Abstract
A novel delivery method is described that incorporates taste stimuli into edible strips for determining n-propylthiouracil (PROP) taster status. Edible strips that contained 400 or 600nanomoles of PROP were prepared for psychophysical studies. Using these strips, we measured taste intensity, taste hedonics, and taste quality responses in a sample of healthy volunteers (n = 118). Participants were also asked to assess a single NaCl strip, a quinine strip, 3 NaCl solutions, and 3 PROP solutions. All psychophysical data were subsequently analyzed as a function of TAS2R38 genotype. The use of PROP strips for distinguishing between individuals with at least 1 PAV allele and individuals with other genotypes was assessed and compared with the use of PROP solutions for making this same distinction. For the 2 PROP strips and PROP solutions, individuals who expressed at least 1 PAV allele could perceive the bitter taste of PROP. Individuals who expressed 2 AVI alleles responded similarly to 400nanomole PROP strips and blank strips. Furthermore, individuals with 2 AVI alleles responded to 0.032 and 0.32mM PROP solutions at intensities that were similar to water, though intensity ratings to 3.2mM PROP solution exceeded water. In general, those with at least 1 PAV allele rated the bitter taste of PROP as unpleasant in both delivery methods (strips or solutions). Psychophysical data from PROP strips and solutions were consistent with TAS2R38 genotype. These results support the validity of edible taste strips as a method for assessing PROP taste perception in humans.
Key words: bitter taste, gustation, NIH Toolbox, PROP, taste blindness, taste strip, taste test
Introduction
There is considerable individual variation in taste sensitivity to the bitter compounds phenylthiocarbamide (PTC), n-propylthiouracil (PROP), and thiouracil (Anonymous 1931; Blakeslee and Fox 1932; Riddell and Wybar 1944; Griffin and Fischer 1960). Variation in the taste of these compounds ranges from imperceptible upwards to extremely intense across the population (Anonymous 1931; Griffin and Fischer 1960; Bartoshuk et al. 1994; Guo and Reed 2001; Hayes and Keast 2011). Differences in taste sensitivity to PROP have been associated with individual differences in food preferences and eating habits (Keller et al. 2002), including preference for vegetables and fatty foods (Dinehart et al. 2006, Sandell and Breslin 2006, Tepper et al. 2009, Duffy et al. 2010).
Although other loci have been proposed (Reed et al. 1999; Drayna et al. 2003), the TAS2R38 (T2R38) gene on the long arm of human chromosome 7 is primarily responsible for encoding the receptor that detects the bitter taste of both PTC and PROP in humans (Drayna et al. 2003; Kim et al. 2003). The human TAS2R38 gene is intron-less and exhibits single nucleotide polymorphisms at 5 different sites, which result in 7 known haplotypes (Kim et al. 2003). These haplotypes primarily explain the wide variability in PTC and PROP taste perception (Kim et al. 2003). Each single nucleotide polymorphism encodes a different amino acid in the receptor protein (at amino acid positions 49, 262, 296, 80 [rare], and 274 [rare]). Of the 7 haplotypes, 2 predominant forms exist at high frequency outside of sub-Saharan Africa (Kim and Drayna 2005). These 2 major haplotypes are primarily responsible for the distinction between those who perceive PTC and PROP as tasteless (nontasters) and those who perceive the bitter taste of these chemicals at moderate to high intensity (tasters). The predominant taster form of the TAS2R38 receptor contains a proline at amino acid position 49, an alanine at position 262, and a valine at position 296 (the PAV haplotype). In contrast, the most common nontaster form of the receptor expresses an alanine, a valine, and an isoleucine at these same 3 amino acid positions (the AVI haplotype). Nucleotide substitution at amino acid residue 49 (A49P) shows the strongest association with taster status, where a proline at this position confers taster status (Drayna 2005). Individuals who express either 1 or 2 PAV-containing alleles are tasters of both PROP and PTC. Individuals who express 2 AVI-containing alleles are nearly always nontasters of both PROP and PTC (Kim et al. 2003; Desai et al. 2011). In the absence of a PAV allele, PVI, AAI, and AAV haplotypes may confer intermediate taste sensitivities to both PTC and PROP (Bufe et al. 2005). Due to their high frequencies, PAV is considered the major taster haplotype, whereas AVI is considered the major nontaster haplotype.
These genetic results support psychophysical studies. For example, threshold measurements can define the PTC/PROP sensitivity phenotype by separating tasters from nontasters. This separation is identified by a positive response at the antimodal concentration (Lawless 1980) or by comparison of PROP thresholds to an antimodal cutoff (Bartoshuk et al. 1994). In addition, PROP taste sensitivity can be classified as nontaster, moderate taster, or supertaster by comparing PROP taste intensities at the suprathreshold level to 3 different NaCl solutions (Tepper et al. 2001).
One limitation to more fully understand the role of bitter taste in human health and nutrition is the lack of a simple, rapid, and reliable method for characterizing PROP taste function in large samples for epidemiological research. Crystals placed directly on the tongue (Blakeslee and Fox 1932), aqueous solutions (Bartoshuk et al. 1994; Tepper et al. 2001), impregnated filter papers (Lawless 1980), or filter paper disks (Zhao et al. 2003) can be used as delivery methods for PTC and PROP. However, impregnated filter paper may produce unacceptable proportions of false negative responses and may not agree with the classification of tasters obtained by taste recognition threshold measurements (Lawless 1980). In addition, the filter paper method may not assure a consistent amount of PROP across the paper (Zhao et al. 2003). PROP solutions can be laborious to prepare (Smutzer et al. 2008), and solutions may introduce temperature as a variable in taste perception (Green and Nachtigal 2012). In addition, the unsaturated ring of PROP absorbs light (Rosseel and Lefebvre 1990), which excites π electrons into π* orbitals. This absorption of light by PROP in solution may in turn lead to photodecomposition (Toxnet 2006). Finally, aqueous solutions must be freshly made on a regular basis and may pose storage concerns.
Edible taste strips were recently introduced for the detection of PROP thresholds, and threshold amounts were equal to or lower than those obtained in previous studies with PROP solutions or PROP-impregnated filter papers (Reed et al. 1995; Smutzer et al. 2008; Desai et al. 2011). However, due to time constraints, threshold methods are seldom employed in large-scale epidemiological studies.
