Abstract
Using data from the National Institutes of Neurological disease and Stroke's (NINDS) study of Parkinson disease (PD), we recently reported that single nucleotide polymorphisms (SNPs) in a region containing the Calpastatin (CAST) gene were associated with PD. Here we follow up this finding with an analysis of the Center for Inherited Disease Research's (CIDR) genome-wide association study in familial PD. After adjusting for population stratification and multiple testing, we find a significant association (p=0.0167) between PD and SNP rs1559085 in CAST. These findings confirm CAST/PD associations in a second, independent, dataset and suggest that CAST be prioritized for further investigation.
Although there are now several single-gene forms of Parkinson disease (PD) known (Klein and Schlossmacher, 2006), the genetic factors that predispose to idiopathic Parkinson disease are largely unknown (de Lau and Breteler, 2006). Two genome-wide association studies (GWASs) have been recently undertaken to address this question. The National Institute of Neurological Disease and Stroke (NINDS) PD study (Fung et al., 2006) uses cases and controls from the NINDS repository, while the Center for Inherited Disease Research (CIDR) genome-wide association study in familial PD study combines data on cases from the PROGENI and GenePD studies with controls (different from those used in NINDS PD) from the NINDS repository (Pankratz et al. 2009). Data from both studies is available through the database of genotypes and phenotypes (dbGap; http://view.ncbi.nlm.nih.gov/dbgap).
Previously, we performed a genome-wide analysis of the NINDS study data (dbGAP accession number phs000089) using three statistical tests; the single-SNP Mantel-Haenszel (MH) test as well as two novel tests based on haplotype sharing, in which the patterns of genetic similarity surrounding each SNP genotyped is compared between case and control participants (Allen and Satten, 2009). We reported a genome-wide significant association between single nucleotide polymorphisms (SNPs) in the calpastatin (CAST [MIM *114090]) gene and PD for one of the haplotype sharing tests. Here we report on the CAST/PD association we find in the CIDR familial PD study.
The CIDR familial PD data, publically released through dbGAP (accession number phs000126.v1.p1), is comprised of genotypes measured on 1943 individuals, of which 1048 are patients with PD and 895 are neurologically normal controls. The Illumina, haplotype tagging human370CNV version 1C array and BeadStudio version 3.1.14 calling algorithm were used for genotyping; 344,301 SNP genotypes were publically released. In comparison, the NINDS PD study augmented the haplotype tagging HumanHap300 array with the gene-centric Illumina Infinium I, resulting in 408,803 unique SNP genotypes.
Following the recommendation of the CIDR PD study investigators, we excluded 176 samples from our analyses due to LRRK2 mutation status, population substructure, and cryptic relatedness. The study investigators also recommended that the data of 38 cases whose DNA was extracted by whole genome amplification be carefully scrutinized. We confirmed that these cases have much lower call rates, lower genotype quality scores, and lower average heterozygosity values than the rest of the data (see supplemental figures 1-4). Thus we elected to exclude these samples from subsequent analyses.
We further subjected the CIDR data to the same quality control and data cleaning measures implemented in our analysis of the NINDS PD study. In particular, we excluded data from SNPs that had extensive missingness (missingness>10%), deviations from Hardy-Weinberg equilibrium (p-value<0.001 in controls), and low minor allele frequency (<0.2%). After this quality control (QC) filtering, data on 334,225 SNPs remained. Using the software package PLINK (Purcell et al., 2007) we searched for but did not find individuals who share more than 12.5% of their SNPs identical by descent, i.e. more than would be reasonably expected in a study of unrelated persons. We also looked for persons whose genotypes suggested that their ancestry was markedly different from the study population using the spectral graph theory approach of Lee et al. (Lee et al., 2009). Following Lee et al. (2009), we filtered the cleaned SNP data to arrive at an approximately independent set of markers distributed throughout the genome. To do so, SNPs were eliminated from regions of known high linkage disequilibrium (LD) and LD was further reduced by pruning SNPs using the ‘--independent-pairwise’ command in PLINK (Purcell et al., 2007). Using this subset of 68,288 approximately independent SNPs, we applied the eigen-analysis methods detailed in Lee et al. (2009) and found that the first 6 eigenvectors were sufficient to describe the genetic variation found in the sample. No further outliers were identified.
