Abstract
The assembly of iron–sulfur (Fe–S) clusters requires dedicated protein factors inside the living cell. Striking similarities between prokaryotic and eukaryotic assembly proteins suggest that plant cells inherited two different pathways through endosymbiosis: the ISC pathway in mitochondria and the SUF pathway in plastids. Fe–S proteins are also found in the cytosol and nucleus, but little is known about how they are assembled in plant cells. Here, we show that neither plastid assembly proteins nor the cytosolic cysteine desulfurase ABA3 are required for the activity of cytosolic aconitase, which depends on a [4Fe–4S] cluster. In contrast, cytosolic aconitase activity depended on the mitochondrial cysteine desulfurase NFS1 and the mitochondrial transporter ATM3. In addition, we were able to complement a yeast mutant in the cytosolic Fe–S cluster assembly pathway, dre2, with the Arabidopsis homologue AtDRE2, but only when expressed together with the diflavin reductase AtTAH18. Spectroscopic characterization showed that purified AtDRE2 could bind up to two Fe–S clusters. Purified AtTAH18 bound one flavin per molecule and was able to accept electrons from NAD(P)H. These results suggest that the proteins involved in cytosolic Fe–S cluster assembly are highly conserved, and that dependence on the mitochondria arose before the second endosymbiosis event leading to plastids.
Keywords: metal cofactor, iron–sulfur, electron paramagnetic resonance, organelle, molybdenum cofactor enzyme
1. Introduction
Iron–sulfur (Fe–S) clusters are cofactors found in a large number of essential proteins [1,2]. Their versatile chemical properties enable enzymes to carry out electron transfer, Lewis acid catalysis, radical chemistry or to provide stability to protein folds. Fe–S-rich sediment may have functioned as a catalytic surface to form the first building blocks of life [3], but when primordial life became dependent on Fe–S chemistry, the catalyst needed to be assembled inside the cell. Whereas simple rhombic and cubane Fe–S clusters can form spontaneously under anaerobic conditions in a test tube, a significant number of dedicated assembly proteins have been uncovered over the past 15 years (reviewed in [4–6]). These are the nif pathway in nitrogen fixing bacteria, which bears similarity to the isc (iron–sulfur cluster) pathway in bacteria and mitochondria. The suf (sulfur mobilization) pathway operates in (cyano)bacteria under oxidative stress and in chloroplasts, while suf BC genes are also commonly found in Archaea. In eukaryotes, the CIA pathway (cytosolic iron–sulfur protein assembly) is involved in the maturation of cytosolic and nuclear Fe–S proteins.
Based on extensive biochemical and cell biological studies, the assembly proteins are needed for controlled delivery of sulfur in the form of persulfide (S0), reduction to sulfide (S2−), pre-assembly together with Fe on a scaffold protein and ATP-dependent transfer of the Fe–S cluster to a target protein. In most bacteria and in organelles, the source of sulfur for Fe–S clusters is cysteine, which is converted to alanine and S0 by a pyridoxal phosphate-dependent cysteine desulfurase. Phylogenetically, the cysteine desulfurases fall into two groups, type I and type II [7]. The mitochondrial cysteine desulfurase NFS1 is type I, whereas the chloroplast CpNifS (also known as SUFS or NFS2) is a type-II enzyme. A third cysteine desulfurase found in the cytosol, named ABA3 for its role in the synthesis of the plant hormone abscisic acid, also belongs to type II. The two types differ in the level of surface exposure of the active site, as well as in the requirement of helper proteins.
While it is clear that the mitochondrial NFS1 and the plastid CpNifS provide sulfur for the assembly of clusters in the mitochondria and plastids, respectively, the source of sulfur for cytosolic/nuclear Fe–S proteins is more enigmatic. In yeast, a small pool of the single NFS1 gene product is localized in the nucleus [8], but does not play a role in Fe–S protein assembly [9]. In human cell lines, a mitochondrial and a cytosolic/nuclear isoform of the NFS1 homologue (called ISCS) have been observed [10,11], but only the mitochondrial form contributes functionally to the assembly of cellular Fe–S proteins [11]. In Arabidopsis, two independent studies found that NFS1::GFP fusion proteins are exclusively targeted to the mitochondria [12,13], although the existence of a minor non-mitochondrial pool can as yet not be ruled out. If the mitochondrial pool of NFS1 provides a sulfur-containing precursor for the CIA pathway, then this needs to be exported to the cytosol via a transporter. A probable candidate is the ABC transporter of the mitochondria, Atm1 in yeast [14,15]. The orthologues in mammals (ABCB7) and plants (ATM3) are also required for the activity of cytosolic Fe–S enzymes [16–18]. However, disruption of Arabidopsis ATM3 is not lethal, although growth is severely compromised [18]. Therefore, in plants the possibility exists that either the plastid CpNifS or the cytosolic ABA3 can provide persulfide to cytosolic Fe–S proteins.
The reduction of S0 to S2−, and possibly the reductive coupling of two rhombic [2Fe–2S] clusters to [4Fe–4S], require electrons. NAD(P)H, ferredoxin reductase and ferredoxin form an electron transfer chain that provides electrons to the mitochondrial ISC pathway in all eukaryotes, including plants [19–21]. In plastids, electrons for the assembly of Fe–S clusters are derived from light or NADPH [22], probably via ferredoxins, but the latter remains to be shown. In the yeast cytosol, the diflavin reductase Tah18 and its partner Dre2 form an electron input module for the CIA pathway [23]. The Tah18 and Dre2 proteins were recently described in Arabidopsis, as ATR3, a homologue of ATR1 and ATR2 (Arabidopsis thaliana P450 reductases), and AtCIAPIN1 (Arabidopsis thaliana homologue of the human cytokine-induced apoptosis inhibitor 1) [24]. In this paper, we use the yeast gene names for the Arabidopsis homologues, with the prefix At for Arabidopsis thaliana. Varadarajan et al. [24] found that AtTAH18 interacted specifically with AtDRE2 identified in a yeast two-hybrid screen. Moreover, cell fractions of yeast or Nicotiana benthamiana overexpressing AtTAH18::GFP were shown to have NADPH-dependent cytochrome c reductase activity. However, the biological role of AtTAH18 and AtDRE2, particularly regarding Fe–S protein biogenesis, was not established, partly owing to lack of viable loss-of-function mutants and incomplete characterization of the yeast proteins at the time.
To investigate the assembly of Fe–S proteins in the plant cytosol, we have addressed the issues of sulfur source and electron input. Arabidopsis mutants in the three cysteine desulfurase genes, encoding the plastid CpNifS, cytosolic ABA3 and mitochondrial NFS1, were analysed for cytosolic Fe–S enzyme activities. Our results show that CpNifS is required for plastid Fe–S enzymes and that ABA3 function is restricted to Molybdenum cofactor (Moco) maturation. For activity of the [4Fe–4S]-dependent enzyme aconitase in the cytosol, NFS1 was required. We also show that AtTAH18 and AtDRE2 could functionally complement the yeast dre2 mutant only when expressed together, indicating that AtDRE2–AtTAH18 complex formation is of functional importance.
