Abstract
Fibroblasts and smooth muscle cells (FSMCs) are principal cell types of connective and adventitial tissues that participate in the development, physiology and pathology of internal organs, with incompletely defined cellular origins. Here, we identify and prospectively isolate from mesothelium a mouse cell lineage that is committed to FSMCs. Mesothelium is an epithelial monolayer covering the vertebrate thoracic and abdominal cavities and internal organs. Time-lapse imaging and transplantation experiments reveal robust generation of FSMCs from the mesothelium. By targeting Mesothelin (MSLN), a surface marker expressed on mesothelial cells, we identify and isolate precursors capable of clonally generating FSMCs. Using a genetic lineage tracing approach, we show that embryonic and adult mesothelium represents a common lineage to trunk FSMCs, and trunk vasculature, with minimal contributions from neural crest, or circulating cells. The isolation of FSMC precursors enables examination of multiple aspects of smooth muscle and fibroblast biology as well as the prospective isolation of these precursors for potential regenerative medicine purposes.
Keywords: epithelial mesenchymal transition, EMT, fibroblast, mesenchyme, mesothelin, mesothelium, stem cell, smooth muscle
Introduction
Fibroblasts and smooth muscle cells (FSMCs) undertake diverse cellular functions during embryonic development and in steady state adult tissues and organs. Morphologically, they are often defined as elongated, spindle-shaped cells that adhere to and migrate over tissue culture substrates. During the development of the internal organs, and their vasculature, FSMCs are the predominant cell types within both stroma and the vasculature’s tunica media and adventitia that synthesize and remodel the extra cellular matrix (ECM), becoming relatively quiescent in adult stages1. Activation of FSMCs, however regulated can impede organ function. As an outcome, FSMCs are the principal cell types that can accumulate in diverse medical conditions, including tissue and organ fibrosis, atherosclerosis, and formation of atheromatous plaque after blood vessel injury2,3. FSMCs may also contribute to cancer progression by contributing to the tumor stroma4,5, a finding that could implicate FSMCs as an important target for anti-cancer therapy6. Based on these similarities in morphology and function, fibroblasts and smooth muscle cells have been proposed to arise from a common lineage7.
Central to our understanding of FSMCs is the question of their origin. Several sources have been proposed for FSMCs of the adult thoracic and abdominal [coelomic] cavities and internal organs. The bone marrow, including hematopoietic stem cells [HSC], were initially presumed to contribute to FSMCs8,9, and to continuously replenish the mesenchymal pool as part of normal tissue homeostasis10; however, we have shown that HSCs in a variety of tissues only give rise to blood cells and platelets11. The embryonic neural crest was thought to contribute to trunk mesenchyme within the internal organs, including smooth muscle12. FSMCs have also been speculated to arise locally from tissue-resident or bone marrow mesenchymal cells13, often mis-characterized as mesenchymal stem cells. However, this idea has largely resulted from the apparent heterogeneity of cultured FSMCs isolated from diverse tissues or from sub-regions within a tissue14.
Another potential source for FSMCs within internal organs is the mesothelium, an epithelial monolayer that lines the vertebrate’s coelomic cavities and internal organs15. The mesothelium provides a non-adhesive layer that facilitates the frictionless movements of organs within the coelomic cavity through the secretion and entrapment of phospholipids via abundant microvilli present on the serosal side. The mesothelium also protects the serosal surfaces from infection, and tumor dissemination16. By synthesizing/secreting a range of cytokines, chemokines and growth factors, the mesothelium reportedly functions in controlling fluid and solute transport, regulation of inflammation, hematopoiesis and wound healing17–20.
Here we set out to explore the developmental potential of the mouse mesothelium, and identified a FSMC precursor within the mesothelium, committed to fibroblasts and smooth muscle of the internal organs, and their vasculature.