This study was designed to demonstrate the validity of using edible taste strips with suprathreshold amounts of taste stimuli for distinguishing PROP tasters and nontasters. Validity was checked by examining psychophysical responses to edible strips that varied in the amount of PROP. These data were then examined as a function of TAS2R38 genotype. Because PROP taster phenotype is known to not perfectly match the genotype (Hayes et al. 2008), taste responses to 3 different suprathreshold PROP solutions were also assessed by TAS2R38 genotype for comparison with edible strip data.
Materials and methods
Edible taste strips
Edible taste strips were prepared as previously described (Smutzer et al. 2008; Desai et al. 2011; Ebba et al. 2012). Pullulan (α-1,4-; α-1,6-glucan) was obtained from NutriScience Innovations, LLC. Pullulan was combined with the polymer hydroxypropyl-methylcellulose (HPMC) (Dow Chemical Co.) at a weight ratio of 11.5:1. For preparation of edible films, HPMC powder was first added to a rapidly stirred solution of distilled water at 75–80 °C, followed by addition of pullulan powder to yield a final concentration of 3.25% wt/vol of polymers. The mixture was cooled to room temperature prior to adding taste stimulus to the mixture. Food coloring was also added at that time to aid in visualization of strips. Films were poured onto nonstick surfaces, dried, cut into 1-inch squares, and stored in the dark at 4 oC until use. Blank (control) strips were prepared as described above except taste stimulus was not included. PROP and quinine HCl were obtained from Sigma Chemical Co., NaCl was obtained from Fisher Scientific, and food coloring (McCormick & Co.) was obtained from a local supermarket. Finally, water was obtained from Deer Park Spring Water, Inc.
Taste solutions
Three suprathreshold PROP solutions (0.032, 0.32, and 3.2mM) and 3 NaCl solutions (0.01, 0.1, and 1.0M) were selected for comparison with taste strips based at the concentrations used by Tepper et al. (2001). All solutions were stored at 4 oC and warmed to room temperature for psychophysical testing.
Test subjects
Healthy subjects (n = 118) were recruited through posted flyers and by word of mouth from Temple University and the surrounding community. Participants included 56 males and 62 females, 47% Caucasian, 32% Asian, 18% African American/Black, and 3% Hispanic, and ranged in age from 18 to 74 years (average age of 25.5±1.1 years). Each subject was asked to refrain from eating or drinking (except water) for a minimum of 30min before his or her scheduled session. All subjects were healthy by self-report, were nonsmokers, and had not been previously diagnosed with any neurological disorders that would compromise their taste function. In addition, no test subjects had visited a dentist in the previous 48h. All subjects provided informed written consent and were reimbursed for their time. The Temple University Human Subjects Review committee fully approved the experimental protocol.
Procedures
Subjects were prescreened by asking a series of questions to determine whether the subject had normal taste function by self-report. Each subject reported demographic data (date of birth, place of birth, gender, ethnicity, race, language spoken at home, and educational level) on a computerized survey. Next, subjects were trained in the use of a computerized version of the general Labeled Magnitude Scale (gLMS) for report of perceived intensity (Bartoshuk et al. 2004). For orientation to the gLMS, each subject was asked to rate light intensities and sound intensities based on their past perceptions. Subjects either touched the scale on a touch screen monitor or used a mouse to place the cursor at a desired position on the vertical scale. In addition, subjects were trained in the placement of edible taste strips in the oral cavity, first placing the strip on their tongue, touching their tongue to the roof of their mouth and then swallowing. Finally, the buccal surface of the oral cavity of each subject was gently brushed in order to obtain a sample of epithelial cells for TAS2R38 genotyping.
Study design
All subjects were presented with 15 taste stimuli (including blank taste strips and water). Blank strips composed of pullulan elicit essentially no background taste (Smutzer et al. 2008). A series of 8 taste strips was presented first, followed by the presentation of 7 aqueous solutions. The order of presentation of taste strips was blank taste strip, 140umole NaCl strip, blank strip, 400nmole PROP strip, blank strip, 600nmole PROP strip, blank strip, and then 148nmole quinine HCl strip. After tasting each strip, subjects rated the taste intensity of the strip on the gLMS, identified the taste as one of sweet, sour, salty, bitter, or other, and finally gave a hedonic rating on a 3-point scale from like (illustrated with a smiling face), neither like nor dislike (illustrated with a neutral face), or dislike (illustrated with a frowning face). Subjects rinsed their mouths twice with water prior to and following each taste strip presentation. After presentation of all the taste strips, subjects were presented with 10ml of water. Subjects were then presented with 3 cups containing 10ml NaCl solutions that varied by 1 log unit (0.01, 0.1, and 1M NaCl), followed by 3 cups containing 10ml of 0.032, 0.32, or 3.2mM PROP solution (based on Tepper et al. 2001). Subjects rated the taste intensity, identified the taste quality, and reported a hedonic rating for the taste solutions in the same manner as they had done for the taste strips. Subjects rinsed their mouths twice with water prior to and following each solution presentation.
TAS2R38 genotyping
Genotyping of TAS2R38 at 3 different polymorphic sites was carried out at the Monell Chemical Senses Center. Genomic DNA was obtained from cheek cells with a sterile swab (Epicentre Biotechnologies). Genomic DNA was amplified using TaqMan primers (Applied Biosystems). Alleles of TAS2R38 were genotyped at each of 3 variant single nucleotide sites using allele-specific fluorescent probes from Applied Biosystems (reference SNP numbers included A49P = rs713598, V262A = rs1726866, and I296V = rs10246939) (Desai et al. 2011). The ability of these nucleotide probes to fully hybridize to genomic DNA resulted in the loss of quenching of the terminal fluorophore and was the criterion for identifying the specific nucleotide at each of the 3 polymorphic sites.