We adjusted for possible population stratification as in our NINDS PD analysis by using the stratification score (Epstein et al., 2007), which we calculated as the probability of being a case given only the genomic information in the 6 eigenvectors described above. Using the values of the stratification score calculated for each individual, we then divided the sample into 5 equal strata based on quintiles of the stratification score. All subsequent statistical tests are based on these five strata. This approach has been shown to successfully correct for population stratification while maintaining good power (Epstein et al., 2007; Sarasua et al., 2009).
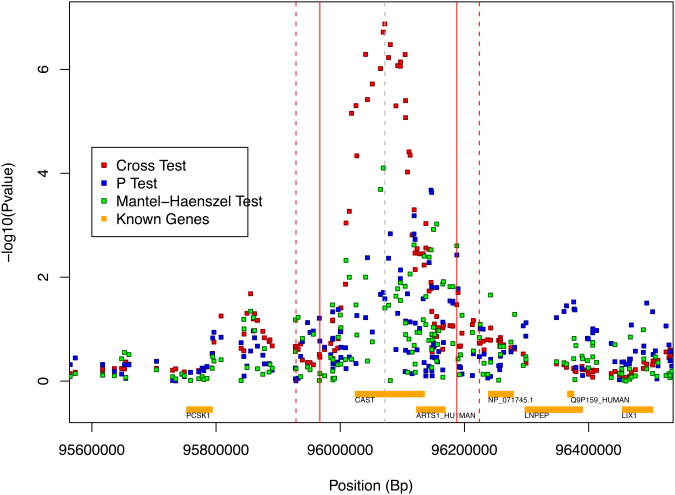
After quality control, data cleaning and adjustment for possible confounding due to population stratification, we examined SNPs within a region containing the CAST gene where we previously found association in the NINDS PD study (see Figure 1—the region, which spans 95967.1 kB to 96187.7 kB on chromosome 5, is indicated by solid red vertical lines). We identified 48 SNPs that passed QC and were within 50kB of CAST. Twenty of these SNPs were found within CAST (both rs2785, the SNP found most associated in NINDS PD, and rs155908, the SNP found most associated here, are among these 20 SNPs). We added two additional SNPs found at either end of the CAST±50kB boundary to arrive at 50 SNPs approximately centered at the peak of the signal found in the NINDS PD analysis. We note that all 50 of these SNPs were also included in the NINDS PD analysis while 23 additional SNPs in this region were included in the NINDS PD analysis but were not genotyped in the CIDR PD study.
Figure 1.
Analysis results from NINDS data reported in Allen and Satten (2009) highlighting follow-up region. Solid red vertical lines denote region comprised of 50 SNPs in the CIDR PD data. Dotted red vertical lines denote extent of SNPs used in haplotype sharing tests.
We tested association at each of the 50 SNPs using the same three stratified tests we used to analyze the NINDS PD data: the standard single-SNP MH test, and the p and cross tests of Allen and Satten (2009) that compare patterns of haplotype similarity between cases and controls. As in our previous analysis of the NINDS data, haplotype similarity tests were calculated using 15-SNP windows centered at each of the 50 SNPs we studied (data from SNPs outside of our 50 SNP region was used to calculate haplotype similarity for windows that were within 7 SNPs of the boundaries of our region). We explicitly adjusted for the fact that we are conducting 150 tests (3 different tests centered at each of 50 SNPs) using the permutation-based step-down procedure of Westfall and Young (1993).
