Abstract
Addictions to cocaine or heroin/prescription opioids [short-acting mu-opioid receptor (MOPr) agonists] involve relapsing cycles, with experimentation/escalating use, withdrawal/abstinence, and relapse/re-escalation. Kappa-opioid receptors (KOPr; encoded by OPRK1), and their endogenous agonists, the dynorphins (encoded by PDYN) have counter-modulatory effects on reward caused by cocaine or MOPr agonist exposure, and exhibit plasticity in addictive-like states. KOPr/dynorphin activation is implicated in depression/anxiety, often co-morbid with addictions. In this Opinion article, we propose that particular stages of the addiction cycle are differentially affected by KOPr/dynorphin systems. Vulnerability and resilience can be due to pre-existing (e.g., genetic) factors, or epigenetic modifications of the OPRK1 or PDYN genes during the addiction cycle. Pharmacotherapeutic approaches limiting changes in KOPr/dynorphin tone, especially with KOPr partial agonists, may hold potential for the treatment of specific drug addictions and psychiatric co-morbidity.
Keywords: addiction, dynorphin, kappa-opioid receptor, heroin, prescription opioids, cocaine
Introduction
The KOPr system, and its endogenous agonist ligands, the dynorphins, have widespread distribution in the central and peripheral nervous system [1–5]. Dynorphins are a class of opioid peptides that arise from the precursor protein prodynorphin. When prodynorphin is cleaved during processing, multiple active peptides are released: dynorphin A, dynorphin B, and α/β-neo-endorphin [6, 7].
Behavioral, perceptual, reward- and mood processes, and neuroendocrine functions [e.g., in the hypothalamic-pituitary-adrenal (HPA) axis] are all modulated by the KOPr/dynorphin system. This system also interacts prominently with dopaminergic circuits, and some of the aforementioned effects of KOPr activation (e.g., on mood and reward) may be secondary to this dopaminergic modulation. Acute agonist- induced activation of the KOPr system can result in aversion/dysphoria/sedation (possibly related to anhedonia and decreased arousal) and also psychotomimesis in humans [8]. Potentially related to these psychotomimetic effects, the widely available hallucinogen salvinorin A (from the plant Salvia divinorum), is a high-efficacy selective KOPr agonist [9].
Administration of high-efficacy KOPr agonists causes depressant-like effects and anhedonia in rodents [10], and causes conditioned place aversion [11, 12]. Upregulation in dynorphin mRNA levels occurs after exposure to stress, or to drugs of abuse (e.g., cocaine or short-acting MOPr agonists, discussed below). Depressant-like or anhedonic effects observed after stress exposure or during cocaine withdrawal can be blocked by KOPr antagonists [13–15]. These findings lead to the postulation that increased endogenous dynorphin-induced activation of KOPr (KOPr “tone”) can result in the above neuro-psychiatric adverse events. Notably, there is considerable co-morbidity of such psychiatric disorders in specific addictive disease patients [16–18].
The KOPr/dynorphin system is also upregulated by exposure to drugs of abuse such as stimulants (e.g., cocaine) and MOPr agonists [19–23]. On a methodological level, it should be noted that plasticity in the KOPr target can be detected both at the mRNA level and the protein level (e.g., with autoradiography). By contrast, most data for plasticity in the dynorphin target is at the mRNA level, since it is more challenging to obtain quantitative data on dynorphin peptides (e.g., due to antibody immunoreactivity and specificity problems). Overall, it may be postulated that KOPr/dynorphin upregulation plays specific roles depending on the stage within an addiction cycle (which, operationally, can include: initiation/escalation of exposure, withdrawal/abstinence and relapse/re-escalation; discussed further below). Other major systems (e.g., the glutamatergic, CRF/AVP and other neuropeptides) are undoubtedly involved, but the scope of this article will be limited to the KOPr/dynorphin.
The focus of this Opinion article is primarily on addictions to cocaine and short-acting MOPr agonists, and not directly on other substances such as nicotine, cannabinoids, or ethanol. Several valuable recent reviews have focused on the role of the KOPr/dynorphin systems in the neurobiology of addiction and comorbid neuropsychiatric states [24–26]. The translational focus of this article is on how specific pharmacotherapeutic approaches focusing on this system (e.g., selective KOPr antagonists or partial agonists) may hold potential at different stages of the operationally defined addiction cycle. We further propose that specific human genetic variants in this system may affect vulnerability and resilience at particular stages of the addiction cycle to specific types of drugs, such as cocaine and other stimulants, or heroin or abused prescription opioids, and may thus further inform clinical treatment efforts.
Addiction states and their cyclical relapsing nature: A framework for the impact of the KOPr/dynorphin system
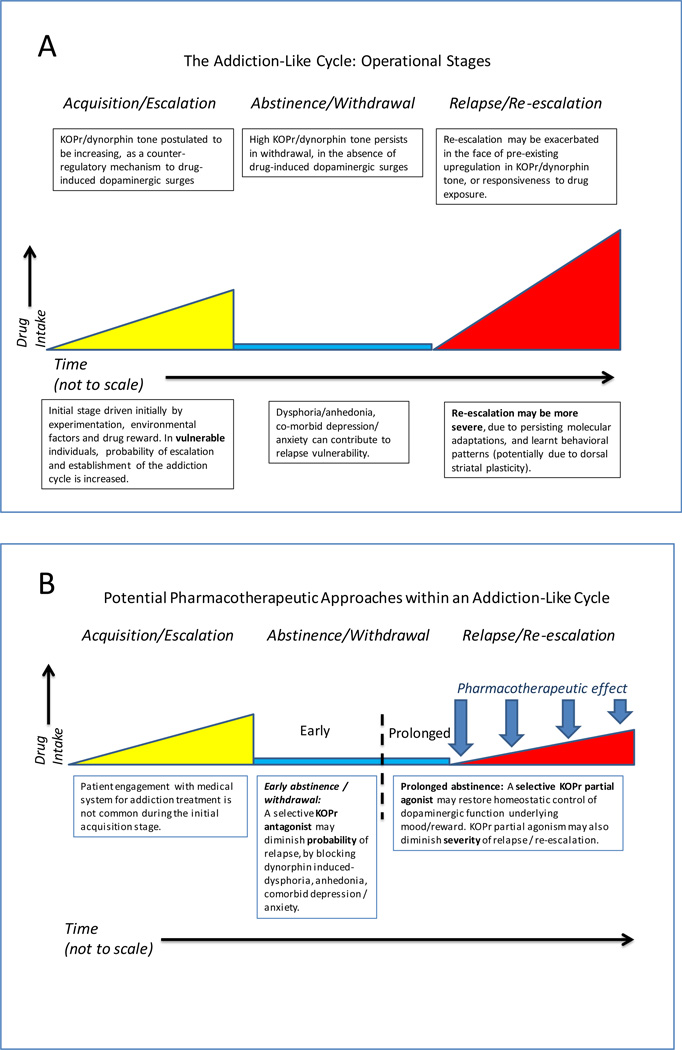
The trajectory of addiction has operational stages including early experimentation, escalating self-exposure, followed by withdrawal/abstinence periods of varying duration, and in vulnerable individuals, relapse/re-escalation, of varying severity (Fig. 1A) [27–32]. Some behavioral and neurobiological manifestations of these stages are specific to each drug of abuse (e.g., stimulants such as cocaine, or heroin/prescription opioids), for example, neurobiological aspects of withdrawal therefrom [33, 34]. By contrast, some downstream effects may be shared across multiple drug classes. For instance, acute dopaminergic activation in nigrostriatal and mesolimbic systems, and activation of a counter-modulatory KOPr/dynorphin response [19, 20, 23, 35, 36], are common responses across different types of drugs of abuse. The dopaminergic systems and the KOPr/dynorphin systems (as well as MOPr) show functional adaptations (some persisting for prolonged periods) after repeated exposure to cocaine or short-acting MOPr agonists, in preclinical models or clinically [20, 21, 23, 37–43]. These adaptations are hypothesized to underlie aspects of relapse and neuropsychiatric co-morbidity. For a variety of practical, catchment, and ethical reasons, it is difficult to obtain human findings at clearly defined stages in the addiction trajectory (especially during the experimentation and initial escalation stages). Thus, evolving preclinical studies have been critical to present understanding in this field.