Results and Discussion
Derivation of FSMCs from the mesothelium
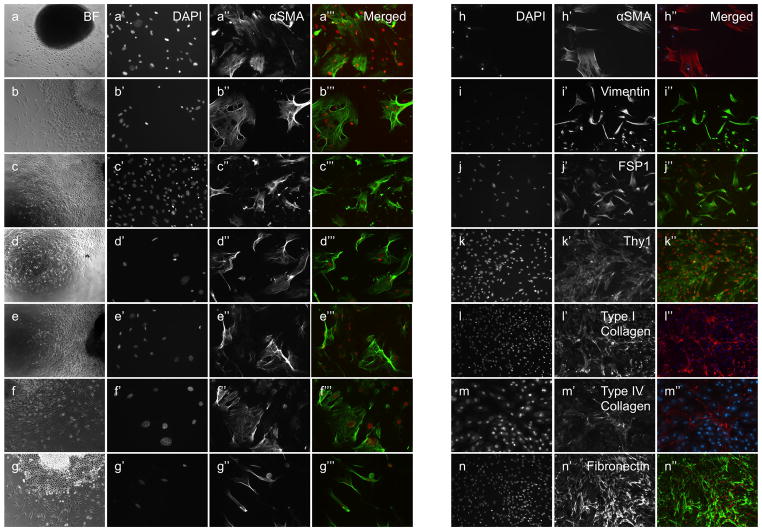
Small patches of mesothelial tissues were removed (see ‘Methods’ section) from the surfaces of the liver, spleen, kidney, lung, intestine, mesentery, diaphragm and peritoneal wall of adult mice, and cultured on tissue culture plates. These patches of tissue were enriched for, but not entirely composed of mesothelial cells. Following the attachments of the mesothelium to the culture plates, fibroblasts/smooth muscle cells (FSMCs) emerged abundantly from all of the tissues’ peripheries (Fig. 1, A–H), and were absent only in regions where attachment of the mesothelium to the culture dish was poor. FSMCs displayed a spindle-shape or a flattened morphology with filopodia/lamellipodia, consistent with a mesenchymal nature (Fig. 1, A‴–G‴) and reached culture confluence within several days. FSMCs from all cultured organs, displayed contractile stress bundles, expressed alpha-Smooth Muscle-Actin protein (αSMA; Fig. 1, A‴–H‴), Vimentin (Fig. 1, I–I″) Fibroblast-Specific Protein-1 (FSP1; Fig. 1, J–J″) and Thy1 protein (CD90; Fig. 1, K–K″), markers that are associated with smooth muscle/fibroblast outcomes (Fig. 1B), as well as Smooth Muscle-Myosin Heavy Chain II and Smooth Muscle-Myosin Heavy Chain XI within cell subsets, indicating smooth muscle-restricted outcomes (Supplementary Fig. 1). FSMCs and the culture plates on which they where grown immunostained for Type I Collagen (Fig. 1, L–L″), Type IV Collagen (Fig. 1, M–M″) and Fibronectin (Fig. 1, N–N″), showing their ability in-vitro to express and secrete components of the extra-cellular matrix.
Figure 1.
Derivation of FSMCs from cultured mesothelium. Liver (a–a‴), spleen (b–b‴), kidney (c–c‴), lung (d–d‴), intestine (e–e‴), mesentery (f–f‴), diaphragm (g–g‴). Bright field (a–g), nuclear DAPI staining (a′–g′, h–n). Mesothelium-derived cells express α-SMA (a″–g″, h–h″), Vimentin (i–i″), FSP1 (j–j″), CD90 (k–k″), Collagen type I (l–l″), Collagen type IV (m–m″) and Fibronectin (n–n″). Merged images (a‴-g‴, h″−n″, DAPI is red, αSMA is green except h″, l″, m″). Original magnifications ×20 (a–n).
Time-lapse video captured the emergence of FSMCs from cultured mesothelial tissues at their leading edges (Svideo1). Emerging FSMCs displayed a spindle-shape or a flattened morphology, were highly motile and contractile, leading to pulling of the tissue explants along the culture plates (Svideo 2). FSMCs did not exhibit directed movement, but rather sampled the tissue culture plates, continuously changing their direction of migration (Svideo 3).
To test the in-vivo potential of the adult mesothelium to generate FSMCs, small (~1mm2) explants of mesothelium were harvested from adult transgenic mice expressing the enhanced green fluorescent protein under the Actin promoter (Actin-eGFP), from mesentery, peritoneum or kidney. Tissues were then transplanted separately, into adult Rag(−/−) gamma chain(−/−) mice (n=4, to prevent tissue rejection), underneath the mesothelium covering the small intestine, liver or peritoneal wall (see methods). Host mice were sacrificed three months post transplantation and the abdominal cavity was analyzed for any presence of donor-derived cells. GFP+ cells were found along the lower digestive system, liver and peritoneum, in areas remote from the site of transplantation (Fig. 2, A–E). Within the lower digestive system, GFP+ cells with a mesenchymal morphology occupied subepithelial and stromal regions of the digestive system (Fig. 2, F, G). We also found individual GFP+ cells and cell foci along blood vessels’ media and adventitia (Fig. 2G, white arrowheads). In the peritoneum where a small patch of mesothelium tissue was transplanted, individual GFP+ cells were scattered throughout and in-between muscle fibers (Fig. 2H). We did not find any contribution of GFP+cells to the organ parenchyma, including the mesothelium. Instead, GFP+ cells ubiquitously displayed mesenchymal morphologies, and expressed markers associated with FSMCs (Fig. 2, I–I‴, J–J‴).
Figure 2.
Derivation of FSMCs from transplantation of mesothelium in vivo. Representative images of the abdominal cavity, from mice three months post transplantation of mesothelium. Lower digestive system (a–c), liver (d) and peritoneum (e). Sections through the lower digestive system (f, g) and peritoneum (h) showing FSMCs and cell foci along a vessel opening (g, white arrowheads). Immunohistochemistry of Vimentin (i–i‴) and FSP1 (j–j‴), showing co-localization of the FSMCs markers with graft-derived GFP+ cells. Original magnifications: ×10 (a, b, f, i, j), ×20 (c–e, g, h).
Mesothelin (MSLN) is a novel marker of FSMC precursors
Because the mesothelium transplants described above were not a pure population of mesothelial cells, we looked for ways to follow only cells derived from mesothelium. Mesothelin (MSLN) is a 40-kDa membrane glycoprotein that is present on normal mesothelium and is over-expressed in a subset of cells in several human tumors, including mesothelioma21. Antibodies to MSLN protein labeled the mesothelium that covers the internal organs, and the parietal mesothelium, including the diaphragm (Supplementary Fig. 2, A–J).