The results of our analysis can be found in table I. After adjusting for multiple comparisons, we found the single SNP MH test to be significant (rs1559085—p=0.0167; unadjusted p=0.0002). In our previous evaluation of the NINDS PD data, we found the cross test was genome-wide significant. In the CIDR PD data, neither haplotype sharing test was significant after adjustment for multiple testing: p test (rs1559085—p=0.1028; unadjusted p=0.0019); cross test (rs152280—p=0.3089; unadjusted p=0.0076). However, the most associated SNP in CIDR PD study (rs1559085) is only 32.7kB away from the SNP (rs27852) that was the center of the association peak we found in the NINDS PD data with the cross test. The two SNPs also show no evidence of recombination between them as the D′ between the SNPs is at its maximal value of 1. The effect size conveyed by rs1559085 is also similar in the two studies with the CIDR PD study showing an allelic odds ratio of 1.43 [95% unadjusted CI=(1.18, 1.74)] while the NINDS PD study shows an allelic odds ratio of 1.56 [95% unadjusted CI=(1.09, 2.21)]. We further note that, although the MH test failed to reach genome-wide significance in the NINDS PD data, the MH test does exhibit some signal in the NINDS PD data. The minimum MH unadjusted p value over the 73 NINDS PD SNPs found in the region from 95967.1 kB to 96187.7 kB on chromosome 5 (indicated by solid red lines in figure 1) was 7.9×10−5 (at rs10053056, which is 35.3kB away from rs1559085 and only 2.6 kB from rs27852). Additionally, 37% of these SNPs (27 out of 73) have unadjusted p-values below 0.05.
Table I.
Results of CAST region follow-up study in CIDR PD data. Fifty loci were tested by 3 different tests (150 tests total). Results sorted by raw p-values. Bold entry denotes significant locus after adjusting for multiple comparisons. Tests: mh—Mantel Haenszel test; p—haplotype sharing p test; c—haplotype sharing cross test.
| SNP | Physical position (Bp) | Test | Raw P-value | Adjusted P-value |
|---|---|---|---|---|
| rs1559085 | 96104458 | mh | 0.0002 | 0.0167 |
| rs1559085 | 96104458 | p | 0.0019 | 0.1022 |
| rs152280 | 96187698 | c | 0.0076 | 0.3054 |
| rs27434 | 96155268 | c | 0.01 | 0.3713 |
| rs10515248 | 96137761 | mh | 0.0101 | 0.3761 |
| rs13362120 | 96096950 | p | 0.012 | 0.4246 |
| rs10515248 | 96137761 | p | 0.0176 | 0.5373 |
| rs10053056 | 96069176 | mh | 0.018 | 0.5442 |
| rs28129 | 96181274 | c | 0.0224 | 0.6054 |
| rs34753 | 96176667 | c | 0.0225 | 0.6074 |
| rs155056 | 96000101 | mh | 0.0247 | 0.6411 |
| rs26618 | 96156592 | c | 0.025 | 0.6411 |
| rs261212 | 95967121 | c | 0.0257 | 0.6495 |
| rs42398 | 96146211 | c | 0.0257 | 0.6495 |
| rs30187 | 96150086 | c | 0.0268 | 0.6624 |
| rs469783 | 96147280 | c | 0.0268 | 0.6626 |
| rs18036 | 96181364 | c | 0.0279 | 0.6712 |
| rs10050860 | 96147966 | c | 0.0307 | 0.7024 |
| rs27037 | 96120450 | mh | 0.0357 | 0.7471 |