Figure Legend 1. Different stages of the addiction cycle and potential KOPr-directed pharmatherapeutic opportunities.
(a) Operational stages of the addiction-cycle to cocaine, heroin or illicitly used prescription opioids. Individual trajectory (e.g., severity of escalation, withdrawal, or risk of relapse) is thought to depend on major interacting factors: extent of drug exposure, concomitant stresses, co-morbid psychiatric / psychological status and genetic predisposition (e.g., in OPRK1/PDYN or OPRM1 genes) [16, 27, 41, 57, 66, 76, 98–100]. Each of these factors may be hypothesized to have particular impact at a specific operational stage. For example, a particular SNP may exacerbate either acquisition/escalation, severity of withdrawal-induced dysphoria, or probability of relapse. (b) Proposed pharmacotherapeutic approaches for addictions and neuropsychiatric co-morbidity (panel B), based on appropriate modulation of KOPr/dynorphin tone with selective antagonists or partial agonists. Selective KOPr antagonists (including recently reported compounds with durations of action that are within the range of most therapeutically used compounds [90]) may reduce relapse probability in the early stages of withdrawal/abstinence, by decreasing anhedonia, dysphoria, stress responsivity or comorbid psychiatric signs that may be secondary to upregulated KOPr/dynorphin tone [14, 15, 46, 50, 51, 90]. It is hypothesized that selective KOPr partial agonists (not currently available, due to lack of KOPr>MOPr selectivity in known ligands) [93, 95, 101] may be beneficial in promoting more prolonged abstinence, as well as decreasing the severity of relapse episodes. More specifically, a selective KOPr partial agonist can be hypothesized to provide a degree of homeostatic tone in the KOPr system, via blocking dynorphin-induced hyper-activation in the system (a factor in relapse; see panel (a)), as well as limiting excessive dopamine surges due to relapse-related exposure to cocaine or heroin/prescription opioids.
Neurobiology of the KOPr/dynorphin system: Impact in the Addiction Cycle
KOPr and dynorphin peptides are localized in areas of the dopaminergic nigrostriatal and mesolimbic–mesocortical systems [2, 3]. They play an important role in the modulation of reward, be it to natural reinforcers (e.g., food, appetitive stimuli) or to drugs of abuse (eg. cocaine,a monoamine reuptake inhibitor, or MOPr agonists such as heroin), presumably through modulation of basal and reward/drug- induced changes in dopaminergic tone. In contrast to most drugs of abuse (e.g., stimulants, MOPr agonists, or ethanol), acutely administered KOPr agonists (such as dynorphin A1–17, which is a high-efficacy endogenous agonist [4, 44], or synthetic ligands such as U50,488 [4]) decrease basal dopamine levels in dopaminergic terminal fields [35, 45, 46]. Endogenous dynorphin tone in the mesolimbic system appears to be low under basal conditions, as shown by the relatively limited effects of administration of a selective KOPr antagonist in rodents not previously challenged with drugs of abuse [47–49].
Endogenous dynorphin activation is thought to mediate, at least in part, dysphoria and anhedonia associated with drug withdrawal, stress-induced aversion states, and stress-induced relapse-like behavior [15, 50–52]. These findings strongly support the hypothesis that the functional status of the endogenous KOPr / dynorphin system is an important feature underlying the cyclical nature of drug addiction trajectory, as well as the co-morbidity observed with psychiatric disorders (e.g., anxiety and mood disorders). This co-morbidity may in itself contribute to exacerbation of the addictive cycle, for example by promoting continued relapse.
Adaptations in the KOPr/dynorphin system after exposure to drugs of abuse
Expression of the PDYN gene is increased on an acute and recurrent basis by drugs of abuse, such as cocaine [19, 20]. Interestingly, this effect becomes more pronounced after chronic high dose exposure, in dorsal striatal areas thought to be involved in compulsive/habit-like behaviors, one of the hallmarks of addictive states [19, 20, 53, 54]. KOPr antagonists can block stress-induced reinstatement of cocaine self-administration or conditioned place preference (CPP), which are models for aspects of relapse [50, 51]. Stress and chronic drug (e.g., cocaine) exposure enhances dynorphin expression, KOPr signaling and KOPr levels, in a time-dependent, neuroanatomically specific manner [19–21, 38, 55, 56]. This leads to the postulation that dynorphin-induced activation of KOPr mediates anxiety- or depressant-like, anhedonic or dysphoric effects after either stress or chronic drug exposure [13, 52, 56]. Furthermore, chronic cocaine exposure also results in adaptations in KOPrs and KOPr-activated second messenger signaling, in areas of the meso-limbic and nigrostriatal system (Figure 1) [21, 55]. Overall, there appears to be a pronounced and relatively broad-based impact of the KOPr/dynorphin system on adaptations to chronic exposure to drugs of abuse and known neuropsychiatric co-morbidity. It may therefore be hypothesized that responsiveness of this system at specific stages of the addiction cycle (e.g., acquisition/escalation, vs. withdrawal/abstinence, vs. relapse, and attendant co-morbidity) may influence vulnerability and resilience. Genetic variation in the KOPr/dynorphin system may thus confer relative resilience or vulnerability at specific stages of an addiction cycle, by acting either on the addictive process per se, on co-morbid psychiatric disorders, or on stress-related brain adaptations (discussed further below) [57, 58]. Genetic association data that clearly separates these three mediating potential mechanisms in the context of addiction is not currently available, and would contribute to the refinement and impact of therapeutic strategies (see Box 1).