Data analysis
Data from 1 participant were not included in the analyses because the average intensity ratings given to blank strips and water for this subject were more than 3 standard deviations above the mean value for all subjects. Taste intensity, taste quality, and taste hedonic ratings were analyzed separately by haplotype group. Comparisons were then made between the major haplotype groups (PAV/PAV, PAV/AVI, and AVI/AVI). Taste intensity data were analyzed as percent of the gLMS scale from 0% to 100% (lowest to highest). Taste quality data and taste hedonic data were converted to binary outcomes. Taste quality reports were coded as identifying the taste as bitter or not (for PROP and quinine) and salty or not (for NaCl). Hedonic data were coded as reporting the taste as disliked or not. ANOVA followed by Newman–Keuls post hoc tests were used to test for haplotype differences in taste intensity, whereas chi-square analyses were used to test for haplotype differences in taste quality and hedonics. Paired t-tests were used to assess differences in intensity ratings for each strip within a haplotype group.
Subject genotypes
After exclusion of the outlier, data from 117 subjects remained. Nine of the remaining individuals had rare haplotypes and were therefore excluded from analyses. Excluded were 3 subjects with AAV/AVI haplotypes, 1 subject with AAI/AVI haplotype, 3 subjects with PAV/AAV haplotypes, and 2 subjects with a PAV/AAI haplotype. The remaining 108 participants included 35 individuals with PAV/PAV haplotypes, 38 individuals with PAV/AVI haplotypes, and 35 subjects with AVI/AVI haplotypes.
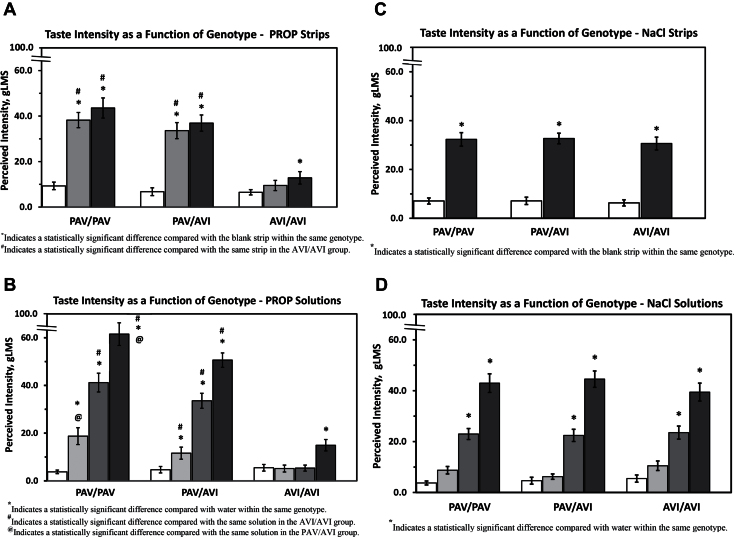
Taste intensity values for PROP strips and PROP solutions
Figure 1A shows the taste intensity results obtained from participants sampling blank strips, 400nmole PROP strips, and 600nmole PROP strips. Results are given separately for each of the 3 major TAS2R38 genotypes. Figure 1B presents taste intensity values obtained from the same participants sampling water and 0.032, 0.32, and 3.2mM PROP solutions.
Figure 1.
Suprathreshold taste intensity response as a function of TAS2R38 genotype. Error bars represent standard error of the mean. (A) Taste intensity responses to 400 and 600nmole PROP strips. Clear columns represent blank strips, gray columns represent 400nmole PROP strips, and black columns represent 600nmole PROP strips. (B) Taste intensity responses to 0.032, 0.32, and 3.2mM PROP solutions. Clear columns represent water controls, light gray columns represent 0.032mM PROP, dark gray columns represent 0.32mM PROP, and black columns represent 3.2mM PROP. (C) Taste intensity responses to a 140 umole NaCl strips. Clear columns represent blank strips, and black columns represent 140umole NaCl strips. (D) Taste intensity responses to 0.01, 0.1, and 1.0M NaCl solutions. Clear columns represent water controls, light gray columns represent 0.01M NaCl, dark gray columns represent 0.10M NaCl, and black columns represent 1.0M NaCl. The same subject population was used in all figures (n = 108).
Genotype groups did not differ in rating of blank strips for the first trial (F(2, 105) = 0.31, P = 0.73). However, there was some indication that PROP exposure may have slightly influenced ratings of blank strips for the PAV/PAV group on the third and fourth trials (trend for a genotype by trial interaction for blank strip trials [F(6, 315) = 1.50, P = 0.18]). Intensity ratings following sampling of both the 400nmole (F(2, 105) =24.5, P < 0.0001) and 600nmole (F(2, 105) =19.8, P < 0.0001) PROP strips were significantly different between genotype groups, with PAV/PAV and PAV/AVI reporting more intense sensations from tasting both of the PROP strips than did AVI/AVI subjects (Newman–Keuls, P < 0.001).
Similar to the results for blank strips, ratings for water did not differ between TAS2R38 genotypes (F(2, 105) = 0.28, P = 0.75). However, taste intensity ratings following sampling of 0.032, 0.32, and 3.2mM PROP solutions were significantly different between genotype groups, with PAV/PAV and PAV/AVI nearly always reporting more intense sensations than AVI/AVI from the PROP solutions. PAV/PAV subjects generally reported more intense sensations than PAV/AVI subjects from PROP solutions (F(2, 105)= 7.28, 40.2, and 48.5, respectively, P < 0.005, Newman–Keuls all Ps < 0.05 except PAV/PAV vs. AVI/AVI for 0.032mM PROP, P = 0.09 and PAV/PAV vs. PAV/AVI for 0.32mM PROP, P = 0.06).
Within each genotype group, participants gave similar intensity ratings to 400 and 600nmole PROP strips (P > 0.05). Intensity ratings for each of the 2 PROP strips were higher than the blank strip for PAV/PAV and PAV/AVI participants (PAV/PAV 400nmole vs. blank, t(34) = 8.75, P < 0.0001; PAV/PAV 600nmole vs. blank, t(34) = 6.48, P < 0.0001; PAV/AVI 400nmole vs. blank, t(37) = 7.12, P < 0.001; PAV/AVI 600nmole vs. blank, t(37) = 8.13, P < 0.0001) but differed only between the 600nmole PROP strip and the blank strip for AVI/AVI individuals (t(34) = 2.88, P < 0.01).