2. Methods
(a). Plant materials and growth
T-DNA insertion mutants in DRE2 (SAIL_1222_B12) and NFS1 (SALK_083681 and WiscDsLox445D06) were obtained from the Nottingham Arabidopsis Stock Centre. nfs1-1/nfs1-2 hemizygous knock-down individuals were obtained by crossing the homozygous SALK_083681 line (nfs1-1/nfs1-1) with heterozygous individuals (nfs1-2/NFS1) of the WiscDsLox445D06 line, followed by PCR genotyping of the F1. The other mutant lines were as previously described: CpNifS [25]; nfu2-1 (SALK_039254) [26,27]; the sir3-3 allele of ABA3 [28]; atm3-1 and atm3-4 [18]. Plants were grown on compost in a controlled growth cabinet at 21 °C, 16 h light : 8 h dark, humidity 65 per cent. For 3-day-old seedlings, seeds were germinated in ½ Murashige and Skoog medium.
(b). Yeast strains, growth and plasmids
Saccharomyces cerevisiae W303-1A (MATa, ura3-1, ade2-1, trp1-1, his3-11,15 and leu2-3,112) was used as wild-type (WT) strain. The Gal-DRE2 and Gal-TAH18 strains (carrying the Gal1-10 promoter) in this study were constructed using the plasmid pYM-N23 [29] as the template for PCR amplification of linear DNA for homologous recombination of the WT strain using nourseothricin selection. A strain having both DRE2 and TAH18 genes under the control of the Gal1-10 promoter was constructed from Gal-DRE2 in a similar fashion, but using the pYM-N23 plasmid in which the NAT gene was replaced by the Schizosaccharomyces pombe HIS5 gene. Correct promoter insertion was confirmed by PCR of chromosomal DNA with primers hybridizing to flanking regions and the inserted cassette. The AtTAH18 coding region was previously isolated [24] and the AtDRE2 coding region was PCR amplified from Arabidopsis thaliana cDNA with gene-specific primers. AtTAH18 and AtDRE2 were cloned into p414 and p416 yeast vectors, respectively [30]. For constructs with natural promoters (NPs) of yeast, 450 bp or 518 bp 5’ of the startcodons of DRE2 or TAH18, respectively, were used. All cloned regions in the plasmids were confirmed by sequencing. Yeast methods were according to [23].
(c). Enzyme assays and spectroscopic analysis
In-gel assays for aldehyde oxidases, xanthine dehydrogenase and aconitases were as previously described [18]; isopropylmalate isomerase (Leu1) activity in yeast cell extracts was determined according to [14]. Spectroscopy and kinetic analysis of horse heart cytochrome c reduction by AtTAH18 was performed as described in [23].
(d). Protein expression and purification
AtDRE2 or AtTAH18 were cloned into the SacI and NcoI sites of the pASK-IBA43plus vector, generating a N-terminal 6xHis- and C-terminal Strep-tag in fusion with the encoded protein. Expression of AtDRE2 or AtTAH18 in E. coli HMS174 was started by inoculation of Terrific Broth medium with 1 per cent (v/v) of an overnight culture. For AtDRE2, the culture at 37 °C was shifted to 25 °C at an OD600 nm of 0.5, followed by the addition of 0.2 mg l−1 anhydrotetracylin. Cells were harvested after 3 h. In the case of AtTAH18, growth was at 20 °C, induction was at OD600 nm = 0.3 and the harvest was after 16 h. Extracts were prepared from defrosted cells with an Avestin liquidizer and clarified by centrifugation for 2 h at 100 000 × g (4 °C). Purification according to the manufacturers’ protocols was with IBA Streptactin Agarose superflow (AtDRE2) or with Ni-NTA HP Sepharose with 20 mM imidazol in the lysis/wash buffer (AtTAH18). AtTAH18 was further purified by Superdex 200pg gel filtration in 25 mM Tris–HCl pH 8.0, 300 mM NaCl buffer using an Äkta system.
3. Results
(a). Plant mitochondria, but not plastids, are required for cytosolic Fe–S enzyme activities
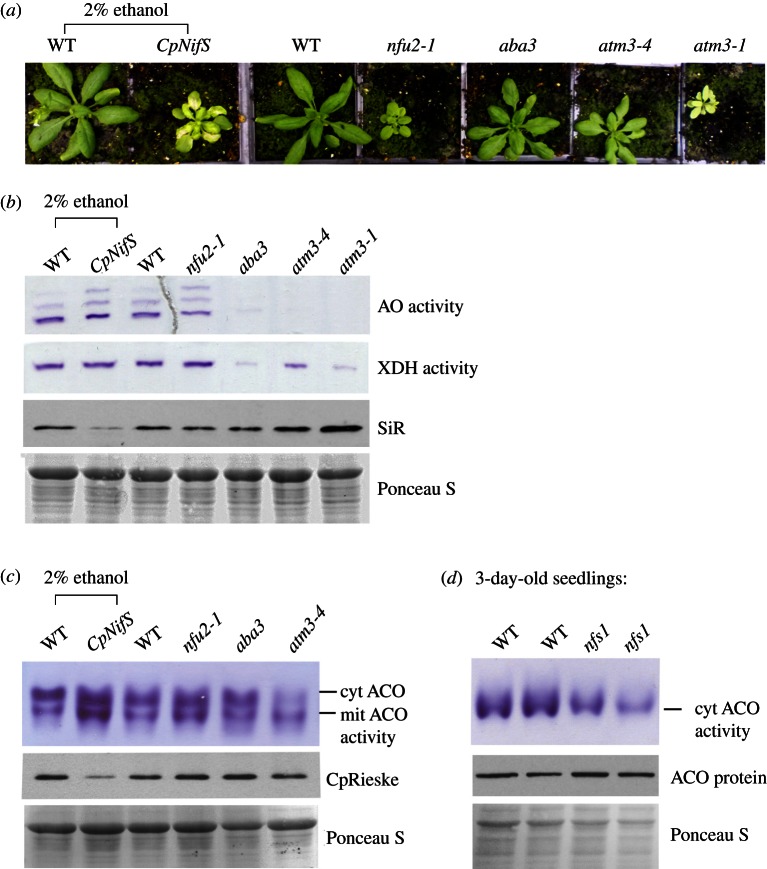
Because plant cells have two functional pathways for Fe–S cluster assembly, ISC in mitochondria and SUF in plastids, each could potentially contribute to the assembly of Fe–S clusters in the cytosol. We have previously shown that the mitochondrial cysteine desulfurase NFS1 is required for activity of cytosolic aldehyde oxidases (AO), which bind two [2Fe–2S] clusters, FAD and Moco [13]. In addition, we have demonstrated that the mitochondrial ABC transporter ATM3 is required for the activities of at least three cytosolic Fe–S enzymes, AO, the structurally related xanthine dehydrogenase (XDH) and the [4Fe–4S] enzyme aconitase (ACO) [18]. To investigate whether the plastid Fe–S assembly pathway also contributes to cytosolic Fe–S cluster assembly, we analysed the activities of cytosolic Fe–S enzymes in two mutants of plastid assembly proteins. Knockout of the cysteine desulfurase CpNifS is lethal, whereas silencing of its expression leads to decreased activity and/or protein stability of all resident plastid Fe–S proteins tested [25]. In contrast, plants lacking the Fe–S scaffold protein NFU2 are viable, but have severely decreased levels of Photosystem I and ferredoxin, while plastid Fe–S proteins such as sulfite reductase (SiR) and nitrite reductase were less affected, and glutamate synthase and Rieske protein not at all [26,27]. Plants expressing an ethanol-inducible RNAi fragment complementary to CpNifS were grown for two weeks and treated with 2 per cent (v/v) ethanol to downregulate CpNifS expression, as previously described [25]. After two weeks, the plants were dwarfed and chlorotic compared with normal growth of WT plants that had undergone the same treatment (figure 1a). In CpNiFS silenced leaves, the levels of SiR and the Fe–S subunit of cytochrome b6f complex (CpRieske) were decreased, but activities of cytosolic AO, XDH and ACO were normal (figure 1b,c). nfu2-1 mutant plants also had normal activities of cytosolic Fe–S enzymes. For comparison, AO, XDH and cytosolic ACO activities were very low or undetectable in plants homozygous for either a weak or strong allele of atm3. Despite the chlorotic appearance and severe growth phenotype of atm3-1 mutant plants, the SiR protein was stable.
