Tobacco addiction is the most significant preventable cause of morbidity and mortality in the Western world, with over 430,000 deaths annually from tobacco-related disease in the United States [1]. While effective treatments are available for smoking cessation (e.g. nicotine replacement therapies, sustained-release bupropion and varenicline), they do not work for all smokers. Thus, the development of more effective medications for smoking cessation based on novel mechanisms is a high priority [2]. This article reviews the links between smoking and monoamine oxidase inhibition towards the development of novel tobacco pharmacotherapies.
Monoamine oxidases (MAO's) are enzymes involved in the catabolism of monoamine neurotransmitters such as dopamine, serotonin and norephinephrine [3]. Interestingly, cigarette smoking produces inhibition of brain MAO activity in humans. Since monoamines are involved in the reinforcing and rewarding effects of tobacco smoking, MAO inhibitors have been proposed as a treatment for tobacco dependence [4-7] .
Monoamine-releasing neurons are stimulated by nicotine from cigarette smoke via activation of pre-synaptic nicotinic acetylcholine receptors (nAChRs). These receptors are widespread in the central nervous system, and are of two general types: low-affinity (e.g. α7) and high-affinity (e.g., α4β2) nAChRs. Activation of these nAChR subtypes lead to influx of Na+ and Ca2+ into neurons, which produces neuronal membrane depolarization and neurotransmitter release [8, 9].
MAO's are flavin adenosine dinucleotide (FAD) co-factor dependent enzymes which are located on the outer membranes of mitochondria. There are two well-characterized isoenzymes: MAO-A, which predominately metabolizes serotonin and norepinephrine and MAO-B, which preferentially metabolizes benzylamine and phenylethylamine [3]. Dopamine and tyramine are metabolized by both isoforms. MAO's catalyze the oxidative deamination of these monoamines. There is considerable overlap of substrate and inhibitors between the two isoforms of MAO.
There are several clinically available subtype-specific MAO inhibitors (MAOI's). Inhibitors targeting the MAO-A subtype typically have antidepressant actions, and include irreversible inhibitors such as phenylzine, tranylcypromine and clorgyline, and the reversible MAO-A inhibitor moclobemide. Non-selective inhibition of MAOs leads to the so-called “cheese effect”, due to accumulation of the dietary monoamine tyramine (which is metabolized by both MAO isoforms) secondary to gastrointestinal tract enzyme inactivation. The typical clinical signs are severe headache, dizziness and hypertension, in what is often referred to as an MAOI-related “hypertensive crisis”. Patients who take these drugs need to avoid foods with high levels of tyramine such as red wine, anchovies and aged cheeses. This phenomenon is negligible with selective (reversible) MAO inhibitors such as moclobemide because of the rapid recover of the MAO enzyme which allows tyramine to be degraded, and therefore reversible inhibitors do not require adherence to MAOI diets. The MAO-B subtype inhibitors include agents such as the irreversible agents selegiline (L-Deprenyl), paragyline and rasagiline, and the reversible inhibitor labazemide. Selegiline, while predominantly an MAO-B inhibitor (at doses ≤10 mg/day), produces inhibition of the A isoform at higher doses (≥20 mg/day) and requires adherence to an MAO diet. Since selegiline is non-selective for MAO subtypes at these higher doses, it also produces antidepressant effects. A transdermal formulation of selegiline (Ensam™) has been approved for the treatment of depression in the United States [10].
There has been a considerable amount of work on the genetics of MAO-A and B (reviewed in [11]), and two known genetic polymorphisms in MAO-A and B genes may influence smoking behavior: a variable number of tandem repeats (VNTR) polymorphism in the promoter region of MAO-A, and a single nucleotide polymorphism (SNP) in the MAO-B gene's coding region comprised of an A→ G transition, which leads to lower enzyme activity, and increased levels of synaptic monoamine concentrations [12]. However, only the MAO-A VNTR appears to have functional consequences for smoking behavior. Interestingly, nicotine dependence scores are higher in males with the 4-repeat allele (Fagerstrom scores of 5.8 versus 4.7 respectively), but in females, the presence of this 4-repeat allele reduces the risk of being a smoker [12]. Clearly, more study of the influence of MAO genetic polymorphisms on smoking behavior is needed.
Besides MAO isoforms, other potential molecular targets of MAOI's should be considered. For example, it is known that at low concentrations, selegiline can inhibit key mitochondrial enzymes such as nitric oxide synthase, cytochrome oxidase IV, as well as mitochondrial hydrogen peroxide production and Ca2+-induced mitochondrial permeability [13]. Moreover, selegiline prevents apoptotic cell death [14] and inhibits the liver metabolic enzyme CYP 2A6, which is involved in the metabolism of nicotine (R. Tyndale, personal communication). It is not clear however if these additional actions contribute to anti-smoking effects of selegiline and other MAOI's.