Taste intensity values for NaCl strips and NaCl solutions
Figure 1C shows taste intensity responses for the blank strips and the 140umole NaCl strip, with results presented separately for each of the 3 major TAS2R38 genotypes. The NaCl strip elicited similar taste intensity responses from participants with all 3 TAS2R38 genotypes (F(2, 105) = 0.25, P = 0.78). Participants reported an average taste intensity value near 30, which corresponds to “strong” on the gLMS (Bartoshuk et al. 2004).
Figure 1D shows taste intensity responses for water and 0.01, 0.1, and 1M NaCl. Consistent with findings for the NaCl strip, there were no significant differences between genotype groups in intensity ratings for NaCl solutions (F’s (2, 105) = 2.18, 0.10, and 0.59, P > 0.05).
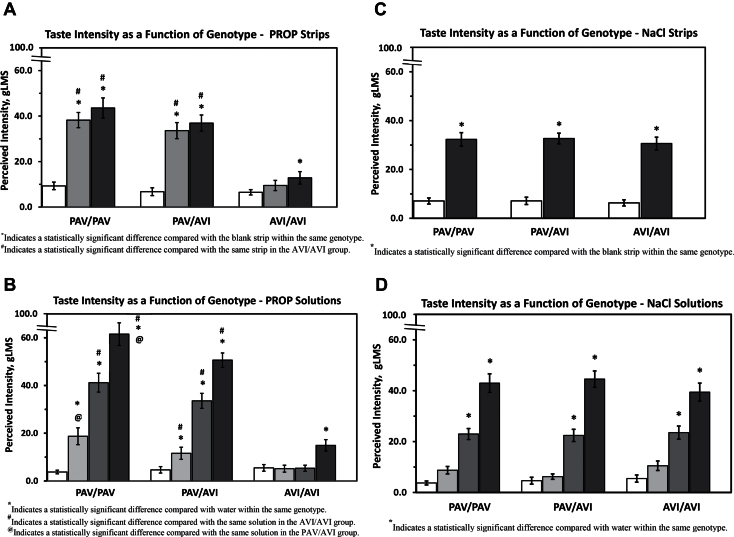
Hedonic data for PROP strips and solutions
Hedonic responses for PROP taste strips separated by TAS2R38 genotype are shown in Figure 2A. Hedonic data differed significantly by genotype for PROP taste strips. The vast majority of PAV/PAV and PAV/AVI subjects indicated dislike for both PROP strips, whereas 40% or fewer of the AVI/AVI subjects indicated dislike for PROP strips (400nmole strip, PAV/AVI vs. AVI/AVI, χ2 = 25.2, P < 0.0001; 600nmole strip, PAV/AVI vs. AVI/AVI, χ2 = 17.4, P < 0.0001; 400nmole strip, PAV/PAV vs. AVI/AVI, χ2 = 30.6, P < 0.0001; 600nmole strip, PAV/PAV vs. AVI/AVI, χ2 = 20.5, P < 0.0001). Fewer than 30% of subjects in any TAS2R38 genotype indicated dislike for the blank strips on any of the trials.
Figure 2.
Hedonic response to taste stimuli as a function of TAS2R38 genotype. Data are plotted as the percentage of respondents who reported a response of dislike. (A) Hedonic responses to 400 and 600nmole PROP taste strips. Clear columns represent blank strips, gray columns represent 400nmole PROP strips, and black columns represent 600nmole PROP strips. (B) Hedonic responses to 0.032, 0.32, and 3.2mM PROP solutions. Clear columns represent water controls, light gray columns represent 0.032mM PROP, dark gray columns represent 0.32mM PROP, and black columns represent 3.2mM PROP. (C) Hedonic responses to a 140umole NaCl strip. Clear columns represent blank strips, and dark columns represent NaCl taste strips for each TAS2R38 genotype. (D) Hedonic responses to 0.01, 0.1, and 1.0M NaCl solutions. Clear columns represent water controls, light gray columns represent 0.01M NaCl, dark gray columns represent 0.10M NaCl, and black columns represent 1.0M NaCl.
Hedonic responses for PROP solutions separated by TAS2R38 genotype are shown in Figure 2B. Hedonic data significantly differed by genotype for solutions. For PROP solutions, hedonic differences between the 2 genotype groups with a PAV allele compared with the AVI/AVI group were most pronounced for 0.32mM PROP solution; the 0.032mM PROP solution elicited a negative hedonic response in fewer individuals across all 3 genotypes, whereas the 3.2mM PROP solution elicited a negative hedonic response in the majority of individuals across all 3 TAS2R38 genotypes. Nevertheless, differences in hedonic response between genotypes were significant for all 3 PROP solution concentrations (χ2 > 8.9, P < 0.01).
Hedonic data for NaCl strips and solutions
Hedonic responses for the NaCl strips separated by TAS2R38 genotype are shown in Figure 2C, whereas hedonic responses for NaCl solutions separated by TAS2R38 genotype are shown in Figure 2D. For both solutions and strips, hedonic responses for NaCl taste did not vary significantly as a function of TAS2R38 genotype—the percentage of respondents choosing “dislike” was similar for all 3 genotypes (P > 0.05). For all 3 genotypes, the percentage reporting “dislike” increased with NaCl concentration in solution.
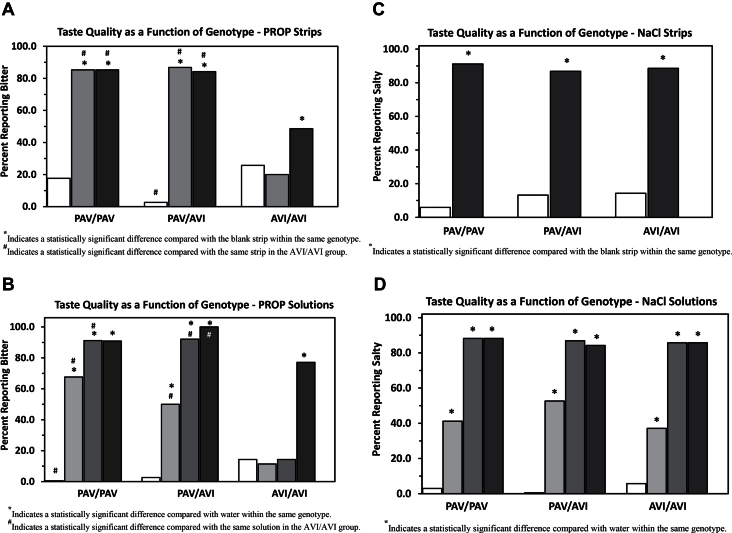
Taste quality data for PROP strips and solutions
Figure 3A shows the taste quality reports (as percent selecting bitter) obtained from participants sampling blank strips, 400nmole PROP strips, and 600nmole PROP strips. Results are given separately for each of the 3 major TAS2R38 genotypes. Figure 3B presents taste quality reports (as percent selecting bitter) obtained from the same participants sampling water and 0.032, 0.32, and 3.2mM PROP solutions.