Figure 1.
Fe–S enzyme activities in Arabidopsis mutants in Fe–S cluster and Moco assembly genes. (a) Growth phenotypes of wild-type (WT) and mutants in the following genes: the plastid cysteine desulfurase CpNifS, downregulated by expression of an ethanol-inducible RNAi; the plastid Fe–S cluster scaffold NFU2; the Moco sulfurase ABA3 (sir3-3 allele); the mitochondrial ABC transporter ATM3, of which a weak allele (atm3-4) and strong allele (atm3-1) are shown. (b) Leaf extracts from 4-week-old plants of WT and the indicated Arabidopsis mutants were separated by native gel electrophoresis and stained for activity of aldehyde oxidases (AO) and xanthine dehydrogenase (XDH). Protein samples of the same extracts (20 μg) were separated by SDS–PAGE, blotted and labelled with antibodies against sulfite reductase (SiR). The blots were stained with Ponceau S to confirm equal protein loading. (c) Leaf extracts from four-week-old WT and mutant plants were separated on native starch-poly acrylamide gels and stained for aconitase activities (ACO) localized in the cytosol (cyt) or mitochondria (mit). Aliquots of 20 μg protein were also separated on denaturing gels, blotted and immuno-labelled for the Rieske Fe–S subunit of cytochrome b6f complex (CpRieske). Ponceau S staining confirmed equal protein loading. (d) Total extracts of 3-day-old seedlings from WT and nfs1 (a mutant in the mitochondrial cysteine desulfurase NFS1) were separated by native gel electrophoresis and stained for aconitase activity. At this developmental stage, only one dominant, cytosolic aconitase isoform is expressed; see the electronic supplementary material, figure S1. Protein of the same extracts (20 μg) were separated by SDS–PAGE, blotted and labelled with antibodies against aconitase, after staining with Ponceau S to confirm equal protein loading.
In addition to the mitochondrial and plastid cysteine desulfurases, plants also contain a cysteine desulfurase in the cytosol, ABA3. The ABA3 protein consists of two domains: an N-terminal cysteine desulfurase domain which transfers a sulfur to Moco bound to the C-terminal domain, prior to transfer and insertion of sulfurated Moco into AO and XDH [31]. Using the same in-gel activity assays, we found that the sir3-3 allele of ABA3 has strongly decreased activities of AO and XDH, but that the cytosolic ACO activity is unaffected (figure 1b,c). These results are in agreement with previous in vitro data suggesting that activity of ABA3 is specific for Moco sulfuration [31,32]. Moreover, the phenotypic features of aba3 mutants, which include a darker green leaf colour and wilty leaves, are very different from atm3 mutants [18,33], also suggesting that ABA3 is not involved in cytosolic Fe–S cluster assembly.
To confirm that the mitochondrial NFS1 is not only required for Fe–S/Moco enzymes like AO [13], but also for cytosolic Fe–S enzymes, we analysed ACO activity in an nfs1 mutant. A previously reported line in which NFS1 was downregulated by transgenic expression of the coding sequence in antisense direction was genetically unstable, whereas T-DNA insertions in the NFS1 coding sequence cause embryo lethality [13]. Therefore, to obtain a viable mutant with consistently decreased NFS1 expression levels, we isolated a mutant with a T-DNA insertion in the 5’ untranslated region of NFS1 (nfs1-1, SALK_083681). Although transcript levels of NFS1 were decreased by approximately 50 per cent in homozygous plants, they had no obvious visual or biochemical phenotypes (data not shown). However, in combination with a knock-out allele (nfs1-2), the hemizygous knock-down seedlings showed delayed germination. During germination of WT Arabidopsis, high activity of cytosolic aconitase (see the electronic supplementary material, figure S1) supports a modified glyoxylate cycle involved in mobilization of storage lipids (Hooks & Balk, manuscript in preparation). In 3-day-old nfs1-1/nfs1-2 seedlings, however, aconitase activity is strongly decreased compared with WT, while the level of aconitase protein is unaffected (figure 1d). These results indicate a deficiency in Fe–S cofactor assembly on cytosolic aconitase and suggest that the mitochondrial cysteine desulfurase is the source of sulfur for cytosolic Fe–S proteins in plants. Moreover, our data show that neither the plastid Fe–S cluster assembly machinery, nor the cytosolic cysteine desulfurase ABA3 play a role in the maturation of Fe–S proteins in the plant cytosol.
(b). The Arabidopsis homologue of DRE2 is essential for embryo development
To investigate proteins downstream of NFS1 and ATM3 that could be involved in the assembly of Fe–S clusters in the plant cytosol, we have previously annotated Arabidopsis and Chlamydomonas homologues of the yeast CIA genes [3,34]. All CIA genes were easily identified, except for CFD1, which is absent in the green lineage [35]. AtDRE2, AtTAH18, AtNBP35, AtNAR1, AtCIA1 and AtMET18 occur as single genes, whereas three genes with homology to CIA2 are found in Arabidopsis (table 1). T-DNA insertion mutants are available for most genes. It has been shown that AtTAH18, AtNBP35, AtNAR1, AtCIA1 and one of the AtCIA2 genes (named AE7) are each essential for embryo development (see table 1 and references). In contrast, Arabidopsis met18 knock-out mutants have no obvious phenotype [37].
Table 1.
Arabidopsis genes of the CIA pathway and their mutant phenotypes.
| locus | gene name | yeast respectively human homologues | mutant allele(s) | phenotype | references |
|---|---|---|---|---|---|
| At5g18400 | DRE2, CIAPIN1 | DRE2, CIAPIN1/anamorsin | T-DNA insertion | embryo lethal | this study |
| At3g02280 | TAH18 , ATR3 | TAH18, NDOR1 | T-DNA insertion | embryo lethal | [24] |
| At5g50960 | NBP35 | NBP35, NUBP2 | T-DNA insertion | embryo lethal | [35,36] |
| At4g16440 | NAR1 | NAR1, IOP1/NARF | T-DNA insertion | ovule abortion or early embryo lethal | [37,38] |
| At2g26060 | CIA1 | CIA1, CIAO1 | T-DNA insertion | embryo lethal | [37] |
| At1g68310 | AE7 | CIA2, MIP18 | T-DNA insertion | embryo lethal | [37] |
| E51K | small, chlorotic, altered leaf architecture; increased DNA damage; aconitase activity decreased, but not aldehyde oxidase | [37,39] | |||
| At3g50845 At3g09380 | AE7-like1(AEL1) AE7-like2(AEL2) | — | none available | N/A | [37] |
| At5g48120 | MET18 | MET18, MMS19 | T-DNA insertion | no phenotype | [37] |
Since the phenotype of atdre2 mutants has not been described, we screened the stock centres for T-DNA insertion mutants. Several lines were obtained, but only one line in the SAIL collection [40] displayed a phenotype. The T-DNA is inserted in the fifth exon, which is likely to cause complete disruption of expression. No homozygous mutant plants were found among the offspring of heterozygous parents. Further inspection of the seed pods revealed inviable seeds among healthy-looking seeds (see the electronic supplementary material, figure S2), at a ratio of 75 : 190 (n = 6 siliques), or approximately 1 : 3 (χ2 = 1.634; χ2.05 = 3.841). Based on the small size of aborted seeds, embryo development is arrested at an early stage, similar to mutants in AtTAH18, AtNBP35, AE7, AtCIA1 and AtNAR1 [37]. Evidently, the CIA genes perform a fundamental function in plants.
