Abstract
Objective
To evaluate the degree to which longitudinal stability of subsyndromal symptoms of depression (SSD) is associated with conversion to dementia in patients with Mild Cognitive Impairment (MCI).
Methods
Data from 405 MCI participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) study were analyzed. Participants were evaluated at baseline and 12 month intervals over three years. Participants were designated as MCI Converters if dementia was diagnosed within 3 years or as Cognitively Stable MCI if dementia was not diagnosed during this interval. SSD were evaluated utilizing the 15-item Geriatric Depression Scale (GDS). Endorsement of specific SSD at baseline and the stability of SSD over 36 months were compared between the two MCI groups.
Results
Baseline symptom endorsement and stability of total GDS scores did not differentiate MCI groups. Worsening of 4 individual items from the GDS over time (memory problems, feelings of helplessness, loss of interest, and preference for staying at home) differentiated MCI converters from cognitively stable MCI (p <0.05 for all). However, only increased endorsement of memory symptoms over time was associated with progression to dementia after controlling for other clinical variables (p=0.05).
Conclusions
SSD in MCI participants largely consist of cognitive symptoms and activity limitations and the stability of SSD over time differentiated the MCI groups better than baseline endorsement of symptoms. However, the only significant predictor of conversion to dementia was increased endorsement of memory problems, which likely represents insight into cognitive problems more than depressive symptomatology in MCI individuals.
Keywords: subsyndromal depression, longitudinal stability, mild cognitive impairment, insight, dementia
Introduction
Subsyndromal symptoms of depression (SSD)(Judd et al 1994), or depressive symptoms not of the severity or frequency to meet criteria for major or minor depression, can include feelings of sadness, helplessness, or worthlessness, decreased energy, sleep disturbance, concentration problems, weight gain, loss of interest in activities, or psychomotor retardation(APA 1994). SSD occur in up to 30% of older community dwelling adults (Cohen et al 2005) and SSD are increasingly being recognized as significant contributors to disability in older adults (Barry et al 2009; Chopra et al 2005). SSD are also among the most commonly reported neuropsychiatric symptoms in Mild Cognitive Impairment (MCI) occurring in up to 50% of MCI individuals (Abizanda et al 2009; Gabryelewicz et al 2004; Geda et al 2008; Lyketsos et al 2002). As such, both major depression and SSD are often conceptualized as being prodromal features of incipient dementia(Brommelhoff et al 2009). However, differentiating symptoms of depression from cognitive symptoms in MCI has remained a significant challenge in older adults due to overlapping symptom criteria, particularly with respect to cognitive symptoms and activity limitations(Steffens 2008). As a result, the degree to which depressive symptoms may serve as phenotypic markers of individuals at increased risk for dementia remains unclear.
Evaluation of the specific types of SSD endorsed by MCI individuals at baseline and the longitudinal stability of these symptoms in relation to conversion to dementia are two significant avenues to clarify this relationship. Previous reports have suggested that anhedonia, sleep disturbance, and concentration problems are all common SSD associated with MCI (Edwards et al 2009; Gabryelewicz et al 2004; Stepaniuk et al 2008). Further, among MCI individuals with major depression, symptoms of depressed affect, in contrast to neurovegetative symptoms or cognitive symptoms, have been shown to differentiate individuals who progressed to dementia from those who remained cognitively stable over time(Houde et al 2008). However, to our knowledge, differential patterns of depressive symptom endorsement for MCI individuals who progress to dementia have not been evaluated specifically among individuals with SSD. Similarly, although several previous studies have demonstrated that cumulative ratings of depressive symptom severity in MCI are relatively stable over time (Li et al 2001; Steinberg et al 2004), one recent study has demonstrated that worsening of total depressive symptom severity over time represents a risk for dementia(Rovner et al 2009). Therefore, there is preliminary evidence to suggest baseline SSD characteristics and stability of SSD over time may differentiate MCI individuals who will develop dementia from those who remain cognitively stable.