Box 1. Outstanding Questions.
Can the main operational stages of the proposed addiction cycle framework (e.g., Fig. 1A) be investigated and potentially validated in a clinical research setting?
What are the behavioral, neurobiological and epigenetic underpinnings of plasticity (eg. in KOPr, dynorphin and MOPr) at specific stages of an experimental addiction-like cycle?
-
Based on the framework and findings of an addiction cycle, are there specific translationally viable behavioral, pharmacological, or pharmaco-epigenetic interventions that can potentially lead to novel therapeutic modalities?
-
○
More specifically:
-
○
Can novel short-acting KOPr selective antagonists (not yet generally available for human studies) have a clinically beneficial effect on addiction trajectory or psychiatric comorbidity?
Can (as yet unavailable) selective KOPr partial agonists (ie. without effects on MOPrs) have a particularly beneficial pharmacotherapeutic profile in this context (Fig. 1B)? The recent exciting report of the crystal structure of KOPr when bound to a synthetic ligand [97] may provide a crucial step forward in the rational design of novel compounds with desirable pharmacodynamic/signaling properties.
How do pre-existing genetic variations (e.g., SNPs, haplotypes, indels), and epigenetic changes in these target genes mechanistically affect vulnerability and resilience at specific stages of an addiction-like cycle? The recent publications of crystal structures for KOPr and MOPr complexed with synthetic ligands may also give further insight into genetic underpinnings of vulnerability and resilience [97, 107].
Is there a causal or mutual relationship between the etiology of specific addictive diseases and specific aspects of psychiatric co-morbidities (e.g., depression and anxiety) based on interaction with KOPr/dynorphin systems, at a genetic, epigenetic or neuroplasticity level?
Genetics of PDYN and vulnerability in the addiction cycle
Genetic polymorphisms in the PDYN gene [e.g., individual single nucleotide polymorphisms (SNPs),haplotypes, repeats, or insertion/deletions [indels]), may affect the efficiency of transcription, or responsivity to environmental or internal stimuli. This would in turn result in downstream neurobiological and behavioral adaptations (e.g., proximally through dynorphins’ agonist actions on KOPr). Therefore, genetic variation at PDYN, or epigenetic changes, could underlie vulnerability and resilience to addictive and co-morbid psychiatric diseases, at specific stages in the addiction cycle.
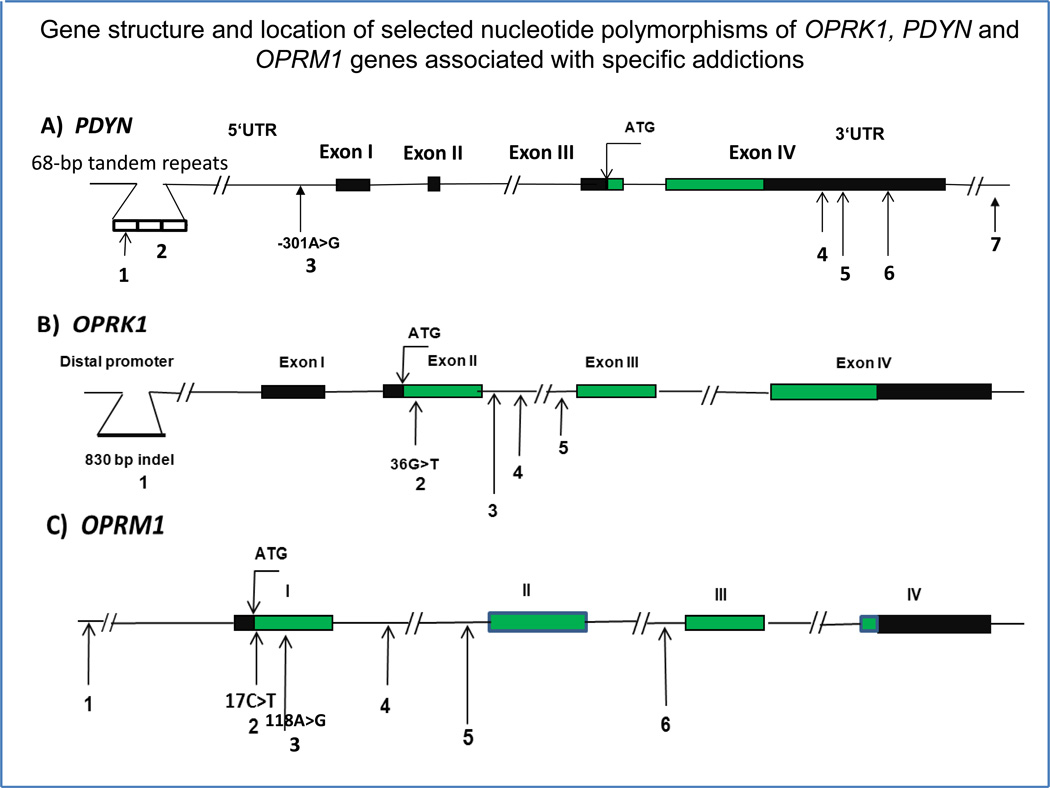
The human PDYN gene contains four exons and is located at chromosome 20p13 (Fig. 2A). Exons 1 and 2 encode the 5′ UTR, exon 3 encodes a signal peptide, and exon 4 encodes the dynorphin peptides, including dynorphin A1–17 and dynorphin B1–13. One of the most studied PDYN polymorphism is a 68-base pair nucleotide tandem repeat polymorphism (rs35286281) located 1250 bp upstream of exon 1 [6] (Table 1). This polymorphism, which contains a putative activator protein-1 (AP-1) transcription complex (including c-Fos/c-Jun) binding site, is found in 1–5 copies [59]. With careful phenotyping, it was found that individuals with three or four copies of the 68-bp tandem repeat showed an increased vulnerability to develop cocaine dependence or cocaine/alcohol codependence, in African-American subjects only [60, 61]. A significant association was reported between the number of 68-bp tandem repeats and methamphetamine dependence; alleles with three or four copies of the 68-bp repeat were found more frequently in Japanese individuals with methamphetamine dependence [62]. Overall, these profiles may be due to drug-specific interactions between gene and drug exposure etiology, and also on differential PDYN polymorphism distribution or allelic frequencies across ethnicities.