(c). AtDRE2 and AtTAH18 fully complement a yeast dre2 mutant when expressed together
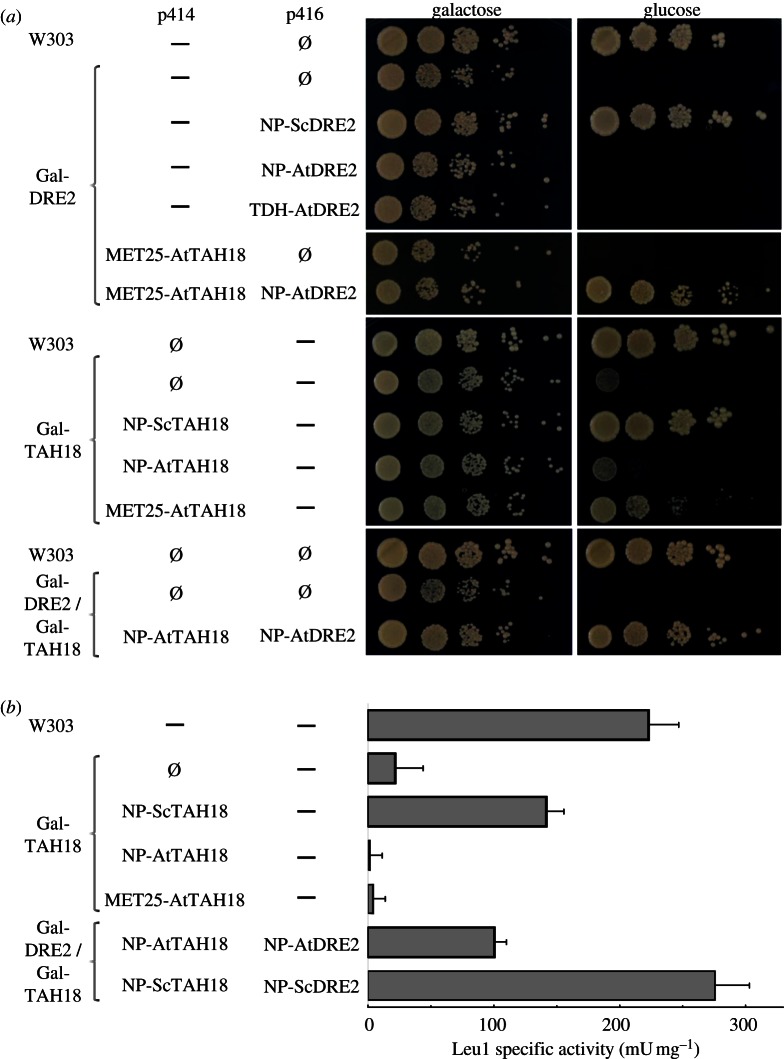
To further investigate the function of AtDRE2, the Arabidopsis gene was cloned and expressed from a plasmid in the yeast Gal1-10-DRE2 regulatable mutant (abbreviated Gal-DRE2). Upon downregulation of DRE2 by changing the carbon source to glucose, the Gal-DRE2 cells stopped growing (figure 2a). While constitutive expression of ScDRE2 was able to rescue growth, expression of AtDRE2 from either the natural yeast DRE2 promoter (NP) or the strong TDH promoter did not rescue.
Figure 2.
Arabidopsis TAH18 and DRE2 complement yeast dre2 when expressed together. (a) The indicated yeast strains were transformed with the listed plasmids. After growth for 40 h on liquid glucose-containing minimal medium, successive 10-fold dilutions of a cell suspension with OD600 nm = 0.5 were spotted on agar plates. Photographs were taken after 40 h of growth at 30 °C. (b) Isopropylmalate isomerase (Leu1) specific activities of cell extracts of yeast strains as in (a). Extracts were prepared from cells grown for 40 h on liquid glucose-containing minimal medium. —, no plasmid; Ø, empty plasmid.
We also investigated whether AtTAH18 could complement a yeast Gal1-10-TAH18 mutant (abbreviated Gal-TAH18). Upon downregulation of the yeast TAH18 gene, constitutive expression of the Arabidopsis homologue could only partially rescue growth when the expression level was increased using the MET25 promoter (figure 2a). Because Dre2 and Tah18 proteins in yeast, human and Arabidopsis are known to form a protein–protein interaction [23,24,41], we tested whether co-expression of AtTAH18 and AtDRE2 could complement dre2 yeast. When expressed from either the NP or MET25 promoter for AtTAH18, the combined expression of the plant genes almost fully complemented growth of a Gal-DRE2 single- and Gal-TAH18/Gal-DRE2 double mutant.
To confirm that yeast growth in the complemented strains was owing to restoration of Fe–S protein biogenesis, the activity of the cytosolic Fe–S enzyme isopropylmalate isomerase (Leu1 protein) was measured. Co-expression of Arabidopsis TAH18 and DRE2 did restore approximately 30 per cent of the Leu1 activity measured in control cells (figure 2b). These data indicate that the AtTAH18 and AtDRE2 proteins form a functional unit that can serve the same function as the respective yeast homologues.
(d). AtTAH18 is a flavoprotein and AtDRE2 binds Fe–S clusters
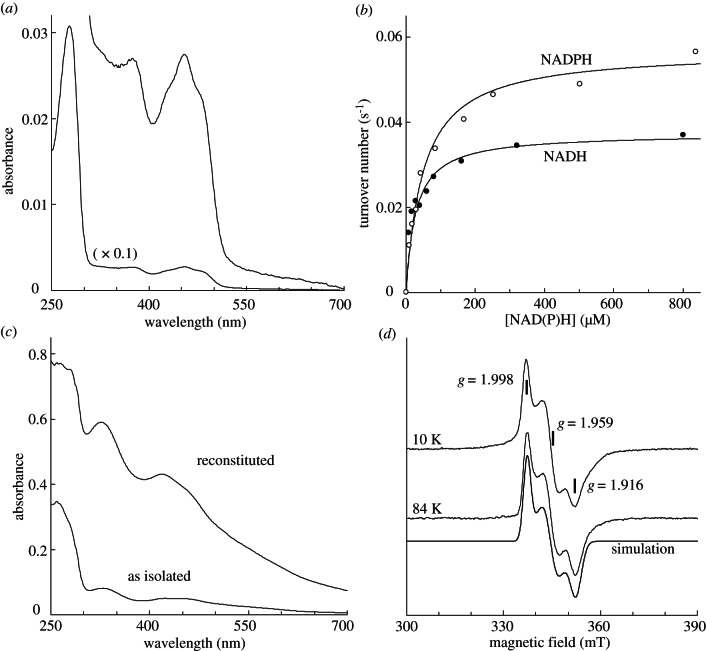
To characterize the cofactors and activities of AtTAH18 and AtDRE2, we expressed these proteins in E. coli. Upon expression at 30 °C the majority of AtTAH18 was found in inclusion bodies. Induction at 20 °C resulted in soluble full-length AtTAH18, which could be purified by Ni-NTA affinity chromatography and gel filtration (0.1 mg g−1 E. coli cells). The yellow monomeric protein contained approximately 1 flavin per molecule, as calculated from the characteristic absorbance maxima at 365 and 450 nm (figure 3a). Electron transfer from NADPH and NADH (0.074 and 0.046 s−1, respectively) to the commonly used electron acceptor cytochrome c (see figure 3b and electronic supplementary material, figure S3) occurred at a rate typical for other diflavin reductases from the same family [23,42]. Km values for cytochrome c, NADPH and NADH were 10, 50 and 28 µM, respectively. The specificity constants (Vmax/Km) are thus almost identical, indicating that in vivo both reduced coenzymes can act as an electron donor.
