EL, O’Reilly EJ, Weisskopf MG, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68(10):764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Reilly EJ, McCullough ML, Chao A, et al. Smokeless tobacco use and the risk of Parkinson’s disease mortality. Mov Disord. 2005;20(10):1383–1384. doi: 10.1002/mds.20587. [DOI] [PubMed] [Google Scholar]
- 46.Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernandez-Crehuet Navajas R. Smoking and Parkinson’s disease: systematic review of prospective studies. Mov Disord. 2004;19(6):614–621. doi: 10.1002/mds.20029. [DOI] [PubMed] [Google Scholar]
- 47.Tanner CM, Goldman SM, Aston DA, et al. Smoking and Parkinson’s disease in twins. Neurology. 2002;58(4):581–588. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- 48.Ross GW, Petrovitch H. Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson’s disease. Drugs Aging. 2001;18(11):797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- 49.Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson’s disease in two prospective studies. Annals of neurology. 2001;50(6):780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- 50.Gorell JM, Rybicki BA, Johnson CC, Peterson EL. Smoking and Parkinson’s disease: a dose-response relationship. Neurology. 1999;52(1):115–119. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- 51.Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br Med Bull. 1996;52(1):58–73. doi: 10.1093/oxfordjournals.bmb.a011533. [DOI] [PubMed] [Google Scholar]
- 52.Castagnoli K, Murugesan T. Tobacco leaf, smoke and smoking, MAO inhibitors, Parkinson’s disease and neuroprotection; are there links? Neurotoxicology. 2004;25(1–2):279–291. doi: 10.1016/S0161-813X(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 53.Castagnoli K, Petzer JB, Steyn SJ, van der Schyf CJ, Castagnoli N., Jr Inhibition of human MAO-A and MAO-B by a compound isolated from flue-cured tobacco leaves and its neuroprotective properties in the MPTP mouse model of neurodegeneration. Inflammopharmacology. 2003;11(2):183–188. doi: 10.1163/156856003765764353. [DOI] [PubMed] [Google Scholar]
- 54.Castagnoli KP, Steyn SJ, Petzer JP, Van der Schyf CJ, Castagnoli N., Jr Neuroprotection in the MPTP Parkinsonian C57BL/6 mouse model by a compound isolated from tobacco. Chem Res Toxicol. 2001;14(5):523–527. doi: 10.1021/tx000224v. [DOI] [PubMed] [Google Scholar]
- 55.LeWitt PA, Taylor DC. Protection against Parkinson’s disease progression: clinical experience. Neurotherapeutics. 2008;5(2):210–225. doi: 10.1016/j.nurt.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinreb O, Amit T, Bar-Am O, Youdim MB. Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog Neurobiol. 2010;92(3):330–344. doi: 10.1016/j.pneurobio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Rascol O, Fitzer-Attas CJ, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease (the ADAGIO study): prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol. 2011;10(5):415–423. doi: 10.1016/S1474-4422(11)70073-4. [DOI] [PubMed] [Google Scholar]
- 58.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53(4):590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 59.Grady SR, Salminen O, Laverty DC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74(8):1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson’s Disease. Pharmacol Rev. 2011;63(4):938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Neill MJ, Murray TK, Lakics V, Visanji NP, Duty S. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr Drug Target CNS Neurol Disord. 2002;1(4):399–411. doi: 10.2174/1568007023339166. [DOI] [PubMed] [Google Scholar]
- 62.Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 63.Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of neurology. 2007;62:588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- 64.Costa G, Abin-Carriquiry JA, Dajas F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain research. 2001;888(2):336–342. doi: 10.1016/s0006-8993(00)03087-0. [DOI] [PubMed] [Google Scholar]
- 65.Dajas F, Costa G, Abin-Carriquiry JA, McGregor R, Urbanavicius J. Involvement of nicotinic acetylcholine receptors in the protection of dopamine terminals in experimental parkinsonism. Funct Neurol. 2001;16(Suppl 4):113–123. [PubMed] [Google Scholar]