Figure Legend 2.
Gene structures and selected loci of genetic variability in human (a) PDYN, (b) OPRK1 and (c) OPRM1 genes in the context of addiction to drugs of abuse (eg. cocaine, heroin, methamphetamine and alcohol). Coding regions of exons are shown in green; 5′- and 3′- untranslated regions (UTR) are in black. The numbers refer to the polymorphisms given in Table 1. The location of the rs1997794 polymorphism (ie. #3) in the PDYN gene is shown in relation to the transcription initiation site; location of 17C>T (#2) and 118A>G (#3) in OPRM1 is shown in relation to ATG (a translation initiation site).
Table 1.
Selected opioid system gene variants reported to be associated with specific drug addictions in human subjects, or on mRNA expression
| Gene | Location on chromosome |
Polymorphism #a |
Gene polymorphism ID |
Polymorphism type |
Location on gene |
Study cohort |
Associated with | Selected Refs |
|---|---|---|---|---|---|---|---|---|
| Prodynorphin (PDYN) |
Chr. 20 | 1 | rs61761346 | SNP | Promoter | N/A | PDYN expression levels, in vitro | [59] |
| 2 | rs35286281 | VNTR | Promoter | AA, His | Opioid, cocaine and methamphetamine dependence | [60, 61] | ||
| 3 | rs1997794 | SNP | Promoter | As, EA | Opioid and alcohol dependence | [63, 65] | ||
| 4 | rs910080 | SNP | 3’-UTR | EA | Cocaine, opioid and alcohol dependence | [66] | ||
| 5 | rs910079 | SNP | 3’-UTR | EA | Cocaine, opioid and alcohol dependence | [63, 65] | ||
| 6 | rs2235749 | SNP | 3’-UTR | As, EA | Cocaine, opioid and alcohol dependence | [66] | ||
| 7 | rs1022563 | SNP | 3’-UTR | As, EA | Opioid dependence | [65] | ||
| Opioid receptor kappa (OPRK1) |
Chr. 8 | 1 | rs35566036 | Indel | Promoter | EA | Alcohol dependence | [76] |
| 2 | rs1051660 | SNP | Exon 1 | EA, His | Opioid dependence | [5] | ||
| 3 | rs6473797 | SNP | Intron 2 | EA | Opioid and alcohol dependence | [74] | ||
| 4 | rs6985606 | SNP | Intron 2 | EA | Cocaine and alcohol dependence | [75] | ||
| 5 | rs997917 | SNP | Intron 2 | EA | Cocaine and alcohol dependence | [75] | ||
| Opioid receptor mu (OPRM1) |
Chr.6 | 1 | rs1074287 | SNP | Promoter | As, EA | Opioid dependence | [102] |
| 2 | rs1799972 | SNP | Exon 1 17C>T) |
AA | Risk of cocaine and alcohol use | [103] | ||
| 3 | rs1799971 | SNP | Exon (118A>G) |
As, E, EA | Opioid and alcohol dependence, severity of HIV nfection and response to HIV treatment |
[81, 99, 104, 105] |
||
| 4 | rs510769 | SNP | Intron 1 | EA | Opioid dependence | [74] | ||
| 5 | rs3778151 | SNP | ntron 1 | As, EA | Opioid dependence | [74] | ||
| 6 | rs2075572 | SNP | Intron 2 | As | Opioid and alcohol dependence | [106] | ||
Polymorphism number corresponds to gene locations shown in Figure 2.
Cohort abbreviations: AA (African-American); E (European); EA (European-American); His (Hispanic); As (Asian)
Several association studies of other PDYN SNPs and different addictions have been reported. Nine PDYN SNPs tested in the large Collaborative Study on Genetics of Alcoholism (COGA) caucasian study cohort (including rs1997794, located in the promoter of PDYN [NM_001190900] or in exon 1 of a splice variant [NM_024411.4]) were associated with alcohol dependence [63]. The minor allele of rs1997794 eliminates a putative binding site for the AP-1 transcription factor complex and modulates PDYN expression [64].
Three SNPs were analyzed in Chinese subjects, for association with heroin dependence [65], resulting in the detection of a sex-specific association (ie., SNP rs1022563 was associated with opioid dependence in females but not males). This illustrates sex as a further stratification variable that may affect the impact of genetic variation of PDYN on this complex disease. An association between three SNPs (rs910080, rs910079 and rs2235749) in the 3′ UTR region, and the haplotype CCT, with both cocaine dependence and cocaine/alcohol codependence, was detected in Caucasians, but not in African Americans [66].
What are the functional consequences of PDYN polymorphisms?
Several studies evaluated the impact of the SNPs on gene expression of PDYN mRNA. An early in vitro study, using a minimal PDYN promoter in a reporter gene expression assay in a rodent neuroblastoma X glioma cell line (NG108), showed that constructs containing three or four copies of the 68-base pair repeat (ie. rs35286281) produced approximately 1.5 greater levels of forskolin-induced transcriptional activity compared with constructs with one or two copies of the repeat [67]. A more recent study in human cell lines transfected with reporter gene expression constructs containing the PDYN promoter with the thirteen most common combinations of the tandem repeat region and the internal SNP (rs61761346) in a control human population, explored this influence more broadly [59]. In neuronal SK-N-SH and H69 cell lines, three or four repeats led to lower expression of luciferase than one or two repeats, which would suggest lower levels of PDYN message and potentially peptide production. However, the opposite effect was found in the human embryonic kidney (HEK293) cell line. The SNP A/G within the repeats (rs61761346, Table 1; Fig. 2A) also had an effect on PDYN gene expression in both SK-N-SH and H69 cells. Thus, promoter forms with the A allele had significantly higher luciferase expression than promoter forms with the G allele [59]. Also, a recent study reported a complex interaction of five polymorphisms in the 5' flanking region (including the polymorphic 68-bp tandem repeat) on regulation of basal expression of PDYN; such regulation was observed both in vitro as well as within two regions of postmortem human brain, the occipital cortex and temporal cortex [68]. Overall, these findings support the importance of the PDYN promoter repeats (and the SNP therein) on gene expression, with a complex pattern of in vitro effects, depending on experimental conditions.