Figure 3.
Cofactors and activity of AtTAH18 and AtDRE2. (a) UV-visible absorbance spectrum of purified AtTAH18 (3 µM) in 25 mM Tris–HCl, pH 8.0, 150 mM NaCl. (b) Electron transfer from NADPH to horse heart cytochrome c (41 µM) by purified AtTAH18. Best fits of experimental points to the Michaelis Menten equation (solid lines) are for a turnover number of 0.074 or 0.046 s−1 (at infinite cytochrome c concentration) and Km of 50 μM or 28 µM for NADPH and NADH, respectively. Buffer as in (a). (c) UV-visible absorbance spectra of 30 µM purified AtDRE2 before (as isolated) and after chemical reconstitution and desalting (reconstituted). Buffer as in (a). (d) X band EPR spectra of purified (as isolated) AtDRE2 (100 μM) reduced with 2 mM sodium dithionite in buffer. EPR conditions: microwave frequency, 9.458 GHz; microwave power, 1.3 mW (10 K) or 20 mW (84 K); modulation amplitude, 1.25 mT; modulation frequency, 100 kHz. Simulation parameters for the EPR signal: gz, gy and gx; 1.998, 1.959 and 1.916 with line widths of 2.3, 4.1 and 3.4 mT, respectively.
AtDRE2 could be obtained in moderate yield following purification on a Strep-tactin column (0.3 mg g−1 E. coli cells). The red-brownish protein had a UV-visible absorbance spectrum with maxima at 315 and 420 nm, suggesting it binds a [2Fe–2S] centre (figure 3c). Reduction of the protein sample revealed an electron paramagnetic resonance (EPR) spectrum characteristic of a rhombic [2Fe–2S]1+ cluster, which was detectable up to 84 K (figure 3d). The g values as determined by simulation of the EPR spectrum (1.998, 1.959 and 1.916) are similar to those reported for the purified yeast Dre2 protein [43,44]. Since chemical analysis detected approximately 1.3 Fe and S per polypeptide, we anaerobically reconstituted AtDRE2. As seen in figure 3c, the amount of visible Fe–S chromophores bound to AtDRE2 increased approximately sevenfold. The intensity of the contribution of the [2Fe–2S]2+ cluster increased and was accompanied by a general increase of less-structured absorbance in the 400–700 nm region. These results indicate that a [4Fe–4S] cluster is present in the holo-form in addition to the [2Fe–2S] cluster.
4. Discussion
The cells of plants and green algae have both mitochondria and plastids, each with an evolutionarily distinct pathway for the assembly of Fe–S clusters, termed ISC and SUF, respectively [6]. Previous reports showed that the pathways act independently: downregulation of the plastid cysteine desulfurase CpNifS did not impact on mitochondrial respiratory function [25]. Mutants in the mitochondrial ABC transporter ATM3 affected cytosolic Fe–S enzymes, but not plastid Fe–S proteins such as Photosystem I, nitrite reductase and chlorophyllide a oxygenase [18]. Here, we extend these observations to include evidence that the cytosolic Fe–S enzymes depend on the mitochondrial assembly pathway, but not the plastid assembly machinery. The findings suggest that the dependence of the cytosolic Fe–S clusters on the mitochondria was established before the second endosymbiosis event leading to plastids, and that this situation was maintained after the introduction of the SUF Fe–S cluster assembly pathway into the cell.
Interestingly, from yeast to human cells to plants, the assembly of cytosolic/nuclear Fe–S proteins depends on a cysteine desulfurase that is localized in the mitochondria. Are there constraints to re-localize certain steps of the cluster assembly process, or is there a specific, biochemical reason for NFS1 to be in the mitochondria? Examples of assembly proteins in unexpected locations have been reported. The Arabidopsis SUFE protein fused to GFP localized to both plastids and mitochondria [45]. The unicellular eukaryote Blastocystis, which has rudimentary mitochondria and no plastids, appears to have acquired a functional SufC–SufB fusion protein by lateral gene transfer. The SufCB protein is localized in the cytosol, alongside putative CIA proteins [46]. Also, as mentioned earlier, the mitochondrial cysteine desulfurase is found in the cytosol/nucleus of yeast and human cells, although this pool does not serve extra-mitochondrial Fe–S protein biogenesis. So given that targeting of a protein is subject to evolutionary change, there must be another reason why the source of sulfide for cytosolic Fe–S clusters is restricted to the mitochondrial matrix. It has been suggested that the ISC pathway is oxygen sensitive, and can only function in the matrix because of lower oxygen partial pressure in this compartment.
The biochemical characterization of Arabidopsis TAH18 and DRE2 reported here, together with other recent studies of plant homologues of the yeast CIA proteins (table 1), allow a first draft of the cytosolic Fe–S cluster assembly pathway in plants (figure 4) as well as comparison with other eukaryotes. Successful complementation of the yeast dre2 mutant with the Arabidopsis TAH18-DRE2 module and characterization of their redox-active cofactors suggest that these proteins also act as an electron transfer chain in the plant CIA machinery. Based on 55Fe incorporation studies in yeast, TAH18-DRE2 act early in the pathway before NBP35, whereas the CIA targeting complex acts late. In plants, NBP35 forms a dimer carrying a stable N-terminal cluster, and a labile C-terminal cluster that spans the two protomers [35,36]. Because of its labile nature, the C-terminal cluster is probably the cluster that is transferred to target apoproteins. The presence of a nucleotide binding domain indicates that NBP35 function needs ATP or GTP, most probably for loading of the Fe–S cluster on the scaffold, as shown in yeast [47]. In fungi and metazoa NBP35 forms a hetero-tetramer with Cfd1/NUBP1. Cfd1 has sequence similarity to the C-terminus of Nbp35, including the nucleotide binding domain and the CxxC motif binding a labile Fe–S cluster. As mentioned before, Cfd1 is absent in the green lineage [35].
Figure 4.