Figure 3.
Taste quality response to bitter and salty taste stimuli as a function of TAS2R38 genotype. Data are plotted as the percentage of respondents who reported a response of bitter for PROP or salty for NaCl. (A) Taste quality responses to 400 and 600nmole PROP strips. Clear columns represent blank strips, gray columns represent 400nmole PROP strips, and black columns represent 600nmole PROP strips. (B) Taste quality responses to 0.032, 0.32, and 3.2mM PROP solutions. Clear columns represent water controls, light gray columns represent 0.032mM PROP, dark gray columns represent 0.32mM PROP, and black columns represent 3.2mM PROP. (C) Taste quality responses to 140umole NaCl strips. Clear columns represent blank strips, and dark columns represent NaCl taste strips for each TAS2R38 genotype. (D) Taste quality responses to 0.01, 0.1, and 1.0M NaCl solutions. Clear columns represent water controls, light gray columns represent 0.01M NaCl, dark gray columns represent 0.10M NaCl, and black columns represent 1.0M NaCl.
Bitter taste quality responses for the blank strips did not differ between TAS2R38 genotypes (P’s > 0.05), except that AVI/AVI subjects reported the blank strips to be bitter more often than did PAV/AVI subjects (χ2 = 5.71, P < 0.02). However, taste quality responses following sampling of both the 400 and 600nmole PROP strips were significantly different between genotype groups, with PAV/PAV and PAV/AVI having a higher proportion of subjects reporting a bitter quality after tasting the strips than did AVI/AVI subjects (χ2’s > 30.3, P’s < 0.0001). Taste quality reports for the 400 and 600nmole PROP strips were similar for the PAV/PAV and PAV/AVI groups. However, for AVI/AVI subjects, the 600nmole strip was reported as bitter more frequently than the 400nmole strip (χ2 = 6.34, P < 0.05).
Bitter taste quality reports for water did not differ between TAS2R38 genotypes except that AVI/AVI reported water to be bitter slightly more often than did PAV/PAV (χ2 = 5.38, P < 0.05). Taste quality reports following sampling of 0.032 and 0.32mM PROP solutions were significantly different between genotype groups, with PAV/PAV and PAV/AVI subjects having a higher percentage reporting bitter after tasting the PROP solutions than did AVI/AVI subjects (χ2’s > 12.5, P < 0.001). Bitter quality reports following sampling of 3.2mM PROP solutions differed between PAV/AVI and AVI/AVI (χ2 = 6.90, P < 0.01) but did not reach significance for PAV/PAV compared with AVI/AVI (χ2 = 1.6, P = 0.20).
Taste quality data for NaCl strips and NaCl solutions
Figure 3C shows the taste quality reports (as percent selecting salty) obtained from participants sampling blank strips or 140umole NaCl strips. Results are given separately for each of the 3 major TAS2R38 genotypes. Figure 3D presents taste quality reports (as percent selecting salty) obtained from the same participants sampling water and 0.01, 0.1, and 1M NaCl solutions. Genotype groups did not significantly differ in reports of saltiness (P’s > 0.05).
Comparison of taste strips and solutions based on 2 trials
We also compared, subject by subject, the number of reversals in expected intensity ratings seen when using data from 2 taste strip trials with the number of reversals seen when using data from 2 taste solution trials. Intensity ratings for the 400nmole PROP strip were compared with intensity ratings for the 140 µumole NaCl strip. Similarly, intensity ratings for the 600nmole PROP strip were compared with intensity ratings for the 140umole NaCl strip. For solutions, intensity ratings for 0.32mM PROP solution was compared with intensity ratings for the 0.1M NaCl solution (based on Tepper et al. 2001). Similarly, intensity ratings for 3.2mM PROP solution were compared with intensity ratings for 1M NaCl. A reversal was considered to have occurred when a PAV/PAV or PAV/AVI individual rated NaCl as more intense than PROP by more than 10% of the gLMS scale, or if an AVI/AVI individual rated PROP as more intense than NaCl by more than 10% of the gLMS scale. Using this rule for classifying reversals, out of all 108 subjects analyzed, there were 17 reversals for the 400nmole PROP strip, 25 for the 600nmole PROP strip, 6 for the 0.32M PROP solution, and 14 for the 3.2mM PROP solution. Based on these reversal rates, the 400nmole PROP strip performed similarly to the 3.2mM PROP solution (P = 0.56) but produced more reversals than did the 0.32mM PROP solution (χ2 = 5.89, P < 0.02). The 600nmole PROP strip also produced more reversals than did the 0.32mM PROP solution (χ2 = 13.6, P < 0.001) and tended to produce more reversals than did the 3.2mM PROP solution, though this did not reach statistical significance (χ2 = 3.79, P = 0.052).