- 66.Huang LZ, Parameswaran N, Bordia T, Michael McIntosh J, Quik M. Nicotine is neuroprotective when administered before but not after nigrostriatal damage in rats and monkeys. J Neurochem. 2009;109(3):826–837. doi: 10.1111/j.1471-4159.2009.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parain K, Marchand V, Dumery B, Hirsch E. Nicotine, but not cotinine, partially protects dopaminergic neurons against MPTP-induced degeneration in mice. Brain research. 2001;890(2):347–350. doi: 10.1016/s0006-8993(00)03198-x. [DOI] [PubMed] [Google Scholar]
- 68.Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132(8):1650–1656. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toulorge D, Guerreiro S, Hild A, Maskos U, Hirsch EC, Michel PP. Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+ FASEB J. 2011;25:2563–2573. doi: 10.1096/fj.11-182824. [DOI] [PubMed] [Google Scholar]
- 70.Visanji NP, O’Neill MJ, Duty S. Nicotine, but neither the alpha4beta2 ligand RJR2403 nor an alpha7 nAChR subtype selective agonist, protects against a partial 6-hydroxydopamine lesion of the rat median forebrain bundle. Neuropharmacology. 2006;51(3):506–516. doi: 10.1016/j.neuropharm.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 71.Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci. 2006;26(17):4681–4689. doi: 10.1523/JNEUROSCI.0215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quik M, Parameswaran N, McCallum SE, et al. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem. 2006;98(6):1866–1875. doi: 10.1111/j.1471-4159.2006.04078.x. [DOI] [PubMed] [Google Scholar]
- 73.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89(1):73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 75.Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. Multiple roles for nicotine in Parkinson’s disease. Biochem Pharmacol. 2009;78(7):677–685. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gotti C, Guiducci S, Tedesco V, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30(15):5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward RJ, Lallemand F, de Witte P, Dexter DT. Neurochemical pathways involved in the protective effects of nicotine and ethanol in preventing the development of Parkinson’s disease: Potential targets for the development of new therapeutic agents. Prog Neurobiol. 2008;85(2):135–147. doi: 10.1016/j.pneurobio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Quik M, Kulak JM. Nicotine and nicotinic receptors; relevance to Parkinson’s disease. Neurotoxicology. 2002;23(4–5):581–594. doi: 10.1016/s0161-813x(02)00036-0. [DOI] [PubMed] [Google Scholar]
- 79.Shimohama S. Nicotinic receptor-mediated neuroprotection in neurodegenerative disease models. Biol Pharm Bull. 2009;32(3):332–336. doi: 10.1248/bpb.32.332. [DOI] [PubMed] [Google Scholar]
- 80.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 81.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 82.Braak H, Muller CM, Rub U, et al. Pathology associated with sporadic Parkinson’s disease--where does it end? J Neural Transm Suppl. 2006;(70):89–97. doi: 10.1007/978-3-211-45295-0_15. [DOI] [PubMed] [Google Scholar]
- 83.Hosur V, Loring RH. alpha4beta2 nicotinic receptors partially mediate anti-inflammatory effects through Janus kinase 2-signal transducer and activator of transcription 3 but not calcium or cAMP signaling. Mol Pharmacol. 2011;79(1):167–174. doi: 10.1124/mol.110.066381. [DOI] [PubMed] [Google Scholar]
- 84.Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25(6):317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Mudo G, Belluardo N, Fuxe K. Nicotinic receptor agonists as neuroprotective/neurotrophic drugs. Progress in molecular mechanisms. J Neural Transm. 2007;114:135–147. doi: 10.1007/s00702-006-0561-z. [DOI] [PubMed] [Google Scholar]
- 86.Quik M. Smoking, nicotine and Parkinson’s disease. Trends Neurosci. 2004;27(9):561–568. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Kawamata J, Shimohama S. Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2011;24 (Suppl 2):95–109. doi: 10.3233/JAD-2011-110173. [DOI] [PubMed] [Google Scholar]