We used an independent approach to ask whether MSLN expression could be used as a marker for FSMCs precursors by looking at its Boolean relationships22. MSLN message showed significant high-to-high relationships with known FSMC markers (Supplementary Fig. 3A, Supplementary Table 1), implying in silico that MSLN expression is highly associated with a FSMC lineage.
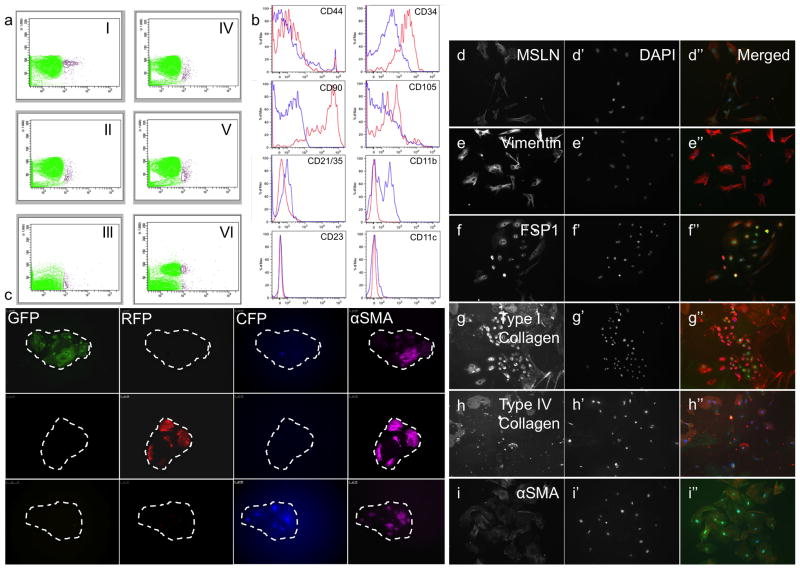
Flow cytometry was then used to isolate FSMC precursors by gating on the absence of Tie2, PECAM-1/CD31 (for endothelial cells), CD45, Ter119 (for blood cells), and presence of MSLN, herein referred to as MSLN+Lin−. A MSLN+Lin− population was present within all adult visceral organs tested (Fig. 3A) and in extremely low numbers within total viable cells (0.2%–0.4%). MSLN+Lin− cells expressed a surface phenotype, that is associated with a mesenchymal nature (Fig. 3B), including Thy1high (CD90), CD34high, CD44low and CD105low, with a mean fluorescent intensity (MFI) of 31,893, 2,294, 52 and 27, respectively. Using flow cytometry, MSLN+Lin− cells were sorted from the internal organs of postnatal day 1 (P1) mice and cultured in-vitro. Cultured MSLN+Lin− cells formed cell foci within several days (Fig. 3C), which expanded throughout subsequent culture days. To determine clonal relationships within these cell foci, 5000 MSLN+Lin− cells were harvested from transgenic mice that stably express separate fluorescent proteins (GFP, RFP, CFP) and mixed prior to culturing. In these tetrachimera cultures, small foci appeared within the culture plates that ubiquitously expressed GFP, RFP or CFP, separately. These outcomes indicate that FSMC foci are monoclonal, hence are derived from expansions of single cells. At the periphery of each focus, cells acquired FSMC morphologies and gradually migrated away from the focus. Subsequently, numerous FSMCs appeared within the culture dish that expressed Mesothelin (Fig. 3, D–D″), Vimentin (Fig. 3, E–E″), FSP1 (Fig. 3, F–F″), Type I Collagen (Fig. 3, G–G″), Type IV Collagen (Fig. 3, H–H″) and αSMA (Fig. 3, I–I″), within all cultured plates.
Figure 3.
Flow cytometry, in-vitro clonal analysis and differentiation of MSLN+Lin− cells. (a) X-axis represents MSLN expression, Y-axis represents side scatter. A population of cells characterized by MSLN+Lin− is present within the heart (I), lung (II), liver (III), peritoneal wall (IV), kidney (V), and thymus (VI). (b) MSLN+Lin− cells express a surface profile associated with a mesenchymal nature. Blue is IgG control, red is antibody. (c) Mixed culture of MSLN+Lin− cells from GFP, RFP and CFP-expressing mice shows that emerging FSMC foci are derived from expansions of single precursors (monoclonal). (d–i) Cultured MSLN+Lin− cells adopt a mesenchymal nature and express MSLN (d), Vimentin (e), FSP1 (f), Type I Collagen (g), Type IV Collagen (h) and αSMA (i) proteins. DAPI (d′–i′). Merged (d″–i″, DAPI in blue, antibody in red). Original magnifications: ×20 (c–i). The overlay of green/red colors within the merged images in g–i, results from spectral overlap of multiple staining per cultured well.