Psychophysical data for quinine taste strips
In order to obtain data from a widely used bitter taste stimulus, a single 148nmole quinine taste strip was included as a positive control. For quinine strips, gLMS values for the 3 most commonly occurring TAS2R38 genotypes averaged 20.4±4.5 (moderate taste intensity) (n = 108), and 21.3±4.5 when all 6 TAS2R38 genotypes (n = 117) were included (graph not shown). As with NaCl strips, taste intensity values for quinine strips did not vary significantly among the 3 major TAS2R38 genotypes (F(2, 105) = 0.48, P = 0.62). However, quinine taste intensities did show considerable variability in our subject population. Five out of 117 subjects reported gLMS values of zero for quinine, whereas another 21 subjects reported gLMS values between 1 and 5. A graph of ranked quinine taste intensity responses did not show break points in the distribution, and no clear separation in gLMS intensity scores could be observed for those who could and could not perceive the bitter taste of the 148nmole quinine strip (see Supplementary data). Finally, only a weak correlation was apparent between subjects with an AVI/AVI genotype and the inability of these subjects to perceive the bitter taste of quinine (r = 0.38, P < 0.03 for 400nmole PROP strips). For all test subjects, there was no correlation for taste intensity values obtained from 400nmole PROP strips and 148nmole quinine strips (r = 0.14, P > 0.05).
Discussion
PROP taste strips for determining taster status
The primary objective of this study was to determine the validity of edible taste strips as a method to identify variations in PROP taste perception. This validation was carried out by comparing psychophysical data obtained from PROP taste strips to TAS2R38 genotype. This study further allowed a direct comparison of PROP taste strips and PROP taste solutions for identifying PROP taster status, as well as how these psychophysical results correlate with TAS2R38 genotype.
Our psychophysical and genetic results indicate that edible taste strips are a suitable delivery method for introducing PROP into the oral cavity at suprathreshold amounts. Our study further demonstrates that edible taste strips are readily adapted to studies on PROP taste perception. Based on TAS2R38 genotype data, the psychophysical results obtained with PROP strips indicate that this new delivery method can accurately assess PROP phenotype. This accuracy was comparable to results obtained with PROP solutions. Similar conclusions have been reported when edible taste strips were used to identify PROP thresholds (Desai et al. 2011).
One notable difference between taste strips and solutions was observed. In this study, blank strips yielded slightly higher gLMS scores compared with water controls. This increase in taste intensity may have been caused by the order of presentation of taste stimuli in the study and/or by the absence of a sufficient water rinse after each trial.
Suprathreshold taste intensity responses to PROP solutions vary between PAV homozygotes and PAV/AVI heterozygotes for 170 and 550 µM PROP solutions (Bufe et al. 2005), with 0.032, 0.32, and 3.2mM PROP solutions (Hayes et al. 2008). Although differences in taste intensity values between the PAV/PAV and PAV/AVI groups for the PROP solutions in this study generally differed for these 2 diplotypes, this difference was less pronounced with strips. PROP strips yielded an average lower taste intensity value for PAV/AVI heterozygotes than for PAV homozygotes, but the taste strip data showed no statistical differences between these 2 diplotypes.
PROP taste intensity data from solutions (Bufe et al. 2005, and this study) suggest that the PAV allele shows a small dosage effect, which could be caused by lower PAV mRNA and/or protein levels in taste receptor cells of heterozygotes than in PAV homozygotes. For example, PAV/AVI individuals may produce fewer PAV variant TAS2R38 receptor proteins within individual bitter taste receptor cells or may have a lower number of taste receptor cells that express this protein. In addition, recent evidence indicates that TAS2Rs form oligomers in heterologous systems (Kuhn et al. 2010). In heterozygotes, PAV and AVI receptor variants could produce functional heteromeric TAS2R38 receptors that cause a small decrease in the perception of PROP. Alternatively, lowered PAV protein levels in taste cells of heterozygotes could decrease the number of functional TAS2R heteromeric receptors that bind PROP. Future genetic and psychophysical studies are required to more clearly identify subtle differences in PROP taste perception between PAV/PAV and PAV/AVI diplotypes.
In this study, a small number of AVI/AVI individuals could perceive the bitter taste of PROP in both taste strips and solutions. Similar findings have been reported with PROP threshold (Hayes et al. 2008; Desai et al. 2011) and suprathreshold studies (Hayes et al. 2008). These results suggest that suprathreshold sensitivities to PROP may be under additional genetic and environmental influences (Bufe et al. 2005). In addition, responsiveness to PROP is associated with salivary levels of II-2 peptide and Ps-1 protein (Cabras et al. 2012), which may be modified in AVI/AVI individuals. Finally, lingual taste papillae density and the number of responsive taste receptor cells to PROP may further modify bitter taste perception in some AVI/AVI individuals (Delwiche et al. 2001; Hayes et al. 2008). Nonetheless, these results support the importance (but not a requirement) of a proline at amino acid 49 of the TAS2R38 receptor for perceiving the bitter taste of PROP.
Quinine taste perception with edible strips
Quinine is an amphophilic compound that exhibits a bitter taste to many individuals and elicits an unpleasant taste quality (Zald et al. 2002). Variations in the ability of humans to perceive the bitter taste of quinine have been known for over 50 years (Fischer and Griffin 1963). In contrast to PROP, taste thresholds for quinine exhibit a Gaussian distribution (Fischer and Griffin 1963). More recently, psychophysical and genetic data have shown that the bitter taste of quinine is independent of TAS2R38 genotype (Hayes et al. 2008; Reed et al. 2010). Other data suggest that quinine may bypass a plasma membrane receptor and directly activate cellular G proteins in taste receptor cells (Naim et al. 1994).
For quinine strips, the percentage of individuals who reported a “dislike” response (including nontasters) ranged from 47% to 63% for all 3 TAS2R38 genotypes, with AVI/AVI individuals reporting the highest proportion of “dislike” responses. For taste quality measurements, the most common response was bitter (62%). The second most common (at 24%) taste quality response for quinine was “no taste” and represented a subset of subjects who also reported gLMS values of zero. This observation indicates that a number of subjects were insensitive (for both taste intensity and taste quality) to 148nmole amounts of quinine. These results are not caused by an unequal distribution of quinine molecules during strip formation because fluorescence emission studies have demonstrated that quinine HCl is evenly distributed in dried films that are used to prepare 1-inch square edible taste strips (Smutzer et al. 2008).
A recent report indicates that several bitter taste receptor gene products respond to quinine in humans (Reed et al. 2010), as well as a proline-rich salivary protein gene on chromosome 12. In addition, single nucleotide polymorphisms have been identified in a candidate quinine taste receptor gene (Reed et al. 2010). These polymorphisms could also modulate variations in quinine taste perception.