- 88.Belluardo N, Mudo G, Blum M, Fuxe K. Central nicotinic receptors, neurotrophic factors and neuroprotection. Behav Brain Res. 2000;113(1–2):21–34. doi: 10.1016/s0166-4328(00)00197-2. [DOI] [PubMed] [Google Scholar]
- 89.Massey KA, Zago WM, Berg DK. BDNF up-regulates alpha7 nicotinic acetylcholine receptor levels on subpopulations of hippocampal interneurons. Mol Cell Neurosci. 2006;33(4):381–388. doi: 10.1016/j.mcn.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou X, Nai Q, Chen M, Dittus JD, Howard MJ, Margiotta JF. Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: relevance to regulating alpha7-containing nicotinic receptors and synaptic function. J Neurosci. 2004;24(18):4340–4350. doi: 10.1523/JNEUROSCI.0055-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Formaggio E, Fazzini F, Dalfini AC, et al. Nicotine increases the expression of neurotrophin receptor tyrosine kinase receptor A in basal forebrain cholinergic neurons. Neuroscience. 2010;166(2):580–589. doi: 10.1016/j.neuroscience.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 92.Park HJ, Lee PH, Ahn YW, et al. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26(1):79–89. doi: 10.1111/j.1460-9568.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- 93.Shi FD, Piao WH, Kuo YP, Campagnolo DI, Vollmer TL, Lukas RJ. Nicotinic attenuation of central nervous system inflammation and autoimmunity. J Immunol. 2009;182(3):1730–1739. doi: 10.4049/jimmunol.182.3.1730. [DOI] [PubMed] [Google Scholar]
- 94.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265(6):663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cormier A, Morin C, Zini R, Tillement JP, Lagrue G. Nicotine protects rat brain mitochondria against experimental injuries. Neuropharmacology. 2003;44(5):642–652. doi: 10.1016/s0028-3908(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 96.Ferger B, Spratt C, Earl CD, Teismann P, Oertel WH, Kuschinsky K. Effects of nicotine on hydroxyl free radical formation in vitro and on MPTP-induced neurotoxicity in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1998;358(3):351–359. doi: 10.1007/pl00005264. [DOI] [PubMed] [Google Scholar]
- 97.Newman MB, Arendash GW, Shytle RD, Bickford PC, Tighe T, Sanberg PR. Nicotine’s oxidative and antioxidant properties in CNS. Life Sci. 2002;71(24):2807–2820. doi: 10.1016/s0024-3205(02)02135-5. [DOI] [PubMed] [Google Scholar]
- 98.Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL. Effects of (−)-nicotine and (−)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol. 2002;64(1):125–135. doi: 10.1016/s0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- 99.Xie YX, Bezard E, Zhao BL. Investigating the receptor-independent neuroprotective mechanisms of nicotine in mitochondria. J Biol Chem. 2005;280(37):32405–32412. doi: 10.1074/jbc.M504664200. [DOI] [PubMed] [Google Scholar]
- 100.Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327(1):239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- 101.Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-dopa-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang L, Grady SR, Quik M. Nicotine Reduces L-Dopa-Induced Dyskinesias by Acting at {beta}2 Nicotinic Receptors. J Pharmacol Exp Ther. 2011;338:932–941. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schneider JS, Pope-Coleman A, Van Velson M, Menzaghi F, Lloyd GK. Effects of SIB-1508Y, a novel neuronal nicotinic acetylcholine receptor agonist, on motor behavior in parkinsonian monkeys. Mov Disord. 1998;13(4):637–642. doi: 10.1002/mds.870130405. [DOI] [PubMed] [Google Scholar]
- 104.Domino EF, Ni L, Zhang H. Nicotine Alone and in Combination with l-DOPA Methyl Ester or the D(2) Agonist N-0923 in MPTP-Induced Chronic Hemiparkinsonian Monkeys. Exp Neurol. 1999;158(2):414–421. doi: 10.1006/exnr.1999.7106. [DOI] [PubMed] [Google Scholar]
- 105.Ishikawa A, Miyatake T. Effects of smoking in patients with early-onset Parkinson’s disease. J Neurol Sci. 1993;117(1–2):28–32. doi: 10.1016/0022-510x(93)90150-w. [DOI] [PubMed] [Google Scholar]
- 106.Fagerstrom KO, Pomerleau O, Giordani B, Stelson F. Nicotine may relieve symptoms of Parkinson’s disease. Psychopharmacology (Berl) 1994;116(1):117–119. doi: 10.1007/BF02244882. [DOI] [PubMed] [Google Scholar]