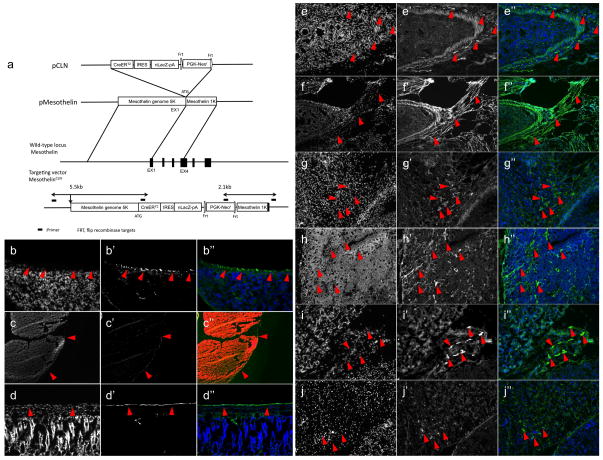
We then knocked into the mouse Mesothelin gene a cassette harboring the CreERT2, nLacZ and the Neomycin resistance constructs (CLN), and created MSLNCLN transgenic mice (Fig. 4A, see ‘Methods’ section). MSLNCLN offspring that were injected with tamoxifen at postnatal day 1 (P1), and sacrificed after 2 days (n=3) showed βgal+ immunostaining within the mesothelium surrounding the lungs, thymus and lower digestive system in a pattern of expression similar to that of MSLN protein (Fig. 4, B–D, red arrowheads). To genetically lineage trace FSMC precursors during embryonic development, MSLNCLN pregnant females where injected with tamoxifen at gestational stage of E10.5 (see materials and methods), sacrificed at gestational stage of E17.5 (n=5), and then processed for histology. Numerous βgal+ fibroblasts were present within the GI tract’s outer serosa and muscular layers, mesentery, thymus and liver (Fig. 4, E–J, red arrowheads). The dermis from cranial, limb and thoracic (dorsal and ventral) regions lacked βgal label (YR, personal observations), in agreement with separate, distinct embryonic origins for these tissue fibroblasts23–25.
Figure 4.
Genetic lineage tracing of fibroblasts within internal organs. (a) Scheme illustrating the transgenic strategy. The CreERT2IRES-lacZ-PGK-neo cassette (pCLN) was introduced into a cassette harboring 6.3-kilobase of the mouse Mesothelin gene (pMesothelin). The Mesothelin-CreERT2-IRES-lacZ construct was subsequently transfected into mouse embryonic stem cells. Selected clones were then injected into C57BL/6 blastocysts following the standard protocol to generate chimeras, and by mating these, MSLNCLN mice. (b–d) βgal staining on sections from MSLNCLN mice, following 2 days post tamoxifen injection. Staining is present within the mesothelium covering the lungs (b, red arrowheads), thymus (c, red arrowheads), and lower digestive system (d, red arrowheads). (e–j) MSLNCLN mice were injected with tamoxifen at e10.5 and analyzed at e17.5. βgal staining is present within fibroblasts in the lower digestive system (e, f, red arrowheads), mesentery (g, red arrowheads), thymus (h, red arrowheads), parathyroid gland (i, red arrowheads) and liver (j, red arrowheads). Original magnifications: ×4 (c), ×20 (b, d–j),
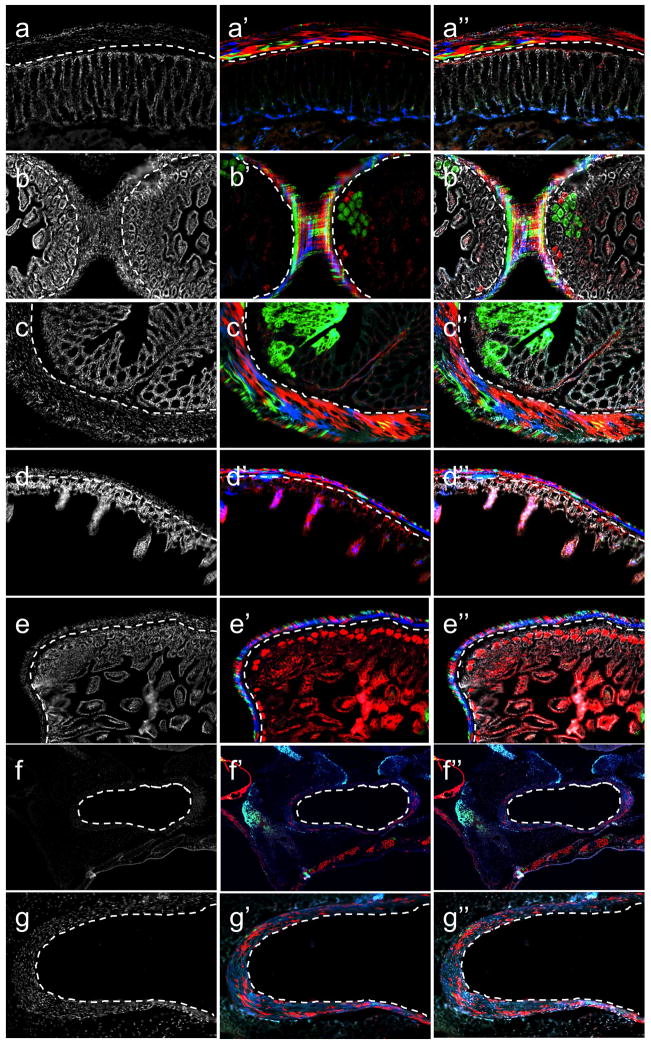
The smooth muscle layer of the lower gastrointestinal system was entirely βgal+ (Fig. 5, A–J). Quantification of βgal label indicated 98%-100% label within the GI tract’s smooth muscle layer that co-localized with αSMA protein expression (Fig. 5, K–K‴). βgal staining (and co-localization with αSMA) was also evident within the submucosal layer of the urinary bladder (Fig. 5, L–L‴), pulmonary arteries (Fig. 5, H, M–M‴), major blood vessels entering the thymus (Fig. 5J, red arrowhead), mesenteric vessels (Fig. 5, N–N‴, red arrowheads), renal arteries and blood vessels (Fig. 5, F, O–O‴, red arrowheads) and within hepatic arteries (Fig. 5, P–P‴, red arrowheads).