As described above, the haplotype CCT in PDYN 3’UTR was associated with cocaine dependence and with combined cocaine/alcohol codependence [66]. Allele-specific gene expression of PDYN was investigated, using SNP rs910079 as a reporter, in postmortem human brains from eight heterozygous subjects, using the allele-specific gene expression assay [69]. Lower expression for the C allele (rs910079), indicating lower expression of the CCT haplotype of PDYN, was observed both in the caudate and nucleus accumbens (NAc) [66]. Analysis of total PDYN expression in 43 postmortem brains (irrespective of clinical status) also showed lower levels of PDYN mRNA in subjects having the “risk” CCT haplotype [66]. The discovery of the haplotype expression differences raises the question of whether this haplotype is functional alone or is linked to other functional variants. For example, this haplotype is in linkage with the functional SNP rs 1997794 in the PDYN promoter region [66]. This study provided evidence that a haplotype with lower mRNA expression of the PDYN gene in human caudate and NAc may be associated with increased vulnerability to develop cocaine dependence. Taking into account the neurobiology of the KOPr/dynorphin system in the setting of preclinical models, and the conceptual framework of the addiction cycle (above), it may be hypothesized that such a functional consequence (e.g., lower pre-existing PDYN mRNA expression), may affect cocaine or cocaine/alcohol addiction in one or more operational stages (for example, in the acquisition/escalation stage).
Preclinical models for assessing the impact of PDYN
Since there are limits to the likelihood of obtaining human post mortem samples at specific stages of the addiction cycle, and at specific times post- drug exposure, preclinical models are critical to further illuminate this issue. Studies in different mouse models show that sensitivity to rewarding effects of drugs of abuse may depend, in part, on the basal level of PDYN gene expression in the striatum. For instance, induction of PDYN by overexpression of cAMP response element binding protein (CREB) in the NAc (prior to cocaine exposure), decreased the acquisition of cocaine-induced CPP [70]. This would be, conceptually, an illustration of a molecular genetic intervention affecting specifically the acquisition stage of the abuse/addiction trajectory.
Interestingly, tissue plasminogen activator (tPA) knockout mice displayed higher basal PDYN mRNA levels in the NAc, and lower acquisition of cocaine-induced CPP in comparison to wild-types [71]. Also, mice of the inbred strain DBA/2J showed higher levels of basal PDYN mRNA in the NAc than C57BL/6J mice, and the former were relatively resistant to the acquisition of morphine-induced CPP [72]. Furthermore, the acquisition of morphine-induced CPP was enhanced in DBA/2J mice pre-treated with the KOPr antagonist nor-binaltorphimine (which would presumably decrease KOPr/dynorphin “tone”). However, complex strain differences (e.g., C57BL/6J vs 129P\3J) in heroin-induced changes in PDYN mRNA levels have also been observed, together with differences in acquisition of heroin-induced CPP [73].
Genetics of OPRK1 and vulnerability at specific stages of the addiction cycle
Similar to the dynorphins, the KOPr gene and protein are also regulated by addiction trajectory in humans and animal models. The human OPRK1 gene is located on chromosome 8q11.2. A recent re-evaluation of the exon/intron structure of the human OPRK1 gene reported four exons and three introns, and a 3'-UTR region of 3096 nucleotides in length (Fig. 2A), similar to rodent Oprk1 [5]. Twelve SNPs in the coding region and intron 1 of the gene were studied, and using logistic regression with heroin dependence status, the 36G>T SNP (rs1051660) exhibited a point-wise significant association with disease status [5] (Table 1). Another study of eleven SNPs (not including SNP rs1051660), reported an association of heroin dependence of the intronic SNP rs6473797 in Caucasians, including Israeli Caucasians [74]. Also, in a study with seven SNPs in EA, three of the SNPs examined (rs1051660, rs6985606 and rs997917) were in association with alcohol or cocaine dependence [75]. A functional polymorphism, 830 bp insertion/deletion (indel, rs35566036, Fig. 2A) was identified in promoter region of OPRK1 located at -1986 bp upstream of the translation start site [76]. The presence of the insertion upstream of the luciferase reporter gene lowered transcription activity of OPRK1 promoter in HepG2 cells [75]. Family-based association analyses in 219 COGA multiplex Caucasian alcohol-dependent families showed significant association of this insertion with alcoholism [76].
Of note, genetic variation in OPRM1 (the gene for MOPr, the main pharmacodynamic target of heroin and prescription opioids) affects vulnerability to addictive diseases and pharmacotherapy (summarized in Figure 2C and Table 1). The KOPr/dynorphin system is counter-modulatory to the MOPr system, and MOPrs also mediate downstream effects of cocaine. Therefore, OPRM1 genetic variation may also contribute to the trajectory of addiction and recovery interactively with the aforementioned systems.
In summary, genetic variation at OPRK1 or PDYN, as well as OPRM1 (and other genes beyond the scope of this article) can have direct functional consequences by altering mRNA transcription, stability or translation, resulting in potential changes in protein structure or protein levels.
Working model of the impact of genetic variation in OPRK1 or PDYN at specific stages of the addiction cycle
Given the role of these receptor / neuropeptide systems in addiction neurobiology (based primarily, but not exclusively, on preclinical studies), it may be rationally postulated that specific genetic variations can result in greater vulnerability or resilience at specific stages of an addiction cycle. Overall, a working model can be presented, in which high pre-existing expression of the PDYN gene (and thus high KOPr/dynorphin tone) results in decreased vulnerability during the initial acquisition/escalation phase in models of addiction trajectory, possibly by blunting the dopaminergic response to cocaine or MOPr-agonists. By contrast, it is reasonable to postulate that high KOPr/dynorphin tone in other stages in the addiction cycle (e.g., in withdrawal/abstinence, or relapse), may conversely exacerbate vulnerability, by enhancing dysphoria, anhedonia and psychiatric comorbidity (Fig. 1 A). High KOPr/dynorphin tone at these stages could be potentially due to epigenetic changes in either OPRK1 or PDYN occurring in response to drug exposure history. Specific sites of methylation of OPRK1 have been observed in P19 mouse embryonal carcinoma cells [77, 78], and of PDYN in human brain [79, 80], potentially paralleled by methylation patterns in peripheral blood mononuclear cells, as a biomarker [80]. Appropriately designed clinical genetic association studies would be critical to determine the applicability of this model. Such studies would include detailed analysis of addiction trajectory and clinical course (e.g., slow vs. rapid escalation, frequency and severity of relapse), and potential association with genetic polymorphisms, or with epigenetic marks at defined stages in addiction trajectory.