| rs155040 | 95995910 | p | 0.0446 | 0.8173 |
| rs27042 | 96142696 | c | 0.046 | 0.8238 |
| rs6885297 | 95974758 | c | 0.0565 | 0.8713 |
| rs17086432 | 95996726 | mh | 0.0717 | 0.9214 |
| rs261234 | 95988209 | p | 0.073 | 0.9217 |
| rs27042 | 96142696 | mh | 0.082 | 0.9446 |
| rs3853203 | 95987808 | p | 0.0834 | 0.9447 |
| rs2611713 | 95984706 | c | 0.0861 | 0.9482 |
| rs4869304 | 96009225 | mh | 0.0951 | 0.9618 |
| rs10515248 | 96137761 | c | 0.0991 | 0.9654 |
| rs10515240 | 95987585 | p | 0.1011 | 0.9684 |
| rs261235 | 95988173 | p | 0.1066 | 0.9742 |
| rs4869305 | 96018123 | mh | 0.1155 | 0.9801 |
| rs469783 | 96147280 | mh | 0.1208 | 0.984 |
| rs10515240 | 95987585 | c | 0.1219 | 0.9841 |
| rs2611713 | 95984706 | p | 0.1227 | 0.9846 |
| rs28081 | 96118746 | c | 0.1296 | 0.9873 |
| rs17086408 | 95990991 | p | 0.1331 | 0.9888 |
| rs27037 | 96120450 | c | 0.1344 | 0.9893 |
| rs1559085 | 96104458 | c | 0.1366 | 0.9903 |
| rs152005 | 96051603 | mh | 0.1368 | 0.9904 |
| rs27037 | 96120450 | p | 0.1388 | 0.9904 |
| rs18036 | 96181364 | p | 0.1411 | 0.9915 |
| rs261212 | 95967121 | mh | 0.1589 | 0.9955 |
| rs10053056 | 96069176 | c | 0.159 | 0.9955 |
| rs152005 | 96051603 | c | 0.1666 | 0.9955 |
| rs27852 | 96071795 | mh | 0.2042 | 0.9992 |
| rs26618 | 96156592 | p | 0.2053 | 0.9993 |
| rs27429 | 96120729 | c | 0.2141 | 0.9993 |
| rs27524 | 96127700 | c | 0.2145 | 0.9993 |
| rs469532 | 96077746 | c | 0.2188 | 0.9993 |
| rs27851 | 96108157 | c | 0.2253 | 0.9993 |
| rs27524 | 96127700 | mh | 0.2255 | 0.9993 |
| rs28096 | 96135000 | c | 0.2414 | 0.9997 |
| rs151835 | 96096738 | c | 0.2417 | 0.9997 |
| rs18036 | 96181364 | mh | 0.2421 | 0.9997 |
| rs4254932 | 96000943 | mh | 0.2425 | 0.9997 |
| rs34753 | 96176667 | p | 0.2454 | 0.9997 |
| rs28129 | 96181274 | p | 0.253 | 0.9998 |
| rs469783 | 96147280 | p | 0.2564 | 0.9999 |
| rs10050860 | 96147966 | mh | 0.2578 | 0.9999 |
| rs17086432 | 95996726 | p | 0.261 | 0.9999 |
| rs3756623 | 96110704 | mh | 0.2622 | 0.9999 |
| rs469532 | 96077746 | mh | 0.2645 | 0.9999 |
| rs27852 | 96071795 | c | 0.2698 | 0.9999 |
| rs261234 | 95988209 | c | 0.2711 | 1 |
| rs27429 | 96120729 | p | 0.2734 | 1 |
| rs28081 | 96118746 | p | 0.28 | 1 |
| rs2611713 | 95984706 | mh | 0.2848 | 1 |
| rs27772 | 96115732 | mh | 0.2863 | 1 |
| rs27851 | 96108157 | p | 0.2912 | 1 |
| rs13362120 | 96096950 | c | 0.2921 | 1 |
| rs17086512 | 96040813 | mh | 0.2924 | 1 |
| rs26482 | 96043712 | c | 0.2975 | 1 |
| rs27434 | 96155268 | p | 0.3043 | 1 |
| rs10515241 | 95988347 | p | 0.3059 | 1 |
| rs27772 | 96115732 | c | 0.3157 | 1 |
| rs27434 | 96155268 | mh | 0.3212 | 1 |
| rs17086512 | 96040813 | c | 0.3221 | 1 |
| rs261235 | 95988173 | c | 0.3266 | 1 |
| rs4869304 | 96009225 | c | 0.3305 | 1 |
| rs10515240 | 95987585 | mh | 0.3309 | 1 |
| rs3853203 | 95987808 | c | 0.3383 | 1 |
| rs151835 | 96096738 | mh | 0.3395 | 1 |
| rs10515241 | 95988347 | mh | 0.3432 | 1 |
| rs155054 | 96001058 | mh | 0.3442 | 1 |