Figure 5.
Genetic lineage tracing of smooth muscle within internal organs, and its vasculature. βgal (a–j, in green) staining is present within the muscular layer of the duodenum (a, b), colon (c) and stomach (d), urinary bladder (e) and ureter (f). βgal staining within blood vessels of the mesentery (g), lungs (h), liver (i, red arrowhead) and thymus (j, red arrowhead). Note βgal staining within lung mesothelium (h, red arrowhead). Co-expression of βgal with αSMA protein in the duodenum (k–k‴), urinary bladder (l–l‴), pulmonary vasculature (m–m‴), mesentery vasculature (n–n‴, red arrowheads), renal vasculature (o–o‴, red arrowhead) and liver vasculature (p–p‴, red arrowheads). Nuclear DAPI staining (k–p), βgal staining (k′–p′), αSMA staining (k″–p″), merged images (k‴–p‴, βgal staining in green, αSMA staining in red). Dotted lines outline the smooth muscle layer within the urinary bladder (l–l‴) and pulmonary vasculature (m–m‴). Original magnifications: ×10 (a–p).
We then analyzed the clonal makeup of trunk smooth muscle by generating tetrachimeric mice, made by injection of mouse embryonic stem cells that stably express separate fluorescent proteins (GFP-mES, RFP-mES, CFP-mES) into wild-type blastocysts that were then implanted into pregnant females26. Tetrachimeric mice (n=3) were sacrificed at postnatal stages of development, at which time internal organs, including their vasculature were harvested, sectioned and the derived fluorescent patterns were analyzed. We found a polyclonal pattern, with multiple, separate clones (of the same color) occupying the muscular layer of the stomach, small and large intestines (Fig. 6, A–E). Each of the patchy clones shared, to some extent, the same color with a patch of overlying mesothelium, indicating that the mesothelial lining includes self-renewing cells, perhaps stem cells. In the smooth muscle linings of the blood vessels we found a similar polyclonal pattern, with multiple clones occupying the media and adventitia of the blood vessels (Fig. 6, F–G). Thus, multiple FSMCs most likely seed and contribute cumulatively to the smooth muscle layers within the internal organs and their vasculature. We then lineage traced MSLN+ precursors during the 1st postnatal month, a period associated with active organ growth and enlargement. Mice that received tamoxifen at postnatal day 1 (P1), and sacrificed following 30 days from injections (n=3) showed substantial βgal stain within the smooth muscle layers of the GI tract. Quantification of βgal label revealed 60–74% staining within the GI tract’s smooth muscle layers, indicating that a significant investment to smooth muscle takes place during this stage. βgal also stained the smooth muscle layer of the urinary bladder in patches (Fig. 7, A, B). Within the liver, heart/lungs and thymus, βgal stained their mesothelial lining (Fig. 7, C–E), and their vasculature’s smooth muscle layer (Fig. 7F). We then lineage traced individual MSLN+ cells within the 1st postnatal month by crossing MSLNCLN mice to ‘Rainbow’ mice21, a multicolor Cre-dependent marker system that harbors a four-color reporter construct within the ROSA locus (red, yellow, green, blue; R26VT2/GK3). MSLNCLN; R26VT2/GK3 offspring at P1 of age were injected with tamoxifen and sacrificed following one-month (n=3). The analyses that followed indicate that multiple clones contribute cumulatively to the expanding smooth muscle layers during the 1st postnatal month. The smooth muscle layers surrounding the intestine, stomach and urinary bladder (Fig. 7, G–I) included multiple new clones of smooth muscle, in agreement with the tetrachimera analysis. A substantial investment of smooth muscle was also noted within the internal vasculature including renal and mesenteric arteries (Fig. 7, J, K) in which a similar polyclonal pattern emerged. In both instances, we failed to note a similar contribution to interstitial fibroblasts in these organs during the 1st postnatal month.
Figure 6.