The short form of the Geriatric Depression Scale (GDS) is one of the most frequently utilized measures of self-reported depressive symptoms for older adults (Chopra et al 2008; Debruyne et al 2009; Geda et al 2006; Lach et al 2010; Yesavage et al 1982). However, to date, relatively little is known about baseline characteristics and longitudinal stability of SSD obtained from the GDS in MCI populations, particularly with respect to dementia outcomes. Given the prevalence of SSD in MCI populations such evaluations are warranted and could improve treatment outcomes through earlier identification of individuals at higher risk for developing dementia. However, despite studies which have validated the short form of the GDS in MCI populations (Debruyne et al 2009), item 10 of this scale, i.e. “Do you feel you have more problems with memory than most” is often viewed more as a cognitive symptom of MCI rather than a symptom of depression. As a result, many researchers have excluded this item from analyses conducted to evaluate the impact of SSD on cognitive decline(Rovner et al 2009). Given that memory impairment is a core feature of both MCI and dementia, and not a symptom of depression, the degree to which endorsement and stability of this symptom differentiates individuals who progress to dementia from those who remain cognitively stable in contrast to other depressive symptoms is of particular interest.
The present study was conducted with three primary aims: 1) To identify the most common types of SSD endorsed on the short form of the GDS in a large sample of older adults diagnosed with MCI, 2) To evaluate if SSD endorsement at baseline differentiates individuals who progress to dementia within three years from those who remain cognitively stable during this interval, and 3) To compare the longitudinal stability of SSD endorsement on the GDS (total scores, individual items) between MCI individuals who convert to dementia relative to cognitively stable MCI participants. Based on previous studies we hypothesize that SSD will be common in MCI, that individuals who progress to dementia will endorse more affective symptoms at baseline than stable MCI participants, and that SSD of individuals who convert to dementia will exhibit less stable responses (i.e. show worsening over time) on GDS total scores and individual items of the GDS evaluating affective symptoms. Additionally we hypothesize that the majority of MCI participants in this sample will endorse memory problems on the GDS and therefore this item will have limited utility in differentiating individuals who develop dementia from cognitively stable MCI participants.
Methods
Setting
The Alzheimer's Disease Neuroimaging Initiative (ADNI). ADNI was launched in 2004 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies, and non-profit organizations. More than 800 participants, ages 55-90, have been recruited from 59 sites across the U.S. and Canada to be followed for 2-3 years. The primary goal of ADNI is to determine whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can accurately measure the progression of MCI and early AD. The identification of specific biomarkers of early AD and disease progression will provide a useful tool for researchers and clinicians in both the diagnosis of early AD and in the development, assessment and monitoring of new treatments. For additional information about ADNI, see www.adni-info.org. Demographic information and clinical data utilized for this study were downloaded from the ADNI clinical data repository (https://www.loni.ucla.edu/ADNI/Data/ADCS_Download.jsp). The July 19, 2009 version of the ADNI clinical database was used for all analyses
Participants
Four hundred and five participants who met the criteria for MCI at baseline constituted the sample for this study. The criteria for diagnosis of MCI included: 1) age between 55 and 90 years, 2) complaints of memory loss by the patient and confirmed by a family relative, 3) Mini-Mental State Exam(Folstein et al 1983) score of 24 and higher, 4) overall Clinical Dementia Rating scale(Morris 1993) score of 0.5, and 5) quantitative evidence of memory impairment relative to age and education matched peers. Exclusion criteria at baseline included: 1) the presence of Major Depressive Disorder or significant symptoms of depression (Geriatric Depression Scale(Yesavage et al 1982) score of 6 or higher), 2) modified Hachinski ischemia score greater than 5, 3) significant neurological or psychiatric illness, 4) use of antidepressant drugs with anticholinergic side effects, and 5) high dose of neuroleptics or chronic sedatives or hypnotics, antiparkinsonian medication, and use of narcotic analgesics. Diagnoses of dementia were made by consensus panel of experts based on NINCDS/ADRDA criteria(McKhann et al 1984).
Assessment of Disability
The Functional Activities Questionnaire (FAQ)(Pfeffer et al 1982)
The FAQ evaluates the ability of participants to complete 10 domains of complex activities (e.g. managing finances) and scores range from 0-20 with higher scores representing increased disability.
Clinical Dementia Rating (CDR) Scale(Morris 1993)
The CDR is a screening measure utilized to assess functional declines in older adults caused by cognitive impairments in order to classify stages of dementia.
Assessment of Symptoms of Depression
Geriatric Depression Scale(GDS)(Yesavage et al 1982)
The short form of the GDS consists of 15 “yes”/”no” questions and total scores range from 0-15 with higher scores exhibiting more depressive symptoms. For this study, individuals scoring >0 and ≤5 on the GDS were classified as exhibiting subsyndromal symptoms of depression. At follow up evaluations individuals scoring ≥ 6 on the GDS were classified as exhibiting symptoms of Major Depression(Marc et al 2008).