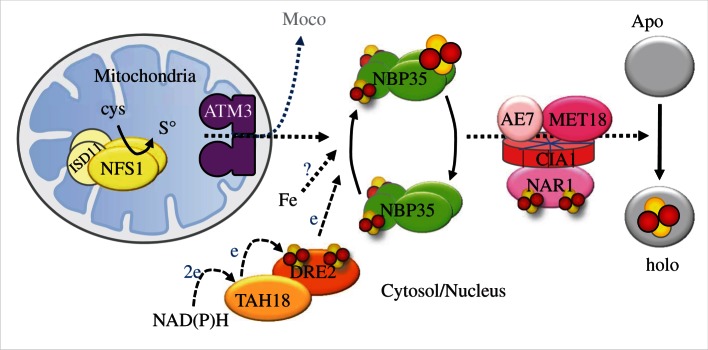
Diagram summarizing proteins required for the assembly of cytosolic and nuclear Fe–S protein in plants. The mitochondrial cysteine desulfurase NFS1 and its partner protein ISD11 are providing sulfur, which is exported from the mitochondria in an as yet unknown form. The requirement of other mitochondrial ISC proteins remains to be demonstrated in plants. The ABC transporter ATM3 is likely to be involved in transport of the sulfur compound, and also supports the biosynthesis of molybdenum cofactor (Moco). Fe–S cofactors are depicted by a cluster of two yellow and two red balls. Labile clusters that are thought to be transferred during the assembly process are enlarged relative to the stable/permanent clusters. Solid arrows indicate steps for which there is experimental evidence; broken arrows with e indicate electron transfer; dotted arrows indicate steps in the process for which there is as yet little or no experimental evidence. Protein interactions are based on Refs. [24,37]. There is no CFD1 homologue in the green lineage.
The CIA targeting complex consists of four proteins: NAR1 has intriguing similarity to [Fe–Fe] hydrogenases; the CIA1 protein is thought to act as a protein binding platform of the complex; the AE7 protein has an hyper-reactive thiol; and the MET18 protein belongs to the HEAT repeat proteins (figure 4). Although the precise molecular functions of these proteins remain to be determined, the formation of the complex and its biological function is conserved from yeast to humans and plants [37,48,49]. A universal phenotype of cia mutants is the loss of nuclear genome integrity. Interestingly, early observations in Arabidopsis already pointed to altered nuclear morphology and increased expression of DNA repair proteins in an atm3 mutant [12]. Also, AtTAH18 was suggested to have a role in DNA replication [24]. These observations are now understood because replicative DNA polymerases have been shown to require an Fe–S cluster [50]. However, differences between eukaryote species have also been observed. For example, the Arabidopsis met18 mutant has no obvious phenotype, although in combination with a single allele of a partial ae7 mutant, the phenotype is strongly enhanced compared with the ae7 homozygote. In contrast, met18 mutants in yeast do have a slow-growth phenotype and methionine auxotrophy, whereas mouse embryos lacking the MET18 homologue MMS19 are embryo lethal [36,47].
Key questions that remain with respect to the assembly of cytosolic/nuclear Fe–S clusters are (i) what is the sulfur-containing substrate of the mitochondrial ABC transporters? (ii) What is the source of Fe and how is it delivered to NBP35? While fast progress has been made in yeast, studies in other eukaryotes are also providing new information and different perspectives.
Acknowledgements
We would like to thank our colleagues who donated Arabidopsis mutant lines: M. Pilon for CpNifS; S. Lobréaux for nfu2-1; Y. Zhao for the sir3-3 allele of ABA3 and atm3-4, and V. Hinoux (née Delorme) and R. C. Coolbaugh for the AtTAH18 coding sequence; antibodies against SiR were kindly provided by R. Hell, and antibodies against CpRieske by J. C. Gray. We would also like to thank J. K. D. Harrison for laboratory assistance. The research was supported by a Marie Curie Intra-European Fellowship to D.B. and a Royal Society Fellowship to J. B. We are most grateful to R. Lill for supporting the work of D.J.A.N., T. J. L. and A.J.P.
Glossary
| ABA3 | cysteine desulfurase enzyme encoded by the ABA3 gene (ABSCISIC ACID3). The enzyme is required for sulfuration of Moco in aldehyde oxidases and xanthine dehydrogenases. Aldehyde oxidase 3 carries out the final step in the biosynthesis of abscisic acid, an important plant hormone. |
| ABC | ATP-binding cassette |
| ABCB7 | ATP-binding cassette transporter, B group, number 7 |
| ACO | aconitase |
| AO | aldehyde oxidase |
| At | as prefix to gene and protein names, meaning Arabidopsis thaliana |
| Atm1 | ATP-binding cassette (ABC) transporter of the mitochondria in Saccharomyces cerevisiae |
| ATM3 | ATP-binding cassette (ABC) transporter of the mitochondria in Arabidopsis thaliana |
| ATR1, ATR2 or ATR3 | Arabidopsis thaliana P450 reductase |
| CFD1 | cytosolic Fe–S cluster deficient |
| CIA genes | genes identified based on their requirement for the assembly of Fe–S clusters on cytosolic and nuclear proteins, but not on mitochondrial Fe–S proteins |
| CIA pathway | cytosolic iron–sulfur protein assembly pathway |
| CIAPIN1 | cytokine-induced apoptosis inhibitor 1; human homologue of DRE2 |
| CpNifS | chloroplast NifS-like (nitrogenase fixation S-like) protein, the cysteine desulfurase enzyme localized in chloroplasts |
| CxxC | cysteine-x-x-cysteine amino acid sequence, with x denoting any amino acid |
| DRE2 | Fe–S binding protein encoded by the DRE2 gene, derepressed for ribosomal protein S14 expression |
| EPR | electron paramagnetic resonance |
| Fe–S | iron–sulfur |
| Gal | galactose; in this text used as an abbreviation for galactose-inducible promoter |
| GFP | green fluorescent protein |
| HEAT repeat | tandemly repeated, 37–47 amino acid long-module occurring in a number of cytoplasmic proteins, including the four name-giving proteins huntingtin, elongation factor 3, alpha regulatory subunit of protein phosphatase 2A and TOR1 |
| isc gene cluster | cluster of seven genes in bacteria required for iron–sulfur cluster assembly |
| ISC pathway | iron–sulfur cluster (assembly) pathway |
| ISCS | human homologue of the bacterial IscS protein, a type-I cysteine desulfurase |
| MET25 | yeast gene involved in methionine synthesis. The promoter is used in this study to drive constitutive expression of transgenes |
| Moco | molybdenum cofactor |
| NFS1 | NifS-like gene encoding a cysteine desulfurase |
| NFU2 | NifU-like protein 2 |
| NP | natural promoter |
| Sc | as prefix to gene and protein names, meaning Saccharomyces cerevisiae |
| SiR | sulfite reductase |
| sir3-3 | sirtinol resistant allele 3–3 (not to be confused with sulfite reductase!) |
| suf operon | bacterial operon of six genes involved in sulfur mobilization for the biosynthesis of Fe–S clusters under oxidative stress and iron limitation. |
| SUF pathway | sulfur mobilization pathway |
| TAH18 | gene that was first identified in a mutant screen for Top1T722A Hypersensitivity. |
| TDH | yeast gene encoding a Triose-phosphate DeHydrogenase. The promoter is used in this study to drive the expression of transgenes at very high levels. |
| T-DNA | transfer DNA; large segment of DNA transferred by Agrobacterium into the plant genome |