Polyclonal origins for smooth muscle revealed by clonal analysis of tetrachimeric mice. Sections through jejunum (a–a″), colon (b–b″, c–c″), duodenum (d–d″), cecum (e–e″), cardiovascular (f–f″) and pulmonary artery (g–g″). Nuclear DAPI staining (a–g), tetrachimera image (a′–g′), merged image (a″–g″). Original magnifications: x4 (f), x10 (a–e, g). The dotted lines in each figure (a–e) are between the epithelial base of the intestine and the overlying serosal layer, including the mesothelium and the underlying, mainly circumferential smooth muscle. In many places, e.g. in b, one can see the clones extending from mesentery to serosal mesothelium; a subset of the overlying mesothelial clone is continuous with a larger patch of smooth muscle, while other mesothelial cells have different color smooth muscle under them. As noted previously (26), the epithelial crypts are always a single color, and up to dozens of adjacent crypts are derived from a single intestinal stem cell.
Figure 7.
Polyclonal contributions of MSLN+ precursors to smooth muscle during the 1st postnatal month. Sections through the intestine (a–a″), urinary bladder (b–b″), liver (c–c″), heart atrium (d–d″), thymus (e–e″) and lungs (f–f″) from MSLNCLN transgenic mice. Significant contribution of MSLN+ precursors is evident in the smooth muscle layers of the GI-tract and urinary bladder, as well as trunk vasculature. Sections through the intestine (g–g″), stomach (h–h″), urinary bladder (i–i″), mesenteric vasculature (j–j″) and renal vasculature (k–k″) from MSLNCLNR26VT2/GK3 transgenic mice. Multiple MSLN+ precursors contribute cumulatively to the postnatal growth of smooth muscle. Original magnifications: ×10 (a–k).
To analyze the contributions of MSLN+ precursors to the maintenance of the internal organs and their vasculature, tamoxifen was injected into MSLNCLN mice at P30 and followed for up to 4-months (n=3), at which time mice were sacrificed and the internal organs were processed for βgal staining. Even though the duration of lineage tracing was 4-times as long, we found significantly less contributions to trunk smooth muscle at these adult stages. MSLN+ precursors mainly contributed to the smooth muscle layers of the stomach, with some patchy contributions to the intestinal smooth muscle (Supplementary Fig. 4). Quantifications of βgal label indicated 19–23% investment into the smooth muscle at this stage, collectively demonstrating that the smooth muscle of the GI tract continuously undergoes cellular maintenance throughout postnatal and adult life. We did not find any significant contributions to trunk vasculature or interstitial fibroblasts at these adult stages.
We then analyzed the response of MSLN+ precursors to organ damage by exposing MSLNCLN mice to sublethal doses of whole-body irradiation (n=4), which causes acute injury to the GI tract and the hematopoietic system27. Mice were sacrificed after 1 month (to allow the cumulative contributions of MSLN+ precursors [early and late] triggered by the injury response, at which time organs were sectioned and analyzed for βgal staining. We found substantial contributions to the smooth-muscle layers of the stomach, intestine and urinary bladder (Fig. 8, A–C) that co-localized with αSMA protein in these sites.
Figure 8.
Developmental restriction of MSLN+ precursors to FSMCs following sub-lethal irradiation. MSLNCLN transgenic mice received tamoxifen followed by a single dose of whole-body irradiation at 650 rads. Sections through the stomach (a–a″), intestine (b–b″), urinary bladder (c–c″), uterine horns (d–d″), seminal vesicle (e–e″), thymus (f–f″) and lungs (g–g″). Co-staining of beta-galactosidase with αSMA in the intestine (h–h″), stomach (i–i″), urinary bladder (j–j″), seminal vesicle (k–k″) and renal vasculature (l–l″). Original magnifications: ×10 (a–k), x20 (l).
We also noticed a substantial contribution to interstitial fibroblasts of the intestine following the injury response (Fig. 8, B, H). Within the female reproductive system, we found a significant contribution to the smooth muscle layers of the uterine horns (Fig. 8D). Within the male reproductive system, we found contributions to the interstitial fibroblasts of the seminal vesicle. MSLN+ derived cells were also found to contribute to the thymic mesenchyme (Fig. 8, E, F, respectively). We found no significant contribution to heart fibroblasts or to heart, lung and mesenteric vessels’ smooth muscle. These results indicate that MSLN+ precursors respond locally rather then uniformly to whole-body irradiation, with the most significant response within the urogenital and GI tract (Supplementary Fig. 5, A–D). We found no evidence of contributions to other germ layers or to other mesoderm lineages, including the organ’s parenchyma following the injury. Instead, MSLN+ precursors retained fate-restriction to FSMCs, co-staining with αSMA (Fig. 8, H–L) within these sites.