Assessment of Cognitive Functioning
The Alzheimer's Disease Assessment Scale- Cognitive Scale (ADAS-Cog) (Rosen et al 1984)
The ADAS-cog is a widely utilized 11-item instrument devised to assess the severity of cognitive impairment in patients with AD. The total score range is 0 to 70 points, with a higher score indicating poorer cognitive performance.
Statistical Analyses
Participants were classified “MCI Converters” if they were diagnosed with dementia within three years of their baseline evaluation or “MCI Cognitively Stable” if they did not progress to dementia during this interval. Diagnoses of dementia were evaluated at 12 month intervals over this 36 month period. The MCI groups were compared with respect to demographic and clinical characteristics using Wilcoxon Rank Sum tests (age, education, MMSE, CDR, ADASc, FAQ) and a Fisher's Exact test (Gender). To evaluate if the endorsement of specific symptoms or GDS total score differed between the MCI groups at baseline a Fisher's exact test was utilized. Subsequently, a linear mixed effects model was employed to evaluate if MCI group status influenced total GDS scores over three years after accounting for the contributions of age and time. To evaluate the longitudinal stability of depressive symptoms over three years for the MCI groups, two approaches were utilized. First, the proportion of individuals who remained stable, declined, improved, or fluctuated with respect to SSD categorical status over three years was calculated and compared between groups utilizing Fisher's Exact test. SSD categories were defined as “depression free”; GDS=0, SSD; GDS 1-5, and Major Depression; GDS>5. Second, the stability (item endorsed, not endorsed) of each of the GDS 15 items was calculated for the three year interval relative to baseline between MCI groups utilizing odds ratios (95% Confidence Interval). Lastly, for each item of the GDS that showed differential stability of symptom endorsement between the two MCI groups a generalized linear mixed model was utilized to evaluate the degree to which symptom endorsement on individual items of the GDS was predicted by MCI group, age, and time.
Results
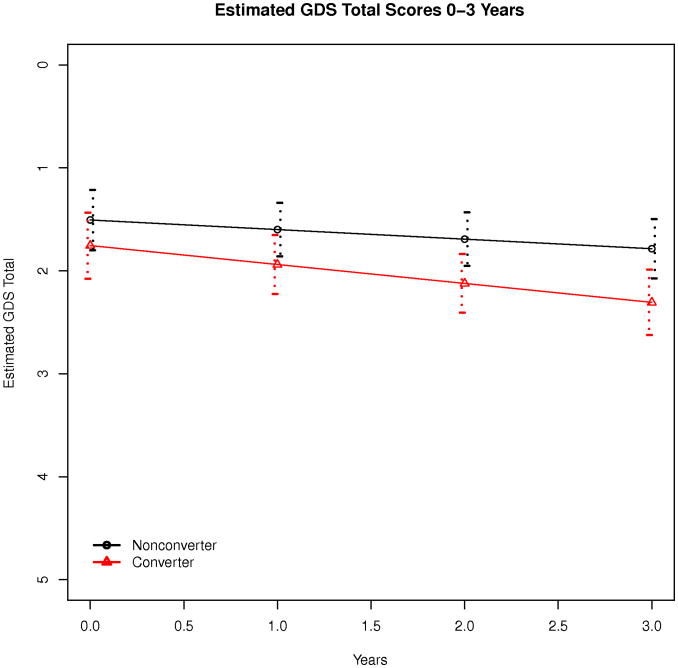
Of the total sample, 312 participants (77%) exhibited SSD at baseline utilizing responses to all 15 items of the GDS and 43% of the sample endorsed item 10 (“Do you have more problems with memory than most?”). Excluding item 10 from analyses resulted in SSD being reported by 55% of the sample. Three year follow up data was available for 227 participants and 103 (45%) individuals converted to dementia during this interval. At baseline, MCI converters exhibited poorer performance on measures of overall cognitive functioning (MMSE, p<0.01; ADAS Cog, p<0.01) and disability (CDR, p<0.01; FAQ, p<0.01) than cognitively stable MCI participants but the two groups did not differ with respect to age, education, gender, or overall depression severity (Table 1). Of individuals completing a three year follow up evaluation, cognitively stable MCI participants did not differ from MCI converters with respect to endorsement of any of the specific GDS items at baseline (Table 2) and the most commonly endorsed SSD for the sample included memory problems, lack of energy, preference for staying at home, and dropping of activities and interests. No differences in GDS total score rate of change was found between the two MCI groups (βyears:converter = 0.09, p = 0.19, Figure 1). Over 36 months, stability of depressive symptom categories did not differ between the cognitively stable MCI participants and MCI converters (p = 0.61) and 49% of the sample exhibited stable SSD symptoms, 16% exhibited worsening symptoms, 8% improved, and 27% of the sample demonstrated fluctuations between depression categories. Further, 11 (9%) of MCI cognitively stable and 16 (16%) of MCI converters developed symptoms of major depression over three years, but the proportion of individuals developing major depression did not differ between groups (OR = 1.88, p = 0.15).