Pharmacotherapeutic implications: Targeting neurobiological adaptations in the KOPr/ dynorphin system at different stages of the addiction cycle
As detailed above, polymorphisms in genes of the opioid receptor system, or of genes encoding cognate endogenous neuropeptides, are postulated to be associated with vulnerability during specific stages of the addiction cycle. In some cases, information on such stage-specific roles is available. For example, polymorphisms in OPRM1 are associated with particular aspects of addiction vulnerability or treatment success [81]. It is also known that sufficient drug exposure (e.g., to cocaine) - independent of genetic predisposition - can result in increases in KOPr/dynorphin tone, as detected in animal models (discussed above). Such an increase in KOPr/dynorphin tone could exacerbate dysphoria, anhedonia and psychiatric co-morbidity, and thus, increase vulnerability to relapse and re-escalation.
Pharmacological strategies based on the KOPr/dynorphin system
High efficacy synthetic KOPr agonists could decrease ongoing reinforcing/rewarding effects of cocaine [82–84]. However, administration of high efficacy KOPr agonists in humans results in dose-limiting sedation or dysphoria, as well as psychotomimetic side effects (to which tolerance could potentially develop), complicating potential use of such compounds in the clinic [85].
Based on a more recent synthesis of the status of the KOPr/dynorphin system within models of addiction trajectory (and psychiatric co-morbidity), it can be proposed that a relative decrease in KOPr/dynorphin tone may be a more desirable strategy. This could be postulated to promote abstinence and minimize relapse probability or severity, and also to allow greater subject engagement with “standard of care” social and psychological support. Since most treatment-seeking patients make contact with the health system after initial acquisition/escalation have already occurred, pharmacotherapeutic approaches have to be potentially effective at later stages in the addiction cycle (Fig. 1B). Early and even prolonged withdrawal/abstinence may be associated with the consequences of upregulated dynorphin activation at KOPr. This could result in anhedonia, dysphoria, as well as co-morbid signs of anxiety or depression. Of note, early withdrawal from MOPr agonists (e.g., up to several days) is also associated with classic signs of autonomic and HPA axis activation; these likely impact other systems, and can be managed with supportive pharmacological treatment [86]. However, the clinical management of withdrawal from MOPr agonists, while necessary, is not sufficient to sustain a decrease in relapse risk over the medium or long term [30, 32]. Therefore in addition to well validated pharmacotherapeutic approaches for heroin or prescription opioid addiction, such as methadone or buprenorphine maintenance therapy, a relative blockade of KOPr/dynorphin tone may be investigated for therapeutic impact.
KOPr/dynorphin upregulated tone has been shown to decline spontaneously in intermediate duration withdrawal/abstinence models in rats [87]. An accelerated decrease in such tone could potentially be achieved with selective KOPr antagonists (which have not yet been studied to date in the clinic, due to lack of FDA-approved compounds). Current selective KOPr antagonists (e.g., nor-binaltorphimine) exhibit extraordinary durations of action in vivo [88, 89], for reasons that may include adaptations downstream of KOPr [90]. These features complicate their study and application in the clinic, since dosing strategies, reversibility of any undesired effects, and study design, would all be impacted. However, newer selective ligands with more therapeutically-relevant durations of action are now available, and are important for proof-of-concept studies [90, 91].
Given the importance of the KOPr/dynorphin system as a homeostatic regulator of natural rewards, as well as drugs of abuse, selective KOPr partial agonists may be a desirable approach, as alternate or sequential pharmacotherapeutic modality during more prolonged abstinence and in relapse prevention [92]. Thus, chronic KOPr partial agonist treatment is postulated to a) provide a stable counter-modulatory tone to dopaminergic surges (e.g., due to drug exposure in relapse), and b) cause a relative blockade of excessive or fluctuating KOPr/dynorphin tone (i.e., a partial KOPr agonist would cause a relative blockade of the effects of endogenous dynorphins, which are high efficacy KOPr agonists). Both of these potential KOPr effects may mitigate against relapse and re-escalation. From a translational perspective, all clinically available medications with KOPr partial agonist effects (e.g., buprenorphine, nalmefene) are not selective. These medications also have prominent MOPr antagonist or MOPr partial agonist effects [4, 93–95]. Of translational relevance, the neuroendocrine biomarker prolactin (which is responsive to both MOPr and KOPr activation) could be used to quantify antagonist, partial agonist, or full agonist, effects in preclinical models or human subjects (eg. [93]). The development of novel selective KOPr partial agonists could be based on recently reported synthetic heterocyclic scaffolds, or natural product scaffolds, such as salvinorin [90, 96], aided by the recently reported crystal structure of human KOPr [97].
Concluding remarks
The KOPr/dynorphin system has emerged as a powerful regulator of neuro-behavioral consequences of acute and prolonged exposure to cocaine, heroin, or illicitly used prescription opioids. This system may also contribute to co-morbid anxiety and depression, which may exacerbate particular stages in the addiction cycle. Genetic polymorphisms in PDYN and OPRK1 may be associated with vulnerability at different stages, by conferring relative risk or protection in a) initial escalation, b) withdrawal/abstinence and attendant anhedonia or dysphoria, c) relapse/re-escalation, or d) on psychiatric co-morbidity. Selective KOPr antagonists and partial agonists, administered either sequentially, or based on the neuro-behavioral status and neurogenetic profile of individual patients, are now thought, by our group and others, to be a potential strategy in the treatment of specific addictive diseases.
Acknowledgements
The authors gratefully acknowledge funding from the National Institutes of Health, National Institute on Drug Abuse (grant P60 DA05130), National Institute of Mental Health grant (MH70880), and National Center for Research Resources (Center for Clinical and Translational Science; grant UL1RR024143).