| WT | wild-type |
| XDH | xanthine dehydrogenase |
References
- 1.Beinert H. 2000. Iron–sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5, 2–15 10.1007/s007750050002 (doi:10.1007/s007750050002) [DOI] [PubMed] [Google Scholar]
- 2.Imsande J. 1999. Iron–sulfur clusters: formation, perturbation, and physiological functions. Plant Physiol. Biochem. 37, 87–97 10.1016/S0981-9428(99)80070-9 (doi:10.1016/S0981-9428(99)80070-9) [DOI] [Google Scholar]
- 3.Martin W, Russell MJ. 2003. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil. Trans. R. Soc. Lond. B 358, 59–85 10.1098/rstb.2002.1183 (doi:10.1098/rstb.2002.1183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Py B, Barras F. 2010. Building Fe–S proteins: bacterial strategies. Nat. Rev. Microbiol. 8, 436–446 10.1038/nrmicro2356 (doi:10.1038/nrmicro2356) [DOI] [PubMed] [Google Scholar]
- 5.Lill R. 2009. Function and biogenesis of iron–sulphur proteins. Nature 460, 831–838 10.1038/nature08301 (doi:10.1038/nature08301) [DOI] [PubMed] [Google Scholar]
- 6.Balk J, Pilon M. 2011. Ancient and essential: the assembly of iron–sulfur clusters in plants. Trends Plant Sci. 16, 218–226 10.1016/j.tplants.2010.12.006 (doi:10.1016/j.tplants.2010.12.006) [DOI] [PubMed] [Google Scholar]
- 7.Mihara H, Esaki N. 2002. Bacterial cysteine desulfurases: their function and mechanisms. Appl. Microbiol. Biotechnol. 60, 12–23 10.1007/s00253-002-1107-4 (doi:10.1007/s00253-002-1107-4) [DOI] [PubMed] [Google Scholar]
- 8.Nakai Y, Nakai M, Hayashi H, Kagamiyama H. 2001. Nuclear localization of yeast Nfs1p is required for cell survival. J. Biol. Chem. 276, 8314–8320 10.1074/jbc.M007878200 (doi:10.1074/jbc.M007878200) [DOI] [PubMed] [Google Scholar]
- 9.Mühlenhoff U, Balk J, Richhardt N, Kaiser JT, Sipos K, Kispal G, Lill R. 2004. Functional characterization of the eukaryotic cysteine desulfurase Nfs1p from Saccharomyces cerevisiae. J. Biol. Chem. 279, 36 906–36 915 10.1074/jbc.M406516200 (doi:10.1074/jbc.M406516200) [DOI] [PubMed] [Google Scholar]
- 10.Tong WH, Rouault T. 2000. Distinct iron–sulfur cluster assembly complexes exist in the cytosol and mitochondria of human cells. EMBO J. 19, 5692–5700 10.1093/emboj/19.21.5692 (doi:10.1093/emboj/19.21.5692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biederbick A, Stehling O, Rosser R, Niggemeyer B, Nakai Y, Elsasser HP, Lill R. 2006. Role of human mitochondrial Nfs1 in cytosolic iron–sulfur protein biogenesis and iron regulation. Mol. Cell Biol. 26, 5675–5687 10.1128/MCB.00112-06 (doi:10.1128/MCB.00112-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushnir S, et al. 2001. A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 13, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazzon AP, Ramirez MV, Warek U, Balk J, Frazzon J, Dean DR, Winkel BS. 2007. Functional analysis of Arabidopsis genes involved in mitochondrial iron–sulfur cluster assembly. Plant Mol. Biol. 64, 225–240 10.1007/s11103-007-9147-x (doi:10.1007/s11103-007-9147-x) [DOI] [PubMed] [Google Scholar]
- 14.Kispal G, Csere P, Prohl C, Lill R. 1999. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 18, 3981–3989 10.1093/emboj/18.14.3981 (doi:10.1093/emboj/18.14.3981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhnke G, Neumann K, Mühlenhoff U, Lill R. 2006. Stimulation of the ATPase activity of the yeast mitochondrial ABC transporter Atm1p by thiol compounds. Mol. Membr. Biol. 23, 173–184 10.1080/09687860500473630 (doi:10.1080/09687860500473630) [DOI] [PubMed] [Google Scholar]
- 16.Pondarré C, et al. 2006. The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron–sulfur cluster biogenesis. Hum. Mol. Genet. 15, 953–964 10.1093/hmg/ddl012 (doi:10.1093/hmg/ddl012) [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Sánchez-Fernández R, Lyver ER, Dancis A, Rea PA. 2007. Functional characterization of AtATM1, AtATM2, and AtATM3, a subfamily of Arabidopsis half-molecule ATP-binding cassette transporters implicated in iron homeostasis. J. Biol. Chem. 282, 21 561–21 571 10.1074/jbc.M702383200 (doi:10.1074/jbc.M702383200) [DOI] [PubMed] [Google Scholar]
- 18.Bernard DG, Cheng Y, Zhao Y, Balk J. 2009. An allelic mutant series of ATM3 reveals its key role in the biogenesis of cytosolic iron–sulfur proteins in Arabidopsis. Plant Physiol. 151, 590–602 10.1104/pp.109.143651 (doi:10.1104/pp.109.143651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange H, Kaut A, Kispal G, Lill R. 2000. A mitochondrial ferredoxin is essential for biogenesis of cellular iron–sulfur proteins. Proc. Natl Acad. Sci. USA 97, 1050–1055 10.1073/pnas.97.3.1050 (doi:10.1073/pnas.97.3.1050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheftel AD, et al. 2010. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl Acad. Sci. USA 107, 11 775–11 780 10.1073/pnas.1004250107 (doi:10.1073/pnas.1004250107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picciocchi A, Douce R, Alban C. 2003. The plant biotin synthase reaction. Identification and characterization of essential mitochondrial accessory protein components. J. Biol. Chem. 278, 24 966–24 975 10.1074/jbc.M302154200 (doi:10.1074/jbc.M302154200) [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, Mitsui A, Matsubara H. 1991. Formation of the Fe–S cluster of ferredoxin in lysed spinach chloroplasts. Plant Physiol. 95, 97–103 10.1104/pp.95.1.97 (doi:10.1104/pp.95.1.97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netz DJ, Stümpfig M, Doré C, Mühlenhoff U, Pierik AJ, Lill R. 2010. Tah18 transfers electrons to Dre2 in cytosolic iron–sulfur protein biogenesis. Nat. Chem. Biol. 6, 758–765 10.1038/nchembio.432 (doi:10.1038/nchembio.432) [DOI] [PubMed] [Google Scholar]
- 24.Varadarajan J, Guilleminot J, Dupas CSJ, Piegu B, Chaboute M-E, Gomord V, Coolbaugh RC, Devic M, Delmore V. 2010. ATR3 encodes a diflavin reductase essential for Arabidopsis embryo development. New Phytol. 187, 67–82 10.1111/j.1469-8137.2010.03254.x (doi:10.1111/j.1469-8137.2010.03254.x) [DOI] [PubMed] [Google Scholar]
- 25.Van Hoewyk D, Abdel-Ghany SE, Cohu CM, Herbert SK, Kugrens P, Pilon M, Pilon-Smits EA. 2007. The Arabidopsis cysteine desulfurase CpNifS is essential for maturation of iron–sulfur cluster proteins, photosynthesis, and chloroplast development. Proc. Natl Acad. Sci. USA 104, 5686–5691 10.1073/pnas.0700774104 (doi:10.1073/pnas.0700774104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Touraine B, Boutin J, Marion-Poll A, Briat JF, Peltier G, Lobréaux S. 2004. Nfu2: a scaffold protein required for [4Fe–4S] and ferredoxin iron–sulfur cluster assembly in Arabidopsis chloroplasts. Plant J. 40, 101–111 10.1111/j.1365-313X.2004.02189.x (doi:10.1111/j.1365-313X.2004.02189.x) [DOI] [PubMed] [Google Scholar]