Table 1. Demographic and Clinical Characteristics of MCI Groups at Baseline (n=227).
| Cognitively Stable MCI | MCI Converters | p | |||
|---|---|---|---|---|---|
| n | Female (%) | n | Female (%) | ||
| Gender | 124 | 37 (29.8) | 103 | 34 (33) | 0.67a |
| n | Mean (sd) | n | Mean (sd) | ||
| Age [years] | 124 | 74.9 (7.6) | 103 | 74.8 (6.7) | 0.69 b |
| Education [years] | 124 | 16.0 (2.8) | 103 | 15.9 (2.9) | 0.72 b |
| MMSE | 124 | 27.7 (1.7) | 103 | 26.7 (1.6) | <0.01 b |
| ADAS Cog | 124 | 9.4 (3.9) | 103 | 12.7 (3.9) | <0.01 b |
| Geriatric Depression Scale | 124 | 1.5 (1.4) | 103 | 1.6 (1.3) | 0.38 b |
| CDR b | 124 | 1.4 (0.7) | 103 | 1.8 (1.0) | <0.01 b |
| FAQ | 122 | 2.0 (3.1) | 102 | 5.7 (4.7) | <0.01 b |
MMSE= Mini Mental Status Exam, ADAS Cog= Alzheimer's Disease Assessment Scale Cognitive, GDS= Geriatric Depression Scale, CDR= Clinical Dementia Rating Scale, FAQ= Functional Activities Questionnaire.
Fisher's Exact test
Wilcoxon Rank Sum tests
Table 2. Endorsement of 15 Item Geriatric Depression Scale Items at Baseline for MCI groups (n=227).
| Stable MCI n (%) | MCI Converters n (%) | OR | P | |
|---|---|---|---|---|
| 1. Basically satisfied with your life? | 6 (5) | 5 (5) | 1.00 | 1.00 |
| 2. Dropped many of your activities and interests | 16 (13) | 21 (20) | 1.72 | 0.15 |
| 3. Feel your life is empty | 2 (2) | 4 (4) | 2.46 | 0.41 |
| 4. Often get bored | 8 (7) | 8 (8) | 1.22 | 0.80 |
| 5. In good spirits most of the time | 6 (5) | 3 (3) | 1.69 | 0.52 |
| 6. Afraid something bad is going to happen | 8 (7) | 9 (9) | 1.39 | 0.62 |
| 7. Feelings of happiness | 6 (5) | 4 (4) | 1.26 | 1.00 |
| 8. Feelings of helplessness | 4 (3) | 4 (4) | 1.21 | 1.00 |
| 9. Preference for staying at home | 32 (26) | 23 (22) | 0.83 | 0.64 |
| 10. More problems with memory than most | 61 (49) | 55 (53) | 1.18 | 0.59 |
| 11. Think wonderful to be alive | 1 (1) | 3 (3) | 0.27 | 0.33 |
| 12. Feelings of worthless | 2 (2) | 1 (1) | 0.60 | 1.00 |
| 13. Feel full of energy | 29 (23) | 27 (26) | 0.86 | 0.63 |
| 14. Situation is hopeless | 1 (1) | 0 (0) | -- | 1.00 |
| 15. Better off than others | 4 (3) | 1 (1) | 0.30 | 0.38 |
Figure 1. Estimated GDS Scores Total Score Over 3 Years (n=227).