References
- 1.Goldstein A, et al. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansour A, et al. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- 3.Simonin F, et al. kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc. Natl. Acad. Sci U S A. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, et al. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPgammaS binding to membranes: determination of potencies and efficacies of ligands. Journal of Pharmacology and Experimental Therapeutics. 1997;282:676–684. [PubMed] [Google Scholar]
- 5.Yuferov V, et al. Redefinition of the human kappa opioid receptor gene (OPRK1) structure and association of haplotypes with opiate addiction. Pharmacogenetics. 2004;14:793–804. doi: 10.1097/00008571-200412000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horikawa S, et al. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983;306:611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- 7.Mansour A, et al. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer A, et al. Psychotomimesis Mediated by Kappa Opiate Receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 9.Roth BL, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc. Natl. Acad. Sci U S A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlezon WA, Jr., et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. Journal of Pharmacology and Experimental Therapeutics. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology. 2004;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 13.Shirayama Y, et al. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, et al. Central kappa-opioid receptor-mediated antidepressant-like effects of nor-Binaltorphimine: behavioral and BDNF mRNA expression studies. Eur J Pharmacol. 2007;570:89–96. doi: 10.1016/j.ejphar.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chartoff E, et al. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regier DA, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 17.Kranzler HR, et al. Validity of the SCID in substance abuse patients. Addiction. 1996;91:859–868. [PubMed] [Google Scholar]
- 18.Mason BJ, et al. Psychiatric comorbidity in methadone maintained patients. J. Addict. Dis. 1998;17:75–89. doi: 10.1300/J069v17n03_07. [DOI] [PubMed] [Google Scholar]
- 19.Daunais JB, et al. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Spangler R, et al. 'Binge' cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res. Mol. Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- 21.Unterwald EM, et al. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- 22.Tjon GH, et al. Delayed occurrence of enhanced striatal preprodynorphin gene expression in behaviorally sensitized rats: differential long-term effects of intermittent and chronic morphine administration. Neuroscience. 1997;76:167–176. doi: 10.1016/s0306-4522(96)00363-6. [DOI] [PubMed] [Google Scholar]
- 23.Wang XM, et al. Acute intermittent morphine increases preprodynorphin and kappa opioid receptor mRNA levels in the rat brain. Brain Res Mol Brain Res. 1999;66:184–187. doi: 10.1016/s0169-328x(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 24.Bruchas MR, et al. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tejeda HA, et al. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell. Mol. Life. Sci. 2012;69:857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 28.Mantsch JR, et al. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- 29.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann N Y Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 31.Kakko J, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361:662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 32.Dunn KE, et al. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend. 2011;119:1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. Curr Top Behav Neurosci. 2010;3:119–178. doi: 10.1007/7854_2009_20. [DOI] [PubMed] [Google Scholar]
- 34.Han MH, et al. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. Journal of Pharmacology and Experimental Therapeutics. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 36.Spanagel R, et al. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimic dopamine release. Psychopharmacology. 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- 37.Unterwald EM, et al. Chronic cocaine alters brain mu opioid receptors. Brain Res. 1992;584:314–318. doi: 10.1016/0006-8993(92)90912-s. [DOI] [PubMed] [Google Scholar]
- 38.Spangler R, et al. Regulation of kappa opioid receptor mRNA in the rat brain by "binge' pattern cocaine administration and correlation with preprodynorphin mRNA. Brain Res Mol Brain Res. 1996;38:71–76. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- 39.Tsukada H, et al. Effects of binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain: an in vivo study using positron emission tomography. J Neurosci. 1996;16:7670–7677. doi: 10.1523/JNEUROSCI.16-23-07670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zubieta JK, et al. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2:1225–1229. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
- 41.Unterwald EM, et al. The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res. 2001;900:103–109. doi: 10.1016/s0006-8993(01)02269-7. [DOI] [PubMed] [Google Scholar]
- 42.Volkow ND, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 43.Bencherif B, et al. Mu-opioid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Biol Psychiatry. 2004;55:255–262. doi: 10.1016/j.biopsych.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Remmers AE, et al. Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. Journal of Pharmacology and Experimental Therapeutics. 1999;288:827–833. [PubMed] [Google Scholar]
- 45.Spanagel R, et al. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, et al. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- 47.Chefer VI, et al. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Doyon WM, et al. kappa-Opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindholm S, et al. Ethanol alters the effect of kappa receptor ligands on dopamine release in the nucleus accumbens. Physiol Behav. 2007;92:167–171. doi: 10.1016/j.physbeh.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 50.Beardsley PM, et al. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 51.Carey AN, et al. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur. J. Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Land BB, et al. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fagergren P, et al. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur. J. Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- 54.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piras AP, et al. Acute withdrawal from chronic escalating-dose binge cocaine administration alters kappa opioid receptor stimulation of [35S] guanosine 5'-O-[gamma-thio]triphosphate acid binding in the rat ventral tegmental area. Neuroscience. 2010;169:751–757. doi: 10.1016/j.neuroscience.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knoll AT, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreek MJ, et al. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 58.Kreek MJ, et al. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- 59.Rouault M, et al. Cell-specific effects of variants of the 68-base pair tandem repeat on prodynorphin gene promoter activity. Addict. Biol. 2011;16:334–346. doi: 10.1111/j.1369-1600.2010.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dahl JP, et al. Confirmation of the association between a polymorphism in the promoter region of the prodynorphin gene and cocaine dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;139B:106–108. doi: 10.1002/ajmg.b.30238. [DOI] [PubMed] [Google Scholar]