- 27.Yabe T, Morimoto K, Kikuchi S, Nishio K, Terashima I, Nakai M. 2004. The Arabidopsis chloroplastic NifU-like protein CnfU, which can act as an iron–sulfur cluster scaffold protein, is required for biogenesis of ferredoxin and photosystem I. Plant Cell 16, 993–1007 10.1105/tpc.020511 (doi:10.1105/tpc.020511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai X, Hayashi K, Nozaki H, Cheng Y, Zhao Y. 2005. Genetic and chemical analyses of the action mechanisms of sirtinol in Arabidopsis. Proc. Natl Acad. Sci. USA 102, 3129–3134 10.1073/pnas.0500185102 (doi:10.1073/pnas.0500185102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janke C, et al. 2004. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 10.1002/yea.1142 (doi:10.1002/yea.1142) [DOI] [PubMed] [Google Scholar]
- 30.Mumberg D, Müller R, Funk M. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119–122 10.1016/0378-1119(95)00037-7 (doi:10.1016/0378-1119(95)00037-7) [DOI] [PubMed] [Google Scholar]
- 31.Wollers S, Heidenreich T, Zarepour M, Zachmann D, Kraft C, Zhao Y, Mendel RR, Bittner F. 2008. Binding of sulfurated molybdenum cofactor to the C-terminal domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J. Biol. Chem. 283, 9642–9650 10.1074/jbc.M708549200 (doi:10.1074/jbc.M708549200) [DOI] [PubMed] [Google Scholar]
- 32.Heidenreich T, Wollers S, Mendel RR, Bittner F. 2005. Characterization of the NifS-like domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J. Biol. Chem. 280, 4213–4218 10.1074/jbc.M411195200 (doi:10.1074/jbc.M411195200) [DOI] [PubMed] [Google Scholar]
- 33.Xiong L, Ishitani M, Lee H, Zhu JK. 2001. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13, 2063–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godman J, Balk J. 2008. Genome analysis of Chlamydomonas reinhardtii reveals the existence of multiple, compartmentalized iron–sulfur protein assembly machineries of different evolutionary origins. Genetics 179, 59–68 10.1534/genetics.107.086033 (doi:10.1534/genetics.107.086033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bych K, Netz DJ, Vigani G, Bill E, Lill R, Pierik AJ, Balk J. 2008. The essential cytosolic iron–sulfur protein Nbp35 acts without Cfd1 partner in the green lineage. J. Biol. Chem. 283, 35 797–35 804 10.1074/jbc.M807303200 (doi:10.1074/jbc.M807303200) [DOI] [PubMed] [Google Scholar]
- 36.Kohbushi H, Nakai Y, Kikuchi S, Yabe T, Hori H, Nakai M. 2009. Arabidopsis cytosolic Nbp35 homodimer can assemble both [2Fe–2S] and [4Fe–4S] clusters in two distinct domains. Biochem. Biophys. Res. Commun. 378, 810–815 10.1016/j.bbrc.2008.11.138 (doi:10.1016/j.bbrc.2008.11.138) [DOI] [PubMed] [Google Scholar]
- 37.Luo D, Bernard DG, Balk J, Hai H, Cui X. 2012. The DUF59 family gene AE7 acts in the cytosolic iron–sulfur cluster assembly pathway to maintain nuclear genome integrity in Arabidopsis. Plant Cell 24, 4135–4148 10.1105/tpc.112.102608 (doi:10.1105/tpc.112.102608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavazza C, Martin L, Mondy S, Gaillard J, Ratet P, Fontecilla-Camps JC. 2008. The possible role of an [FeFe]-hydrogenase-like protein in the plant responses to changing atmospheric oxygen levels. J. Inorg. Biochem. 102, 1359–1365 10.1016/j.jinorgbio.2008.01.027 (doi:10.1016/j.jinorgbio.2008.01.027) [DOI] [PubMed] [Google Scholar]
- 39.Yuan Z, Luo D, Li G, Yao X, Wang H, Zeng M, Huang H, Cui X. 2010. Characterization of the AE7 gene in Arabidopsis suggests that normal cell proliferation is essential for leaf polarity establishment. Plant J. 64, 331–342 10.1111/j.1365-313X.2010.04326.x (doi:10.1111/j.1365-313X.2010.04326.x) [DOI] [PubMed] [Google Scholar]
- 40.McElver J, et al. 2001. Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics 159, 1751–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vernis L, Facca C, Delagoutte E, Soler N, Chanet R, Guiard B, Faye G, Baldacci G. 2009. A newly identified essential complex, Dre2-Tah18, controls mitochondrial integrity and cell death after oxidative stress in yeast. PLoS ONE 4, e4376. 10.1371/journal.pone.0004376 (doi:10.1371/journal.pone.0004376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paine MJ, Garner AP, Powell D, Sibbald J, Sales M, Pratt N, Smith T, Tew DG, Wolf CR. 2000. Cloning and characterization of a novel human dual flavin reductase. J. Biol. Chem. 275, 1471–1478 10.1074/jbc.275.2.1471 (doi:10.1074/jbc.275.2.1471) [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, et al. 2008. Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol. Cell Biol. 28, 5569–5582 10.1128/MCB.00642-08 (doi:10.1128/MCB.00642-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Netz DJ, Pierik AJ, Stümpfig M, Mühlenhoff U, Lill R. 2007. The Cfd1-Nbp35 complex acts as a scaffold for iron–sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 3, 278–286 10.1038/nchembio872 (doi:10.1038/nchembio872) [DOI] [PubMed] [Google Scholar]
- 45.Xu XM, Møller SG. 2006. AtSufE is an essential activator of plastidic and mitochondrial desulfurases in Arabidopsis. EMBO J. 25, 900–909 10.1038/sj.emboj.7600968 (doi:10.1038/sj.emboj.7600968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsaousis AD, et al. 2012. Evolution of Fe/S cluster biogenesis in the anaerobic parasite Blastocystis. Proc. Natl Acad. Sci. USA 109, 10 426–10 431 10.1073/pnas.1116067109 (doi:10.1073/pnas.1116067109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Netz DJ, Pierik AJ, Stumpfig M, Bill E, Sharma AK, Pallesen LJ, Walden WE, Lill R. 2012. A bridging [4Fe–4S] cluster and nucleotide binding are essential for function of the Cfd1-Nbp35 complex as a scaffold in iron–sulfur protein maturation. J. Biol. Chem. 287, 12365–12378 10.1074/jbc.M111.328914 (doi:10.1074/jbc.M111.328914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gari K, León Ortiz AM, Borel V, Flynn H, Skehel JM, Boulton SJ. 2012. MMS19 links cytoplasmic iron–sulfur cluster assembly to DNA metabolism. Science 337, 243–245 10.1126/science.1219664 (doi:10.1126/science.1219664) [DOI] [PubMed] [Google Scholar]
- 49.Stehling O, Vashisht AA, Mascarenhas J, Jonsson ZO, Sharma T, Netz DJA, Pierik AJ, Wohlschlegel JA, Lill R. 2012. MMS19 assembles iron–sulfur proteins required for DNA metabolism and genomic integrity. Science 337, 195–199 10.1126/science.1219723 (doi:10.1126/science.1219723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Netz DJ, et al. 2011. Eukaryotic DNA polymerases require an iron–sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 8, 125–132 10.1038/nchembio.721 (doi:10.1038/nchembio.721) [DOI] [PMC free article] [PubMed] [Google Scholar]