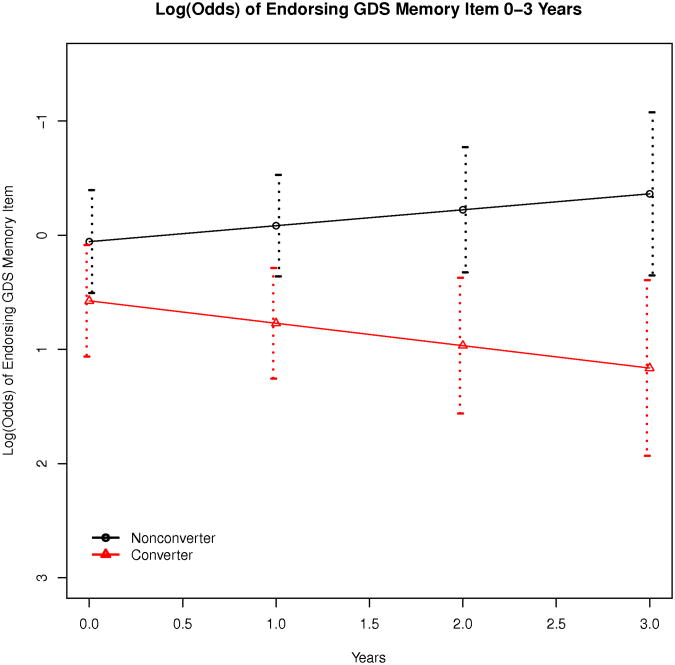
An evaluation of stability of specific GDS items for each of the two MCI groups (Table 3) revealed that 4 individual items from the GDS were less stable for MCI converters than MCI cognitively stable participants (memory problems, feelings of helplessness, preference for staying at home, dropped activities and interests) and in each case, increased endorsement of these symptoms over time (i.e. worsening of symptoms) was evident in the MCI converter group. A generalized linear mixed model (Table 4) was subsequently utilized to evaluate the degree to which symptom endorsement on these 4 GDS items was predicted by MCI group, age, and time and only stability of memory symptoms was predicted by MCI group (Log(OR)years:converter = 0.34, p = 0.05; Figure 2).
Table 3. Stability of Depressive Symptoms of Geriatric Depression Scale-15 Ites for MCI groups (n=227).
| Stable MCI (%) | MCI Converters (%) | Test | P | |
|---|---|---|---|---|
| 1. Basically satisfied with your life? | 84.7 | 80.6 | 0.75 | 0.48 |
| 2. Dropped many of your activities and interests | 71.0 | 48.5 | 0.39 | <0.01 |
| 3. Feel your life is empty | 88.7 | 89.3 | 1.06 | 1.00 |
| 4. Often get bored | 81.5 | 75.7 | 0.71 | 0.33 |
| 5. In good spirits most of the time | 91.1 | 87.4 | 0.68 | 0.39 |
| 6. Afraid something bad is going to happen | 81.5 | 82.5 | 1.07 | 0.86 |
| 7. Feelings of happiness | 86.3 | 85.4 | 0.93 | 0.85 |
| 8. Feelings of helplessness | 87.9 | 71.8 | 0.35 | <0.01 |
| 9. Preference for staying at home | 68.5 | 51.5 | 0.49 | 0.01 |
| 10. More problems with memory than most | 59.7 | 42.7 | 0.51 | 0.01 |
| 11. Think wonderful to be alive | 94.4 | 93.2 | 0.82 | 0.79 |
| 12. Feelings of worthless | 91.1 | 84.5 | 0.53 | 0.15 |
| 13. Feel full of energy | 58.9 | 59.2 | 1.01 | 1.00 |
| 14. Situation is hopeless | 91.9 | 88.3 | 0.67 | 0.38 |
| 15. Better off than others | 90.3 | 91.3 | 1.12 | 1.00 |
Table 4. Comparison of Worsening on Selected GDS Items for MCI Groups (n=227).
| Log(OR)years:converter | Std. Error | Z | P | |
|---|---|---|---|---|
| Dropped many activities and interests | 0.01 | 0.29 | 0.04 | 0.97 |
| Feelings of helplessness | 0.05 | 0.34 | 0.16 | 0.16 |
| Preference of staying at home | -0.08 | 0.19 | -0.44 | 0.66 |
| Feelings of increased memory problems | 0.34 | 0.17 | 1.94 | 0.05 |
Figure 2. Endorsement of Memory Problems Over Three Years for MCI Groups (n=227).
Discussion
Our major findings include the following: 1) SSD is common in MCI and the most frequently endorsed symptoms from the GDS included memory problems, lack of energy, preference for staying at home, and limitations in activities, 2) Total SSD severity and endorsement of specific depressive symptoms did not differ at baseline between MCI groups, 3) The majority of MCI participants demonstrated either stable or fluctuating SSD and stability of SSD total severity scores did not differ between MCI groups, and 4) Stability of 4 specific depressive symptoms from the GDS (memory problems, activity and interest limitations, preference for staying at home, and feelings of helplessness) over 36 months differentiated cognitively stable MCI participants from MCI converters. However, only endorsement of memory problems was associated with conversion to dementia after accounting for other clinical variables. Each of these findings will be discussed below.