Negligible contributions to trunk FSMCs from embryonic neural crest or circulating cells
We then analyzed the contribution of the embryonic neural crest to the developing trunk, using the Wnt1Cre transgenic mouse, which permanently labels early migratory neural crest populations at all axial levels excluding the forebrain28. Wnt1Cre transgenic mice were crossed with R26mTmG, a double-fluorescent reporter mouse that replaces the expression of tomato red with green fluorescent protein (GFP) after Cre-mediated excision29. Wnt1Cre;R26mTmG offspring were sacrificed at postnatal stages (n=6), at which time internal organs including their vasculature were harvested, sectioned and the localizations of GFP+ cells were analyzed. Within the digestive and urogenital systems, we found numerous GFP+ cells within the outer serosa and inner muscular layers. However, GFP+ cells did not exhibit the ubiquitous pattern of seeding of FSMCs (as observed by βgal staining, or tetrachimeric mice), displayed long thin cellular processes that penetrated the smooth muscle layers, were mutually exclusive from αSMA protein expression within these sites (Supplementary Fig. 6, A–C), but stained positive for β3-tubulin (Supplementary Fig. 6, H–J) indicative of peripheral nerves. Within the trunk vasculature, GFP+ cells remained within peripheral/circumferential sites, displayed long processes of peripheral nerves, did not appreciably contribute to smooth muscle layers of the blood vessels, and were mutually exclusive from αSMA protein expression (Supplementary Fig. 6, D–G), except for the aortic arch (YR personal observations). Furthermore, cultured mesothelial tissues from the intestine, stomach, urinary bladder, liver and heart of Wnt1Cre;R26mTmG mice gave rise to FSMCs that expressed tomato red (mT), but not GFP (Supplementary Fig. 6, K–O), indicating they do not share lineage relationship with embryonic neural crest.
We further analyzed the contributions of the embryonic neural crest to the developing trunk by analyzing two additional neural crest reporters30,31 Sox9Cre and MpzCre. Sox9Cre transgenic mice were crossed with R26mTmG mice. Within adult Sox9Cre;R26mTmG offspring (n=3), we failed to see any investments of GFP+ cells to smooth muscle of the internal organs, or to their vasculature (Supplementary Fig. 6, P–U). MpzCre transgenic mice were crossed with R26lacZ transgenic mice that express beta-galactosidase under the promoter of the ROSA26 locus. Within the internal organs from MpzCreR26lacZ transgenic offspring (n=2), we found no evidence of contributions to the smooth muscle layer of the GI tract, with small lacZ-positive foci that were interspersed within the smooth muscle layers and which morphologically resembled neural plexuses (Supplementary Fig. 7, A, A′, white arrows). LacZ positive staining was present within neural plexuses that closely associated with the tunica media/adventitia of the intestinal and mesenteric blood vessels (Supplementary Fig. 7, A″, A‴, doted lines), but was absent from the smooth muscle layers of the intestinal, mesenteric, renal, hepatic and cardiac vasculatures (Supplementary Fig. 7, A–D), except the aortic arch, which showed substantial lacZ label within its smooth muscle layer (Supplementary Fig. 7, D′, D″).
Cumulatively, these results show that trunk FSMCs do not originate from neural crest (with the exception of the aortic arch) both in-vivo and in-vitro, and are consistent with findings of neural crest derived cardiovascular malformations with normal smooth muscle differentiation32.
We then tested whether any circulating cells could contribute to FSMCs of the internal organs, by creating pairs of genetically marked parabiotic mice that have a shared anastomosed blood circulatory system33. Wild-type mice that were surgically conjoined to mice expressing GFP under the chicken β-actin promoter were left parabiosed for 1 year (n=3), at which time the internal organs from parabiosed wild-type mice were analyzed for the presence of donor-derived GFP+ cells. Donor derived GFP+ cells were present within numerous sites within internal organs (Supplementary Fig. 8, A–D) that immunoassayed for the pan-hematopoietic marker CD45 and did not contribute to the respective organ’s mesothelium, FSMCs and vasculature (Supplementary Fig. 8, E–I). These observations are in agreement with a previous publication that shows minimal contributions of transplanted hematopoietic stem cells, to non hematopoietic tissues11.
Early work in chick embryos, has proposed that the proepicardium, a mesothelial rudiment of the heart, gives rise to cardiac muscle, endothelium, smooth muscle and fibroblasts34–36. However these results were largely based on xenotransplantation of bulk embryonic tissues, not on targeting of lineage specific markers.
More recently, using genetic lineage tracing in mice, the heart proepicardium has been proposed as a multipotent cell source for myocardium, endothelium, smooth muscle37–39 even mesenchymal stem cells, giving rise to ectoderm and endoderm derivatives40. These outcomes however were largely based on genetic tracing of genes that within the heart is not specific to the mesothelium41–43. Our lineage tracing of the mesothelium, reveals a developmental restriction to smooth muscle and fibroblasts that is maintained throughout development, postnatal growth and maintenance and following irradiation-induced injury. We find no evidence for contributions to heart muscle or heart endothelium. In fact, our genetic lineage tracing reveals that MSLN+ precursors, and not neural crest represent the major contributing lineage to smooth muscle of the trunk. Lineage restriction of embryonic mesothelium to FSMCs was reported for the vasculature of the developing intestine44 and lungs45 using the Wt1Cre system, which does not appear to label all trunk FSMCs, most notably those contributing to the GI tract and urogenital system. Therefore, MSLN+ cells most likely are hierarchically upstream to Wt1+ cells. Although transplantability and engraftability were not addressed here (and are potential therapeutic challenges), the identification and prospective isolation of FSMC precursors represents a major advancement towards their targeting for regenerative medicine purposes, including the clinical investigation into the etiology and progression of their respective tumors. It also implies that soft tissue sarcomas of connective and smooth muscle tissues share a common embryonic origin.