As opposed to the bimodal distribution in PROP taster status (Fischer and Griffin 1963; Kranzler et al. 1996;Bufe et al. 2005), the range in gLMS taste intensity values for quinine showed no sharp delineation between tasters and nontasters. These results suggest that possible genetic modifications at the receptor level and nongenetic factors may affect quinine perception in humans. Finally, this variation in quinine taste perception should be useful for identifying candidate receptors for this bitter taste stimulus.
Taste perception in the oral cavity
Our results indicate that 400 and 600nmole strips yield psychophysical data that are similar to 0.32mM PROP solutions. However, the amount of PROP that is introduced into the oral cavity that elicits a robust taste response in strips is considerably lower than the amount that is required for a comparable taste intensity response with PROP solutions. This edible strip delivery method allows lower amounts of PROP to be presented to test subjects for psychophysical testing. This lower amount should be especially advantageous for examining taste sensitivity in children, young adults, and clinical populations.
One-inch square polymer-based taste strips are dissolved by a relatively small volume of saliva, which permits this delivery method to present taste stimuli to more localized areas of the oral cavity. After strips dissolve in saliva, a higher local concentration of taste stimulus may come in contact with a smaller number of taste papillae (Desai et al. 2011). In contrast, whole mouth testing with suprathreshold amounts of aqueous 10ml solutions allows taste stimuli to come in contact with more papillae in the oral cavity, but this delivery method dilutes the concentration of the taste stimulus. With aqueous delivery methods, higher amounts of taste stimuli are required. Taken together, these studies indicate that the area of the oral cavity that is exposed to edible strips is smaller but requires less tastant to yield an intensity response that is comparable to aqueous solutions.
Spatial summation in the oral cavity predicts that the perceived taste intensity of PROP should increase among individuals who can perceive PROP as more papillae come in contact with this taste stimulus (Miller and Reedy 1990; Delwiche et al. 2001). Our results with edible taste strips would argue against a major role for spatial summation in the perception of bitter taste stimuli such as PROP at the suprathreshold level.
In general, our 3-point hedonic scale showed that individuals who could perceive the bitter taste of PROP were more likely to report a hedonic response of “dislike.” In adult populations, 9-point hedonic scales (Peryam and Girardot 1952), bipolar hedonic scales based on the gLMS (Duffy et al. 2004), and LMS hedonic category-ratio scales (Lim et al. 2009) are more commonly used in psychophysical taste research. However, none of these scales have been validated for use in young children. Our aim for including this hedonic scale was to develop a simplified response scale that would be appropriate for use in young children. This population was targeted by the NIH Toolbox, which provided funding for this research. Prior work with young children and hedonic ratings for PROP has employed a forced choice methodology employing a simple like versus dislike decision (Mennella et al. 2005). The 3-point scale was selected here to be consistent with other NIH Toolbox assessments.
In summary, the comparison of suprathreshold taste intensity, hedonics, and taste quality data of PROP strips with TAS2R38 genotype analysis, along with comparisons between strips and solutions, validates the use of edible taste strips for identifying PROP taste perception in humans. These results further indicate that 400 and 600nmole PROP strips yield taste responses that are sufficient for suprathreshold studies on this bitter taste stimulus. Finally, this study underscores the usefulness of edible strips for delivering suprathreshold amounts of primary taste stimulus to the oral cavity. Edible taste strips should continue to be useful for examining taste sensitivity, presentation of hydrophobic stimuli such as capsaicin and capsiate, genetic variation in taste receptors, regional testing on the tongue surface (Smutzer et al. 2012), the role of spatial summation in taste perception, and bitter taste perception in clinical populations (Brewer et al. 2012). This method is an attractive alternative to PROP solutions or the filter paper method for examining taste blindness. Finally, this novel delivery method is a valuable tool for determining the possible role of taste sensitivity on human health, nutrition, and food selection for large population-based studies.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/.
Funding
This work was supported by a contract awarded to Richard C. Gershon from the institutes and centers that form the National Institutes of Health Blueprint for Neuroscience Research (The NIH Toolbox for Neurological and Behavioral Function [contract number HHS-N-260-2006-00007-C]) and National Institutes of Health, National Institute on Deafness and Other Communication Disorders [2R44 DC007291]. Dr. Griffith also wishes to acknowledge the support of the Research Foundation of Flanders [FWO; GP.035.11N].
Supplementary Material
Acknowledgements
The authors thank Judith Stull, Ray Abarintos, Leonard P. Nelson, and Janis Zambrano for their valuable assistance. The authors also thank Danielle Reed and Fujiko Duke for assistance with TAS2R38 genotyping and Dow Chemical Company for the HPMC that was used in developing the taste strips.