With respect to the overall prevalence of SSD at baseline in this sample, 77% exhibited one or more symptoms of depression on the GDS. If item 10 (memory problems) was excluded from analysis, 55% of the sample endorsed SSD on this measure demonstrating that 22% of the sample endorsed isolated memory problems. Our results suggest that in addition to memory problems, lack of energy, preference for staying at home, and dropping of activities and interests were the most commonly endorsed SSD for the sample.
Overall, the prevalence of SSD obtained from the entire GDS in our sample was higher than estimates of SSD in MCI using other measures of depression, which typically report the prevalence of SSD of approximately 30%-50% (Abizanda et al 2009; Gabryelewicz et al 2004; Geda et al 2008; Lyketsos et al 2002). However, if item 10 of the GDS was removed from analysis, our results would be more consistent with these previous estimates. As memory problems are not considered to be symptoms of depression by most diagnostic criteria(APA 1994), we would recommend that when utilizing the GDS to estimate the prevalence of SSD in MCI populations, item 10 should be excluded (Rovner et al 2009). However, we also recognize that endorsement of this item may in fact represent difficulties with concentration which is a symptom of depression(APA 1994) and further research may be necessary to determine the validity of this item of the GDS for use in MCI populations. Further, while there is not a consensus criterion for defining SSD, our methodology for designating SSD included individuals with only one reported depressive symptom consistent with many previous studies evaluating neuropsychiatric symptoms in MCI (Abizanda et al 2009; Gabryelewicz et al 2004; Lyketsos et al 2002). However, we do recognize that SSD is also often utilized to denote individuals with two or more depressive symptoms (Judd et al 1994) (Lyness et al 2009) a distinction which is particularly important when comparing the prevalence of SSD across different patient populations.
Our findings also suggested that at baseline, individuals with stable MCI do not differ from individuals who progress to dementia with respect to overall severity of SSD or the endorsement of specific items. These findings were unexpected given recent findings that depressive symptom severity and endorsement of specific affective symptoms at baseline differentiated individuals who converted to dementia from cognitively stable MCI participants in other studies (Boyle et al 2010) (Houde et al 2008). We interpret our findings to suggest that SSD in MCI, unlike more significant symptoms of depression, are primarily a generalized consequence or concurrent feature of the MCI syndrome but these symptoms are not pathognomic features of individuals at higher risk for dementia. Further, given that the most commonly endorsed SSD in this sample included items that could be attributed to cognitive decline and resulting activity limitations, our results could be interpreted to suggest that SSD in MCI populations does not represent an a concurrent affective component and instead reflects a significant overlap in symptoms between the two syndromes.
When we evaluated the stability of depressive symptoms categorically over time we found that the majority of patients in the sample demonstrated stable, fluctuating, or improvement in SSD with only a relatively small proportion of the sample (16%) exhibiting worsening of SSD. This finding was consistent with previous studies demonstrating that cumulative ratings of depressive symptom severity in MCI are relatively stable over time (Li et al 2001; Steinberg et al 2004). However, unexpectedly we did not see significant differences between our two MCI groups with respect to categorical evaluations of stability of total SSD severity as might be expected given a recent study suggesting that increased SSD severity over time represented a risk for dementia (Rovner et al 2009). Unlike this previous study, however, we utilized categorical ratings of SSD rather than total symptom score, which may have contributed to our divergent findings. We utilized this categorical approach as we felt this represented the most clinically meaningful analysis of the data as by definition SSD represent subthreshold symptoms of depression.
In contrast, our findings indicate that the worsening of four specific items of the GDS (memory problems, dropped activities and interests, preference for staying at home, and feelings of helplessness) over time relative to baseline endorsement were associated with conversion to dementia. As we feel these four items are most strongly associated with cognitive problems and functional limitations which are core aspects of a diagnosis of dementia, these findings are not surprising but do illustrate the complexity of distinguishing mood symptoms from cognitive symptoms among individuals with MCI(Steffens 2008). However, after accounting for other clinical features, only worsening of memory problems over time was associated with conversion to dementia. Again, as memory problems are not typically viewed as a symptom of depression and represent a core feature of dementia, this finding is not surprising. However, a lack of association between the stability of other SSD that are more reflective of affective symptoms, such as feelings of depressed mood and worthlessness, and conversion to dementia suggests worsening of affective symptoms are not core depressive features of a prodromal phase of incipient dementia.