Several clinical and neuroimaging reports suggest that cigarette smoke contains components which inhibit both MAO isoforms. Human ex vivo studies have suggested that smokers have reduced levels of platelet MAO-A and -B activity as compared to non-smokers [15-17]. Consistent with these results, Positron Emission Tomography (PET) studies using labeled clorgyline and selegiline have shown that binding of these ligands to brain and peripheral organs is reduced in smokers versus non-smokers [18, 19]. Both clinical studies assaying platelet MAO inhibition and PET imaging studies suggest that this inhibition persists for greater than 30 days, consistent with the irreversible inhibition produced by these agents [15, 20]. This inhibition of MAO by smoking is not related to the direct effects of nicotine. Interestingly, in vitro studies have identified several components, namely the alkaloids harman (a specific competitive MAO-A inhibitor; IC50=0.34 μM) and norharman (non-specific competitive MAO-A and B inhibitor, with IC50's of 6.5 and 4.7 μM respectively) as contributing to the inhibitory effects of tobacco smoke on MAO isoforms [21]. Besides harman alkaloids, other components of tobacco smoke may also inhibit MAO-A and –B [22, 23].
Since it has been observed that: 1) MAO inhibition leads to increases in synaptic monoamines which are also increased by nAChR activation; 2) cigarette smoke possesses components which inhibit MAO isoforms, we (and others) have reasoned that MAO inhibitors may be promising candidates for developing medications to aid smokers with tobacco cessation.
The first proof-of-concept study to suggest that MAO inhibitors might be useful for the treatment of tobacco addiction was a clinical trial by investigators in France [4] who conducted a placebo-controlled evaluation of the reversible MAO-A inhibitor moclobemide (up to 400 mg/day). Participants were randomized to either placebo (n=44) or moclobemide (n=44; 400 mg/day for two months and 200 mg/day for the third month) and abstinence was assessed at six months (“end of trial”) and one year after quit date. Self-reported abstinence rates were higher in the moclobemide group than the placebo group (p<0.05 at end of trial, p=0.09 at 1-year follow-up); however, biochemically-verified smoking abstinence rates (using the nicotine metabolite cotinine in plasma) were not significantly different at either trial endpoint (p=0.12) or the 1-year follow-up (p=0.13). There were no differences between the moclobemide and placebo groups in weight gain or cigarette cravings over the course of the trial.
MAO-B inhibitors such as selegiline have also been tested for their potential as smoking cessation agents. A human laboratory study by Houtsmuller and colleagues from Johns Hopkins University [7] found that the “carbon monoxide (CO) boost”– the increase in the inhalation of the smoking combustion product CO by acute cigarette smoking under controlled conditions – was reduced by oral selegiline (10 mg PO) as compared to placebo in n=15 subjects who received both selegiline and placebo using a double-blind, randomized, within-subjects counterbalanced study design. Selegiline also decreased self-reported cravings and decreased interference of smoking-related words during a modified Stroop test as compared to placebo. Subsequently, George and colleagues [6] at Yale University studied selegiline (10 mg; 5 mg bid) compared to placebo during an eight week randomized, double-blind clinical trial of n=40 nicotine dependent smokers. At the end of 8 weeks (“end of trial” - 6 weeks after the quit date), 45% of smokers treated with selegiline achieved biochemically-verified smoking abstinence, with a comparable figure of 15% for the placebo group (p<0.05). Cessation rates were reduced at the 6 month (Week 26) follow-up assessment, with point prevalence cessation rates of 20 versus 5% (p=0.18). During the trial, treatment with selegiline versus placebo was found to reduce the positive aspects of cigarette smoking (p=0.09). A second trial of selegiline combined with nicotine transdermal patch by Biberman and colleagues in Israel compared to placebo plus patch [5] found continuous abstinence rates at one year were 25 versus 11% respectively (n=108; p=0.08). Participants receiving selegiline plus patch reported lower levels of cravings one week after the target quit date than those receiving patch and placebo (p<0.05) and there were no significant differences between the groups on weight gain or adverse events. Finally, a placebo-controlled multi-site evaluation of the reversible MAO-B inhibitor lazabemide (100 and 200 mg/day) in N=330 French cigarette smokers [24] found that this agent dose-dependently increased cessation rates compared to placebo with significant effects on point prevalence abstinence at trial endpoint for the 200 mg/day dose compared to placebo (30% and 17% respectively, p=0.01). However, this clinical trial was discontinued prior to completion and this agent did not reach the market since Phase III trials for other indications suggested substantial liver toxicity.