Supplementary Material
Acknowledgments
We thank A. Mosley for assisting with generating the transgenic MSLNCLN mice, Dr. Ron Kopito, Dr. Michael Brandeis and Kirill Bersuker for usage and their assistance with the time-lapse video. We thank Tori Violante for her assistance with sectioning, Daniel Hunter for his assistance with histochemical staining and Guy Paz and Daniel Montoro for their assistance with figure preparations. The CreERT2IRES-lacZ-PGK-neo cassette was a gift from Dr. Richard J. Gilbertson. This work was supported in part by a grant from the California Institute of Regenerative Medicine (RC1 00354 to ILW), the Smith Family Trust (to ILW) and from the National Institutes of Health (RO1 DK064640 to PXX). Y.R. is supported by the Human Frontier Science Program (HFSP) Long Term Fellowship, and the Machiah Foundation Fellowship.
References and Notes
- 1.Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1979;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- 2.Bochaton-Piallat ML, Gabbiani G. Smooth muscle cell: a key cell for plaque vulnerability regulation? Circ Res. 2006;98:448–9. doi: 10.1161/01.RES.0000214330.15785.46. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy LJ, Weissman IL. Dual origin of intimal cells in cardiac-allograft arteriosclerosis. N Engl J Med. 1971;285:884–887. doi: 10.1056/NEJM197110142851603. [DOI] [PubMed] [Google Scholar]
- 4.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 5.Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–17. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 6.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–47. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 7.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–52. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 8.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 9.Zhang N, et al. Blood-borne stem cells differentiate into vascular and cardiac lineages during normal development. Stem Cells Dev. 2006;15:17–28. doi: 10.1089/scd.2006.15.17. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow- derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–52. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Science. 2002;297:2256–2259. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 13.Sorrell JM, Caplan AI. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 14.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutsaers SE, Wilkosz S. Structure and function of mesothelial cells. Cancer Treat Res. 2007;134:1–19. doi: 10.1007/978-0-387-48993-3_1. [DOI] [PubMed] [Google Scholar]
- 16.Yung S, Chan TM. Intrinsic cells: mesothelial cells -- central players in regulating inflammation and resolution. Perit Dial Int. 2009;29:S21–7. [PubMed] [Google Scholar]
- 17.Herrick SE, Mutsaers SE. Mesothelial progenitor cells and their potential in tissue engineering. Int J Biochem Cell Biol. 2004;36:621–42. doi: 10.1016/j.biocel.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Elmadbouh I, Michel JB, Chachques JC. Mesothelial cell transplantation in myocardial infarction. Int J Artif Organs. 2007;30:541–9. doi: 10.1177/039139880703000612. [DOI] [PubMed] [Google Scholar]
- 19.Mutsaers SE, Di Paolo N. Future directions in mesothelial transplantation research. Int J Artif Organs. 2007;30:557–561. doi: 10.1177/039139880703000614. [DOI] [PubMed] [Google Scholar]
- 20.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–54. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 21.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahoo D, et al. MiDReG: a method of mining developmentally regulated genes using Boolean implications. Proc Natl Acad Sci U S A. 2010;107:5732–5737. doi: 10.1073/pnas.0913635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivera-Martinez I, Missier S, Fraboulet S, Thélu J, Dhouailly D. Differential regulation of the chick dorsal thoracic dermal progenitors from the medial dermomyotome. Development. 2002;129:4763–4772. doi: 10.1242/dev.129.20.4763. [DOI] [PubMed] [Google Scholar]
- 24.Atit R, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 25.Tran TH, et al. Role of canonical Wnt signaling/β-catenin via Dermo1 in cranial dermal cell development. Development. 2010;137:3973–3984. doi: 10.1242/dev.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Kirsch DG, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 29.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global doublefluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi Y, et al. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- 31.Cheung M, et al. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Choudhary B, et al. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev Biol. 2006;289:420–429. doi: 10.1016/j.ydbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Wright DH, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 34.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of smooth muscle cells migrating into the heart along with ingrowth of the pericardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 35.Männer J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 36.Wessels A, Pérez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 37.Smart N, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai CL, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou B, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong JJ, et al. Adult Cardiac-Resident MSC-like Stem Cells with a Proepicardial Origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng B, Ren XF, Cao F, Zhou XY, Zhang J. Developmental patterns and characteristics of epicardial cell markers Tbx18 and Wt1 in murine embryonic heart. J Biomed Sci. 2011;18:67. doi: 10.1186/1423-0127-18-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christoffels VM, et al. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–E9. doi: 10.1038/nature07916. [DOI] [PubMed] [Google Scholar]
- 43.Hosen N, et al. The Wilms’ tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia. 2007;21:1783–1791. doi: 10.1038/sj.leu.2404752. [DOI] [PubMed] [Google Scholar]
- 44.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 45.Que J, et al. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahoo D, Dill DL, Gentles AJ, Tibshirani R, Plevritis SK. Boolean implication networks derived from large scale, whole genome microarray datasets. Genome Biol. 2008;9:R157. doi: 10.1186/gb-2008-9-10-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.