While we expected memory symptoms to be common in MCI individuals, our findings that 42% of the sample endorsed memory problems is significant for several reasons. First, we expected a higher rate of endorsement for this item given that all participants in the study were diagnosed with MCI and demonstrated memory impairments on testing. Our findings indicate that less than one half of participants in the sample reported an awareness of cognitive symptoms that are core diagnostic features of MCI. Given this, we feel that endorsement of memory problems on the GDS may represent a mechanism for evaluating participant's awareness or insight into cognitive deficits and recent studies have suggested that patient awareness of cognitive symptoms may be a particularly useful in differentiating individuals with different forms neurodegenerative disease (Barnes et al 2006; Williamson et al 2009). Our results could extend these previous studies to suggest that awareness of memory problems in MCI, while not typically viewed as SSD, may also be a significant clinical attribute associated with increased risk for conversion to dementia. In addition, previous studies evaluating the agreement between self reported symptoms of depression and informant ratings of depression in MCI and dementia patients have found that item 10 represents the single item of the GDS for which patients and informants show high degrees of agreement(Chopra et al 2008). In combination, these findings would suggest that self-report measures of awareness of memory problems obtained from commonly utilized measures of depression may be a particularly useful, in combination with measures of cognitive functioning and activity limitations, in identifying individuals at risk for continued cognitive decline.
Overall, this study has several strengths including a large sample size, thorough ascertainment of MCI status, and a relatively long follow up period. Despite these strengths, there are several limitations to the study which also should be discussed. First, it is important to note that for our designation of SSD we did not require specific depressive symptoms to be endorsed and we did not exclude any individual items of the GDS from our statistical analyses. Although our intent for this study was not to validate the short form of the GDS or to demonstrate the incidence of SSD in MCI populations, this methodological consideration will limit the generalizability of our findings. Similarly, diagnostic evaluations of minor depression were not conducted and therefore, it is possible that some of the patients that we designated as exhibiting SSD may have in fact met diagnostic criteria for minor depression. Further, as we excluded participants with major depression and significant cerebrovascular disease, our ability to generalize our findings to the larger population is limited. Additionally, while not specific to our study, measurement error may have resulted in the inclusion of some individuals with normal cognitive function or dementia in our baseline sample or the misdiagnosis of dementia over the 36 interval. Lastly, by design, we utilized only one self-report measure of depression to evaluate SSD. Given previous studies demonstrating that ratings of depressive symptoms in MCI often differ from clinician ratings and informant ratings(Chopra et al 2008), this represents a further limitation to our findings.
Conclusions
SSD in this MCI sample was primarily characterized by cognitive complaints, activity limitations, and decreased energy. Neither baseline SSD nor longitudinal stability of SSD total scores differentiated cognitively stable MCI participants from those who developed dementia. Worsening of specific GDS items associated with memory problems, decreased energy, feelings of helplessness and activity limitations was more common in individuals who developed dementia, however only increased endorsement of cognitive complaints over time was associated with progression to dementia after accounting for other clinical variables. Therefore or results suggest that SSD may have limited utility in identifying MCI individuals at increased risk for dementia.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. Data analysis was supported in part by the following grants from the National Institutes of Health: KO8 MH081065, P41 RR023953.