| rs3853203 | 95987808 | mh | 0.3451 | 1 |
| rs28096 | 96135000 | p | 0.3471 | 1 |
| rs4869305 | 96018123 | p | 0.3498 | 1 |
| rs3756623 | 96110704 | c | 0.3651 | 1 |
| rs26618 | 96156592 | mh | 0.3688 | 1 |
| rs6885297 | 95974758 | p | 0.3747 | 1 |
| rs1421911 | 96026703 | c | 0.3784 | 1 |
| rs27042 | 96142696 | p | 0.3866 | 1 |
| rs261235 | 95988173 | mh | 0.389 | 1 |
| rs155056 | 96000101 | c | 0.3976 | 1 |
| rs17086408 | 95990991 | c | 0.4017 | 1 |
| rs13362120 | 96096950 | mh | 0.405 | 1 |
| rs30187 | 96150086 | mh | 0.4112 | 1 |
| rs155054 | 96001058 | p | 0.4116 | 1 |
| rs17086408 | 95990991 | mh | 0.4189 | 1 |
| rs27524 | 96127700 | p | 0.4315 | 1 |
| rs26482 | 96043712 | mh | 0.4319 | 1 |
| rs28129 | 96181274 | mh | 0.4348 | 1 |
| rs4254932 | 96000943 | c | 0.4368 | 1 |
| rs34753 | 96176667 | mh | 0.4405 | 1 |
| rs4254932 | 96000943 | p | 0.442 | 1 |
| rs469532 | 96077746 | p | 0.4447 | 1 |
| rs1862182 | 96025282 | c | 0.4459 | 1 |
| rs1421911 | 96026703 | mh | 0.4528 | 1 |
| rs152280 | 96187698 | mh | 0.4644 | 1 |
| rs261212 | 95967121 | p | 0.4652 | 1 |
| rs1862609 | 96112446 | c | 0.4658 | 1 |
| rs10515242 | 96008431 | p | 0.4659 | 1 |
| rs10515241 | 95988347 | c | 0.4756 | 1 |
| rs30187 | 96150086 | p | 0.4791 | 1 |
| rs10515242 | 96008431 | mh | 0.4925 | 1 |
| rs26482 | 96043712 | p | 0.4989 | 1 |
| rs4869305 | 96018123 | c | 0.4991 | 1 |
| rs10515242 | 96008431 | c | 0.5142 | 1 |
| rs152005 | 96051603 | p | 0.5199 | 1 |
| rs155054 | 96001058 | c | 0.5322 | 1 |
| rs42398 | 96146211 | mh | 0.539 | 1 |
| rs4400148 | 96014543 | c | 0.5499 | 1 |
| rs1862182 | 96025282 | mh | 0.565 | 1 |
| rs1421911 | 96026703 | p | 0.5676 | 1 |
| rs4869304 | 96009225 | p | 0.6087 | 1 |
| rs17086432 | 95996726 | c | 0.6249 | 1 |
| rs42398 | 96146211 | p | 0.6261 | 1 |
| rs27852 | 96071795 | p | 0.6867 | 1 |
| rs6885297 | 95974758 | mh | 0.6879 | 1 |
| rs3756623 | 96110704 | p | 0.6963 | 1 |
| rs155040 | 95995910 | c | 0.6983 | 1 |
| rs261234 | 95988209 | mh | 0.7214 | 1 |
| rs1862609 | 96112446 | mh | 0.731 | 1 |
| rs152280 | 96187698 | p | 0.7346 | 1 |
| rs1862609 | 96112446 | p | 0.755 | 1 |
| rs10050860 | 96147966 | p | 0.7579 | 1 |
| rs1862182 | 96025282 | p | 0.7805 | 1 |
| rs10053056 | 96069176 | p | 0.7833 | 1 |
| rs27772 | 96115732 | p | 0.7925 | 1 |
| rs17086512 | 96040813 | p | 0.7937 | 1 |
| rs28096 | 96135000 | mh | 0.8419 | 1 |
| rs4400148 | 96014543 | p | 0.8439 | 1 |
| rs155040 | 95995910 | mh | 0.8586 | 1 |
| rs28081 | 96118746 | mh | 0.8802 | 1 |
| rs4400148 | 96014543 | mh | 0.8874 | 1 |
| rs151835 | 96096738 | p | 0.9023 | 1 |
| rs155056 | 96000101 | p | 0.9488 | 1 |
| rs27429 | 96120729 | mh | 0.9603 | 1 |
| rs27851 | 96108157 | mh | 0.9974 | 1 |