- 61.Williams TJ, et al. Prodynorphin gene promoter repeat associated with cocaine/alcohol codependence. Addict Biol. 2007;12:496–502. doi: 10.1111/j.1369-1600.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- 62.Nomura A, et al. Genetic variant of prodynorphin gene is risk factor for methamphetamine dependence. Neurosci Lett. 2006;400:158–162. doi: 10.1016/j.neulet.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 63.Xuei X, et al. Association of the kappa-opioid system with alcohol dependence. Mol. Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- 64.Taqi MM, et al. Prodynorphin promoter SNP associated with alcohol dependence forms noncanonical AP-1 binding site that may influence gene expression in human brain. Brain Res. 2011;1385:18–25. doi: 10.1016/j.brainres.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 65.Clarke TK, et al. An association of prodynorphin polymorphisms and opioid dependence in females in a Chinese population. Addict. Biol. 2009;14:366–370. doi: 10.1111/j.1369-1600.2009.00151.x. [DOI] [PubMed] [Google Scholar]
- 66.Yuferov V, et al. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34:1185–1197. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zimprich A, et al. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J. Neurochem. 2000;74:472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]
- 68.Babbitt CC, et al. Multiple Functional Variants in cis Modulate PDYN Expression. Mol. Biol. Evol. 2010;27:465–479. doi: 10.1093/molbev/msp276. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, et al. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 70.Carlezon WA, Jr., et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 71.Maiya R, et al. Tissue plasminogen activator modulates the cellular and behavioral response to cocaine. Proc. Natl. Acad. Sci. USA. 2009;106:1983–1988. doi: 10.1073/pnas.0812491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gieryk A, et al. Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology (Berl) 2010;208:291–300. doi: 10.1007/s00213-009-1730-1. [DOI] [PubMed] [Google Scholar]
- 73.Schlussman SD, et al. Regional mRNA expression of the endogenous opioid and dopaminergic systems in brains of C57Bl/6J and 129P3/J mice: strain and heroin effects. Pharmacol. Biochem. Behav. 2011;100:8–16. doi: 10.1016/j.pbb.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levran O, et al. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, et al. The OPRD1 and OPRK1 loci in alcohol or drug dependence: OPRD1 variation modulates substance dependence risk. Mol. Psychiatry. 2008;13:531–543. doi: 10.1038/sj.mp.4002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edenberg HJ, et al. A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum. Mol. Genet. 2008;17:1783–1789. doi: 10.1093/hmg/ddn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park SW, et al. Retinoic acid-induced chromatin remodeling of mouse kappa opioid receptor gene. J Neurosci. 2005;25:3350–3357. doi: 10.1523/JNEUROSCI.0186-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park SW, et al. Epigenetic regulation of kappa opioid receptor gene in neuronal differentiation. Neuroscience. 2008;151:1034–1041. doi: 10.1016/j.neuroscience.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taqi MM, et al. Prodynorphin CpG-SNPs associated with alcohol dependence: elevated methylation in the brain of human alcoholics. Addict Biol. 2011;16:499–509. doi: 10.1111/j.1369-1600.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuferov V, et al. Tissue-specific DNA methylation of the human prodynorphin gene in post-mortem brain tissues and PBMCs. Pharmacogenet. Genomics. 2011;21:185–196. doi: 10.1097/FPC.0b013e32833eecbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho MK, et al. Breaking barriers in the genomics and pharmacogenetics of drug addiction. Clin. Pharmacol. Ther. 2010;88:779–791. doi: 10.1038/clpt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glick SD, et al. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- 83.Negus SS, et al. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1997;282:44–55. [PubMed] [Google Scholar]
- 84.Schenk S, et al. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 85.Walsh SL, et al. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. Journal of Pharmacology and Experimental Therapeutics. 2001;299:147–158. [PubMed] [Google Scholar]
- 86.Gold MS, et al. Efficacy of clonidine in opiate withdrawal: a study of thirty patients. Drug Alcohol Depend. 1980;6:201–208. doi: 10.1016/0376-8716(80)90323-3. [DOI] [PubMed] [Google Scholar]
- 87.Bailey A, et al. Downregulation of kappa-opioid receptors in basolateral amygdala and septum of rats withdrawn for 14 days from an escalating dose “binge” cocaine administration paradigm. Synapse. 2007;61:820–826. doi: 10.1002/syn.20436. [DOI] [PubMed] [Google Scholar]
- 88.Broadbear JH, et al. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 89.Ko MC, et al. Ultra-long antagonism of kappa opioid agonist-induced diuresis by intracisternal nor-binaltorphimine in monkeys. Brain Res. 2003;982:38–44. doi: 10.1016/s0006-8993(03)02938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melief EJ, et al. Duration of Action of a Broad Range of Selective Kappa Opioid Receptor Antagonists is Positively Correlated with c-Jun N-Terminal Kinase-1 Activation. Mol. Pharmacol. 2011;80:920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grimwood S, et al. Pharmacological characterization of 2-methyl-N-((2'-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine (PF-04455242), a high-affinity antagonist selective for kappa opioid receptors. Journal of Pharmacology and Experimental Therapeutics. 2011 doi: 10.1124/jpet.111.185108. [DOI] [PubMed] [Google Scholar]
- 92.Kreek MJ, et al. Pharmacotherapy of addictions. Nat. Rev. Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 93.Bart G, et al. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology. 2005;30:2254–2262. doi: 10.1038/sj.npp.1300811. [DOI] [PubMed] [Google Scholar]
- 94.Wentland MP, et al. Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett. 2009;19:2289–2294. doi: 10.1016/j.bmcl.2009.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang B, et al. Synthesis and binding affinity of novel mono- and bivalent morphinan ligands for kappa, mu, and delta opioid receptors. Bioorg Med Chem. 2011;19:2808–2816. doi: 10.1016/j.bmc.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lovell KM, et al. Synthesis of neoclerodane diterpenes and their pharmacological effects. Top. Curr. Chem. 2011;299:141–185. doi: 10.1007/128_2010_82. [DOI] [PubMed] [Google Scholar]
- 97.Wu H, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012 doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuferov V, et al. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- 99.Bart G, et al. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol. Psychiatry. 2004;9:547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Logrip ML, et al. Stress modulation of drug self-administration: Implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bidlack JM, et al. Partial opioids. Medications for the treatment of pain and drug abuse. Ann N Y Acad Sci. 2000;909:1–11. doi: 10.1111/j.1749-6632.2000.tb06672.x. [DOI] [PubMed] [Google Scholar]
- 102.Nielsen DA, et al. Genotype patterns that contribute to increased risk for or protection from developing heroin addiction. Mol. Psychiatry. 2008;13:417–428. doi: 10.1038/sj.mp.4002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crystal HA, et al. A C17T polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African American women. Addict. Biol. 2012;17:181–191. doi: 10.1111/j.1369-1600.2010.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bart G, et al. Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- 105.Proudnikov D, et al. Association of Polymorphisms of the Mu Opioid Receptor Gene with the Severity of HIV Infection and Response to HIV Treatment. J Infect Dis. 2012 doi: 10.1093/infdis/jis264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang D, et al. Effect of mu-opioid receptor gene polymorphisms on heroin-induced subjective responses in a Chinese population. Biol. Psychiatry. 2007;61:1244–1251. doi: 10.1016/j.biopsych.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 107.Manglik A, et al. Crystal structure of the mu-opioid receptor bound to a morphinan antagonist. Nature. 2012 doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]