Footnotes
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI investigators include (complete listing available at http://www.loni.ucla.edu/ADNI/Collaboration/ADNI_Manuscript_Citations.pdf
References
- Abizanda P, Lopez-Jimenez E, Lopez-Ramos B, Romero L, Sanchez-Jurado PM, Leon M, et al. Neuropsychiatric symptoms in mild cognitive impairment and Alzheimer's disease. Rev Esp Geriatr Gerontol. 2009;44:238–243. doi: 10.1016/j.regg.2009.03.018. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4th. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry LC, Allore HG, Bruce ML, Gill TM. Longitudinal association between depressive symptoms and disability burden among older persons. J Gerontol A Biol Sci Med Sci. 2009;64:1325–1332. doi: 10.1093/gerona/glp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle LL, Porsteinsson AP, Cui X, King DA, Lyness JM. Depression predicts cognitive disorders in older primary care patients. J Clin Psychiatry. 2010;71:74–79. doi: 10.4088/JCP.08m04724gry. [DOI] [PubMed] [Google Scholar]
- Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, Pedersen NL. Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychol Aging. 2009;24:373–384. doi: 10.1037/a0015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra MP, Sullivan JR, Feldman Z, Landes RD, Beck C. Self-, collateral- and clinician assessment of depression in persons with cognitive impairment. Aging Ment Health. 2008;12:675–683. doi: 10.1080/13607860801972412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra MP, Zubritsky C, Knott K, Have TT, Hadley T, Coyne JC, et al. Importance of subsyndromal symptoms of depression in elderly patients. Am J Geriatr Psychiatry. 2005;13:597–606. doi: 10.1176/appi.ajgp.13.7.597. [DOI] [PubMed] [Google Scholar]
- Cohen CI, Magai C, Yaffee R, Walcott-Brown L. Racial differences in syndromal and subsyndromal depression in an older urban population. Psychiatr Serv. 2005;56:1556–1563. doi: 10.1176/appi.ps.56.12.1556. [DOI] [PubMed] [Google Scholar]
- Debruyne H, Van Buggenhout M, Le Bastard N, Aries M, Audenaert K, De Deyn PP, et al. Is the geriatric depression scale a reliable screening tool for depressive symptoms in elderly patients with cognitive impairment? Int J Geriatr Psychiatry. 2009;24:556–562. doi: 10.1002/gps.2154. [DOI] [PubMed] [Google Scholar]
- Edwards ER, Spira AP, Barnes DE, Yaffe K. Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry. 2009;24:716–722. doi: 10.1002/gps.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- Gabryelewicz T, Styczynska M, Pfeffer A, Wasiak B, Barczak A, Luczywek E, et al. Prevalence of major and minor depression in elderly persons with mild cognitive impairment--MADRS factor analysis. Int J Geriatr Psychiatry. 2004;19:1168–1172. doi: 10.1002/gps.1235. [DOI] [PubMed] [Google Scholar]
- Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Bergman H, Whitehead V, Chertkow H. A predictive depression pattern in mild cognitive impairment. Int J Geriatr Psychiatry. 2008;23:1028–1033. doi: 10.1002/gps.2028. [DOI] [PubMed] [Google Scholar]
- Judd LL, Rapaport MH, Paulus MP, Brown JL. Subsyndromal symptomatic depression: a new mood disorder? J Clin Psychiatry. 1994;55:18–28. [PubMed] [Google Scholar]
- Lach HW, Chang YP, Edwards D. Can Older Adults with Dementia Accurately Report Depression Using Brief Forms? J Gerontol Nurs. 2010:1–8. doi: 10.3928/00989134-20100303-01. [DOI] [PubMed] [Google Scholar]
- Li YS, Meyer JS, Thornby J. Longitudinal follow-up of depressive symptoms among normal versus cognitively impaired elderly. Int J Geriatr Psychiatry. 2001;16:718–727. doi: 10.1002/gps.423. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Chapman BP, McGriff J, Drayer R, Duberstein PR. One-year outcomes of minor and subsyndromal depression in older primary care patients. Int Psychogeriatr. 2009;21:60–68. doi: 10.1017/S1041610208007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691–698. doi: 10.1176/appi.ajp.162.4.691. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Casten RJ, Leiby BE. Variability in depressive symptoms predicts cognitive decline in age-related macular degeneration. Am J Geriatr Psychiatry. 2009;17:574–581. doi: 10.1097/jgp.0b013e31819a7f46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC. Separating mood disturbance from mild cognitive impairment in geriatric depression. Int Rev Psychiatry. 2008;20:374–381. doi: 10.1080/09540260802094589. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Tschanz JT, Corcoran C, Steffens DC, Norton MC, Lyketsos CG, et al. The persistence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2004;19:19–26. doi: 10.1002/gps.1025. [DOI] [PubMed] [Google Scholar]
- Stepaniuk J, Ritchie LJ, Tuokko H. Neuropsychiatric impairments as predictors of mild cognitive impairment, dementia, and Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2008;23:326–333. doi: 10.1177/1533317508317351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson C, Alcantar O, Rothlind J, Cahn-Weiner D, Miller BL, Rosen HJ. Standardised measurement of self-awareness deficits in FTD and AD. J Neurol Neurosurg Psychiatry. 2009;81:140–145. doi: 10.1136/jnnp.2008.166041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]