Our analysis of the CIDR familial PD data confirms the association between Parkinson's disease and the genomic region we previously identified in the NINDS PD data. Further, the effect sizes and p-values seen in the two studies are quite similar. We are unsure why the strongest haplotype sharing signal in the NINDS PD data (the cross statistic) produced the weakest signal in the CIDR PD data. One reason may be that the NINDS PD study had a higher density of SNPs, especially in gene-centric regions, making it easier to detect sharing patterns. Also, the NINDS PD study sampled isolated PD cases without extensive PD family history (individuals with 3 or more relatives with Parkinsonism were excluded), while the CIDR PD study sampled only probands with close PD relatives. Thus a possible explanation for the difference in the strength of the sharing signal may be due to different types of Parkinson's risk variants being captured in the two study designs. Individuals with close PD relatives (as in CIDR PD) are more likely to have their genetic PD risk explained by highly penetrant but rare variants than are those without close PD relatives (as in NINDS PD). These rare variants may only segregate within a single family in the study population, which would dilute the haplotype sharing signal about such loci (recall that the relatives with PD were not included in the study).
Our analysis of the NINDS and CIDR PD GWASs find PD association in a genomic region containing the calpastatin (CAST) gene. Calpastatin inhibits calpain, a calcium-dependent protease participating in a variety of physiologic processes (Goll et al., 2003). Calpains have been implicated in neurodegenerative disorders such as PD, Alzheimer's and multiple sclerosis (Saito et al., 1993; Mouatt-Prigent et al., 1996; Tsuji et al., 1998; Shields et al., 1999; Adamec et al., 2002; Raynaud and Marcilhac, 2006). The mid-brains of PD patients have been found to have higher levels of calpain (Mouatt-Prigent et al., 1996) and calpain overexpression may play a role in neuronal death (Shukla et al., 2006; Camins et al., 2006). Inhibiting calpain in animal models of PD prevents neuronal and behavioral deficits (Crocker et al., 2003). Thus, variation in calpastatin expression that affects calpain inhibition may explain how CAST genotypes affect PD risk. Our confirmation of the CAST locus/PD association in a second data set suggests that CAST involvement in PD etiology should be further investigated.
Supplementary Material
Supplemental Figure 1: Empirical CDF of call rates. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Supplemental Figure 2: Empirical CDF of 10th percentile of genotype quality score. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Supplemental Figure 3: Empirical CDF of 50th percentile of genotype quality score. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Supplemental Figure 4: Empirical CDF of average heterozygosity. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Table S1: Summary of allele frequencies and Mantel-Haenszel estimated odds-ratios across NINDS PD and CIDR PD studies.