- 107.Clemens P, Baron JA, Coffey D, Reeves A. The short-term effect of nicotine chewing gum in patients with Parkinson’s disease. Psychopharmacology (Berl) 1995;117(2):253–256. doi: 10.1007/BF02245195. [DOI] [PubMed] [Google Scholar]
- 108.Ebersbach G, Stock M, Muller J, Wenning G, Wissel J, Poewe W. Worsening of motor performance in patients with Parkinson’s disease following transdermal nicotine administration. Mov Disord. 1999;14(6):1011–1013. doi: 10.1002/1531-8257(199911)14:6<1011::aid-mds1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 109.Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. The effects of nicotine on Parkinson’s disease. Brain Cogn. 2000;43(1–3):274–282. [PubMed] [Google Scholar]
- 110.Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology. 2001;57(6):1032–1035. doi: 10.1212/wnl.57.6.1032. [DOI] [PubMed] [Google Scholar]
- 111.Villafane G, Cesaro P, Rialland A, et al. Chronic high dose transdermal nicotine in Parkinson’s disease: an open trial. Eur J Neurol. 2007;14:1313–1316. doi: 10.1111/j.1468-1331.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 112.Shoulson I. Randomized placebo-controlled study of the nicotinic agonist SIB-1508Y in Parkinson disease. Neurology. 2006;66(3):408–410. doi: 10.1212/01.wnl.0000196466.99381.5c. [DOI] [PubMed] [Google Scholar]
- 113.Lemay S, Chouinard S, Blanchet P, et al. Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):31–39. doi: 10.1016/S0278-5846(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 114.Quik M, O’Leary K, Tanner CM. Nicotine and Parkinson’s disease: implications for therapy. Mov Disord. 2008;23(12):1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thiriez C, Villafane G, Grapin F, Fenelon G, Remy P, Cesaro P. Can nicotine be used medicinally in Parkinson’s disease? Expert Rev Clin Pharmacol. 2011;4(4):429–436. doi: 10.1586/ecp.11.27. [DOI] [PubMed] [Google Scholar]
- 116.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Biasi M, Dani JA. Reward, Addiction, Withdrawal to Nicotine. Annu Rev Neurosci. 2010;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dwoskin LP, Bardo MT. Targeting nicotinic receptor antagonists as novel pharmacotherapies for tobacco dependence and relapse. Neuropsychopharmacology. 2009;34(1):244–246. doi: 10.1038/npp.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paolini M, De Biasi M. Mechanistic insights into nicotine withdrawal. Biochem Pharmacol. 2011;82:996–1007. doi: 10.1016/j.bcp.2011.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raupach T, van Schayck CP. Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs. 2011;25(5):371–382. doi: 10.2165/11590620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 121.Hawkes CH. The prodromal phase of sporadic Parkinson’s disease: does it exist and if so how long is it? Mov Disord. 2008;23(13):1799–1807. doi: 10.1002/mds.22242. [DOI] [PubMed] [Google Scholar]
- 122.O’Sullivan SS, Williams DR, Gallagher DA, Massey LA, Silveira-Moriyama L, Lees AJ. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord. 2008;23(1):101–106. doi: 10.1002/mds.21813. [DOI] [PubMed] [Google Scholar]
- 123.Changeux JP. Allosteric receptors: from electric organ to cognition. Annu Rev Pharmacol Toxicol. 2010;50:1–38. doi: 10.1146/annurev.pharmtox.010909.105741. [DOI] [PubMed] [Google Scholar]
- 124.Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78(7):658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochem Pharmacol. 2009;78(7):668–676. doi: 10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 126.McIntosh JM, Absalom N, Chebib M, Elgoyhen AB, Vincler M. Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochem Pharmacol. 2009;78(7):693–702. doi: 10.1016/j.bcp.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signalling: roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol Rev. 2009;61(1):39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bacher I, Wu B, Shytle DR, George TP. Mecamylamine - a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opin Pharmacother. 2009;10(16):2709–2721. doi: 10.1517/14656560903329102. [DOI] [PubMed] [Google Scholar]
- 129.Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology (Berl) 2010;212:1–12. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]