Overall, these clinical studies suggested the potential of MAO-A and B inhibitors for the development of tobacco cessation pharmacotherapies. Larger NIH-funded trials with selegiline with both the oral and transdermal formulations for smoking cessation are in progress, and several pharmaceutical companies are developing highly selective inhibitors of MAO isoforms for smoking cessation.
In conclusion, the finding that cigarette smoking is associated with inhibition of both MAO subtypes has lead to the conceptualization that treatment of smokers with MAO subtype-selective inhibitors may represent a novel class of medications to be developed as aids to behavioral therapies for smoking cessation. Initial proof of concept studies in human smokers support the further development of these agents. Given evidence for genetic variation in both MAO-A and –B genes which may alter the function of these enzymes, there will be considerable interest in the application of pharmacogenetic methods to tailoring treatment of smokers to agents which target these MAO isoforms. What is not clear is whether other molecular sites of action of MAOI's may contribute to the anti-smoking effects of these agents. Potential mechanisms to explain the actions of MAO inhibitors in tobacco dependence are depicted in Figure 1. Since the majority of smokers who attempt to quit smoking using existing pharmacotherapies (e.g. nicotine replacement therapies, bupropion and varenicline) are unsuccessful in the long-term, the development of novel smoking cessation pharmacotherapies is of great importance [25]. Therefore, the strategy of exploiting MAO inhibition for the treatment of tobacco dependence derives from a rational series of conceptualizations and empirical studies, which could translate these important basic and clinical research findings into improved pharmacological treatments for tobacco addiction.
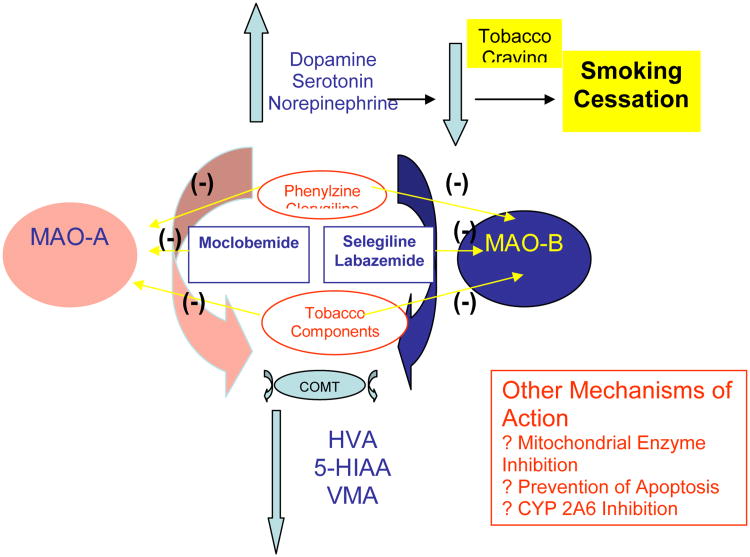
Figure 1. Mechanism of Action of MAO Inhibitors in the Treatment of Tobacco Dependence.
The diagram depicts the effects of MAO subtype inhibition on central monoamine levels. Inhibition of monoamine metabolism by MAO inhibitors leads to increased synaptic levels of dopamine, serotonin and norepinephrine. There is also a compensatory decrease in the respective monoamine metabolites (HVA, 5-HIAA and VMA), which are produced by the sequential actions of MAO and COMT. Overall, the increase in monoamine levels is hypothesized to lead to a decrease in nicotine and tobacco craving and a reduction in the drive to smoke. This process facilitates smoking cessation. Other potential targets of MAO inhibitors are also listed in the box.
Abbreviations
- MAO
monoamine oxidase
- COMT
Catechol-O-methyltransferase
- HVA
homovanillic acid
- 5-HIAA
5-hydroxyindoleacetic acid
- VMA
3-methoxy-4-hydroxymandelic acid
Footnotes
This work was supported in part by grants from the National Institute on Drug Abuse (K02-DA-16611 and R01-DA-15757 to T.P.G. and K12-DA-00167 to A.H.W.).