Acknowledgments
We thank the NINDS and the CIDR whole genome association study in familial Parkinson's Disease study investigators for providing the CIDR PD data through dbGap. Funding support for the CIDR PD study and the genotyping of samples was provided by the NINDS (Foroud/Myers, PI). The dataset used in analyses described in this manuscript was obtained from the NINDS Database found at http://view.ncbi.nlm.nih.gov/dbgap through dbGaP accession number phs000126.v1.p1. A.S.A. acknowledges support from the NIH through NHLBI grant K25 HL077663 and NIMH grant R01 MH084680.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Andrew S. Allen, The Department of Biostatistics and Bioinformatics, Duke Clinical Research Institute, Duke University, Durham.
Glen A. Satten, the Centers for Disease Control and Prevention, Atlanta.
References
- Adamec E, Mohan P, Vonsattel JP, Nixon RA. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol. 2002;104:92–104. doi: 10.1007/s00401-002-0528-6. [DOI] [PubMed] [Google Scholar]
- Allen AS, Satten GA. A novel haplotype-sharing approach for genome-wide case-control association studies implicates the calpastatin gene in Parkinson's disease. Genet Epidemiol. 2009 doi: 10.1002/gepi.20417. Published online: Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camins A, Verdaguer E, Folch J, Pallàs M. Involvement of Calpain Activation in Neurodegenerative Processes. CNS Drug Reviews. 2006;12:135–148. doi: 10.1111/j.1527-3458.2006.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, et al. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. The Journal of Neuroscience. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LML, Breteler MMB. Epidemiology of Parkinson's Disease. Lancet Neurology. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- Epstein MP, Allen AS, Satten GA. A simple and improved correction for population stratification in case-control studies. Am J Hum Genet. 2007;80:921–30. doi: 10.1086/516842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung HC, Scholz S, Matarin M, et al. Genome-wide genotyping in Parkinson's disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurology. 2006;5:911–916. doi: 10.1016/S1474-4422(06)70578-6. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Klein C, Schlossmacher MG. Parkinson disease, 10 years after its genetic revolution: Multiple clues to a complex disorder. Neurology. 2007;69:2093–2104. doi: 10.1212/01.wnl.0000271880.27321.a7. [DOI] [PubMed] [Google Scholar]
- Lee AB, Luca D, Klei L, et al. Discovering genetic ancestry using spectral graph theory. Genetic Epidemiology. 2009 doi: 10.1002/gepi.20434. Published Online: May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatt-Prigent A, Karlsson J, Agid Y, Hirsch E. Increase m-calpain expression in the mesencephalon of patients with Parkinson's disease but not in neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73:979–987. doi: 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH the PSG-PROGENI and GenePD Investigators, Coordinators and Molecular Genetic Laboratories. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis:A possible regulation of Alzheimer's disease. FEBS Journal. 2006;273:3437–3443. doi: 10.1111/j.1742-4658.2006.05352.x. [DOI] [PubMed] [Google Scholar]
- Saito K, Elce J, Hamos J, Nixon R. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: A potential molecular basis for neuronal degeneration. Proc Nat Acad Sci USA. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasua SM, Collins JS, Williamson DM, et al. Effect of population stratification on the identification of significant SNPs in genome wide association studies. To appear in BMC Proceedings. 2009 doi: 10.1186/1753-6561-3-s7-s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci USA. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla M, Rajgopal Y, Babu PP. Activation of calpains, calpastatin and spectrin cleavage in the brain during the pathology of fatal murine cerebral malaria. Neurochemistry International. 2006;48:108–113. doi: 10.1016/j.neuint.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer's disease brains. Neurosci Lett. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Young SS. Resampling-Based Multiple Testing. New York: J Wiley & Sons; 1993. p. 340. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Empirical CDF of call rates. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Supplemental Figure 2: Empirical CDF of 10th percentile of genotype quality score. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Supplemental Figure 3: Empirical CDF of 50th percentile of genotype quality score. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Supplemental Figure 4: Empirical CDF of average heterozygosity. Red lines denote individuals whose DNA did not require amplification. Black lines denote individuals whose DNA was extracted via whole genome applification.
Table S1: Summary of allele frequencies and Mantel-Haenszel estimated odds-ratios across NINDS PD and CIDR PD studies.