References
- Anonymous 1931. Science news: some advances in the sciences during 1931. Science. 74(Suppl):14a [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. 2004. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 82(1):109–114 [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Miller IJ. 1994. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 56(6):1165–1171 [DOI] [PubMed] [Google Scholar]
- Blakeslee AF, Fox AL. 1932. Our different taste worlds: P.T.C. as a demonstration of genetic differences in taste. J Hered. 23:97–107 [Google Scholar]
- Brewer WJ, Lin A, Moberg PJ, Smutzer G, Nelson B, Yung AR, Pantelis C, McGorry PD, Turetsky BI, Wood SJ. 2012. Phenylthiocarbamide (PTC) perception in ultra-high risk for psychosis participants who develop schizophrenia: testing the evidence for an endophenotypic marker. Psychiatry Res. 199(1):8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W. 2005. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 15(4):322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabras T, Melis M, Castagnola M, Padiglia A, Tepper BJ, Messana I, Tomassini Barbarossa I. 2012. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS One. 7(2):e30962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche JF, Buletic Z, Breslin PA. 2001. Relationship of papillae number to bitter intensity of quinine and PROP within and between individuals. Physiol Behav. 74(3):329–337 [DOI] [PubMed] [Google Scholar]
- Desai H, Smutzer G, Coldwell SE, Griffith JW. 2011. Validation of edible taste strips for identifying PROP taste recognition thresholds. Laryngoscope. 121(6):1177–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. 2006. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol Behav. 87(2):304–313 [DOI] [PubMed] [Google Scholar]
- Drayna D. 2005. Human taste genetics. Annu Rev Genomics Hum Genet. 6:217–235 [DOI] [PubMed] [Google Scholar]
- Drayna D, Coon H, Kim UK, Elsner T, Cromer K, Otterud B, Baird L, Peiffer AP, Leppert M. Utah Genetic Reference Project 2003. Genetic analysis of a complex trait in the Utah Genetic Reference Project: a major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome 16p. Hum Genet. 112(5-6):567–572 [DOI] [PubMed] [Google Scholar]
- Duffy VB, Hayes JE, Davidson AC, Kidd JR, Kidd KK, Bartoshuk LM. 2010. Vegetable intake in college-aged adults is explained by oral sensory phenotypes and TAS2R38 genotype. Chemosens Percept. 3(3-4):137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy VB, Peterson JM, Bartoshuk LM. 2004. Associations between taste genetics, oral sensation and alcohol intake. Physiol Behav. 82(2-3):435–445 [DOI] [PubMed] [Google Scholar]
- Ebba S, Abarintos RA, Kim DG, Tiyouh M, Stull JC, Movalia A, Smutzer G. 2012. The examination of fatty acid taste with edible strips. Physiol Behav. 106(5):579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Griffin F. 1963. Quinine dimorphism: a cardinal determinant of taste sensitivity. Nature. 200:343–347 [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D. 2012. Somatosensory factors in taste perception: effects of active tasting and solution temperature. Physiol Behav. 107(4):488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F, Fischer R. 1960. Differential reactivity of saliva from ‘tasters’ and ‘non-tasters’ of 6-n-propylthiouracil. Nature. 187:417–419 [DOI] [PubMed] [Google Scholar]
- Guo SW, Reed DR. 2001. The genetics of phenylthiocarbamide perception. Ann Hum Biol. 28(2):111–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. 2008. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 33(3):255–265 [DOI] [PubMed] [Google Scholar]
- Hayes JE, Keast RS. 2011. Two decades of supertasting: where do we stand? Physiol Behav. 104(5):1072–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KL, Steinmann L, Nurse RJ, Tepper BJ. 2002. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 38(1):3–12 [DOI] [PubMed] [Google Scholar]
- Kim UK, Drayna D. 2005. Genetics of individual differences in bitter taste perception: lessons from the PTC gene. Clin Genet. 67(4):275–280 [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. 2003. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 299(5610):1221–1225 [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Moore PJ, Hesselbrock VM. 1996. No association of PROP taster status and paternal history of alcohol dependence. Alcohol Clin Exp Res. 20(8):1496–1500 [DOI] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Batram C, Meyerhof W. 2010. Oligomerization of TAS2R bitter taste receptors. Chem Senses. 35(5):395–406 [DOI] [PubMed] [Google Scholar]
- Lawless HA. 1980. Comparison of different methods used to assess sensitivity to the taste of phenylthiocarbamide PTC. Chem Senses. 5:247–256 [Google Scholar]
- Lim J, Wood A, Green BG. 2009. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 34(9):739–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Reed DR. 2005. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 115(2):e216–e222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IJ, Jr, Reedy FE., Jr 1990. Variations in human taste bud density and taste intensity perception. Physiol Behav. 47(6):1213–1219 [DOI] [PubMed] [Google Scholar]
- Naim M, Seifert R, Nürnberg B, Grünbaum L, Schultz G. 1994. Some taste substances are direct activators of G-proteins. Biochem J. 297(Pt 3):451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peryam DR, Girardot NF. 1952. Advanced taste-test method. Food Eng. 24:58–61 [Google Scholar]
- Reed DR, Bartoshuk LM, Duffy V, Marino S, Price RA. 1995. Propylthiouracil tasting: determination of underlying threshold distributions using maximum likelihood. Chem Senses. 20(5):529–533 [DOI] [PubMed] [Google Scholar]
- Reed DR, Nanthakumar E, North M, Bell C, Bartoshuk LM, Price RA. 1999. Localization of a gene for bitter-taste perception to human chromosome 5p15. Am J Hum Genet. 64(5):1478–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Zhu G, Breslin PA, Duke FF, Henders AK, Campbell MJ, Montgomery GW, Medland SE, Martin NG, Wright MJ. 2010. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet. 19(21):4278–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell WJB, Wybar KC. 1944. Taste of thiouracil and phenylthiocarbamide. Nature. 154:669 [Google Scholar]
- Rosseel MT, Lefebvre RA. 1990. High-performance liquid chromatographic determination of propylthiouracil in plasma. J Chromatogr. 507:247–251 [DOI] [PubMed] [Google Scholar]
- Sandell MA, Breslin PA. 2006. Variability in a taste-receptor gene determines whether we taste toxins in food. Curr Biol. 16(18):R792–R794 [DOI] [PubMed] [Google Scholar]
- Smutzer G, Lam S, Hastings L, Desai H, Abarintos RA, Sobel M, Sayed N. 2008. A test for measuring gustatory function. Laryngoscope. 118(8):1411–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutzer GS, Abarintos RA, Jimenez JC. 2012. Development of a Regional Taste Test with Edible Circles. XVI International Symposium on Olfaction and Taste. Stockholm, Sweden: Poster #409, 238. [Google Scholar]
- Tepper BJ, Christe nsen CM, Cao J. 2001. Development of brief methods to classify individuals by PROP taster status. Physiol Behav. 73(4):571–577 [DOI] [PubMed] [Google Scholar]
- Tepper BJ, White EA, Koelliker Y, Lanzara C, d’Adamo P, Gasparini P. 2009. Genetic variation in taste sensitivity to 6-n-propylthiouracil and its relationship to taste perception and food selection. Ann NY Acad Sci. 1170:126–139 [DOI] [PubMed] [Google Scholar]
- Toxnet 2006. Toxicology Data Network. National Library of Medicine HSDB Database. Propyl thiouracil Number:3390. Available from: URL http://toxnet.nlm.nih.gov
- Zald DH, Hagen MC, Pardo JV. 2002. Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol. 87(2):1068–1075 [DOI] [PubMed] [Google Scholar]
- Zhao L, Kirkmeyer SV, Tepper BJ. 2003. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 78(4-5):625–633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.