References
- 1.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 2.George TP, O'Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci. 2004;25(1):42–48. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. NeuroToxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Berlin I, Said S, Spreux-Varoquaux O, Launay JM, Olivares R, Millet V, et al. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clin Pharmacol Ther. 1995;58:444–452. doi: 10.1016/0009-9236(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 5.Biberman R, Neumann R, Gerber Y. A randomized controlled trial of oral selegiline plus nicotine skin patch compared with placebo plus nicotine skin patch for smoking cessation. Addiction. 2003;98:1403–1407. doi: 10.1046/j.1360-0443.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 6.George TP, Vessicchio JC, Termine A, Jatlow PI, Kosten TR, O'Malley SS. A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biological Psychiatry. 2003;53:136–143. doi: 10.1016/s0006-3223(02)01454-3. [DOI] [PubMed] [Google Scholar]
- 7.Houtsmuller EJ, Thornton JA, Stitzer ML. Effects of selegiline (L-deprenyl) during smoking and short-term abstinence. Psychopharmacology. 2002;163:213–220. doi: 10.1007/s00213-002-1152-9. [DOI] [PubMed] [Google Scholar]
- 8.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 9.McGehee DS, Iacoviello M, Mitchum R. Cellular and synaptic effects of nicotine (Chapter 3) In: George TP, Weinberger AH, editors. Medication Treatments for Nicotine Dependence. Taylor and Francis; Boca Raton, FL: 2006. [Google Scholar]
- 10.Bodkin JA, Amsterdam JD. Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am J Psychiatry. 2002;159:1869–1875. doi: 10.1176/appi.ajp.159.11.1869. [DOI] [PubMed] [Google Scholar]
- 11.Shih JC, Chen K. Regulation of MAO-A and MAO-B gene expression. Curr Med Chem. 2004;11:1995–2005. doi: 10.2174/0929867043364757. [DOI] [PubMed] [Google Scholar]
- 12.Ito H, Hamajima N, Matsuo K, Okuma K, Sato S, Ueda R, Tajima K. Monoamine oxidase polymorphisms and smoking behaviour in Japanese. Pharmacogenetics. 2003;13:73–79. doi: 10.1097/00008571-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Czerniczyniec A, Bustamante J, Lores-Arnaiz S. Modulation of brain mitochondrial function by deprenyl. Neurochem Int. 2006;48:235–241. doi: 10.1016/j.neuint.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Palfi M, Szoko E, Kalman M. Molecular mechanisms of the neuroprotective effect of (-)deprenyl. Orv Hetil. 2006;147:1251–1257. [PubMed] [Google Scholar]
- 15.Berlin I, Said S, Spreux-Varoquaux O, Olivares R, Launay JM, Puech AJ. Monoamine oxidase activities in heavy smokers. Biol Psychiatry. 1995a;38:756–761. doi: 10.1016/0006-3223(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 16.Berlin I, Spreux-Varoquaux O, Launay JM. Platelet monoamine oxidase activity is inversely associated with plasma cotinine concentration. Nicotine & Tobacco Research. 2000;2:243–246. doi: 10.1080/14622200050147501. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert DG, Zuo Y, Browning RA, Shaw TM, Rabinovich NE, Gilbert-Johnson AM, et al. Platelet monoamine oxidase B activity changes across 31 days of smoking abstinence. Nicotine & Tobacco Res. 2003;5:813–819. doi: 10.1080/14622200310001614575. [DOI] [PubMed] [Google Scholar]
- 18.Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor RR, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996a;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- 19.Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, et al. Brain monoamine oxidase A: inhibition by cigarette smoke. Proc Natl Acad Sci USA. 1996b;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. NeuroToxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 21.Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Comm. 2005;326:378–386. doi: 10.1016/j.bbrc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Hauptman N, Shih JC. 2-Napthylamine, a compound found in cigarette smoke, decreases both monoamine oxidase A and B catalytic activity. Life Sciences. 2001;68:1231–1241. doi: 10.1016/s0024-3205(00)01022-5. [DOI] [PubMed] [Google Scholar]
- 23.Khalil AA, Steyn S, Castagnoli NJ. Isolation and characterization of a monoamine oxidase inhibitor from tobacco leaves. Chem Res Toxicol. 2000;13:31–35. doi: 10.1021/tx990146f. [DOI] [PubMed] [Google Scholar]
- 24.Berlin I, Aubin HJ, Pedarrriosse AM, Rames A, Lancrenon S, Lagrue G, Lazabemide Smoking Cessation Study Investigators Lazabemide, a selective, reversible monoamine oxidase B inhibitor, as an aid to smoking cessation. Addiction. 2002;97:1347–1354. doi: 10.1046/j.1360-0443.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 25.George TP. Medication treatments for nicotine dependence. 1st. Boca Raton, FL; Taylor and Francis: 2006. p. 327. [Google Scholar]