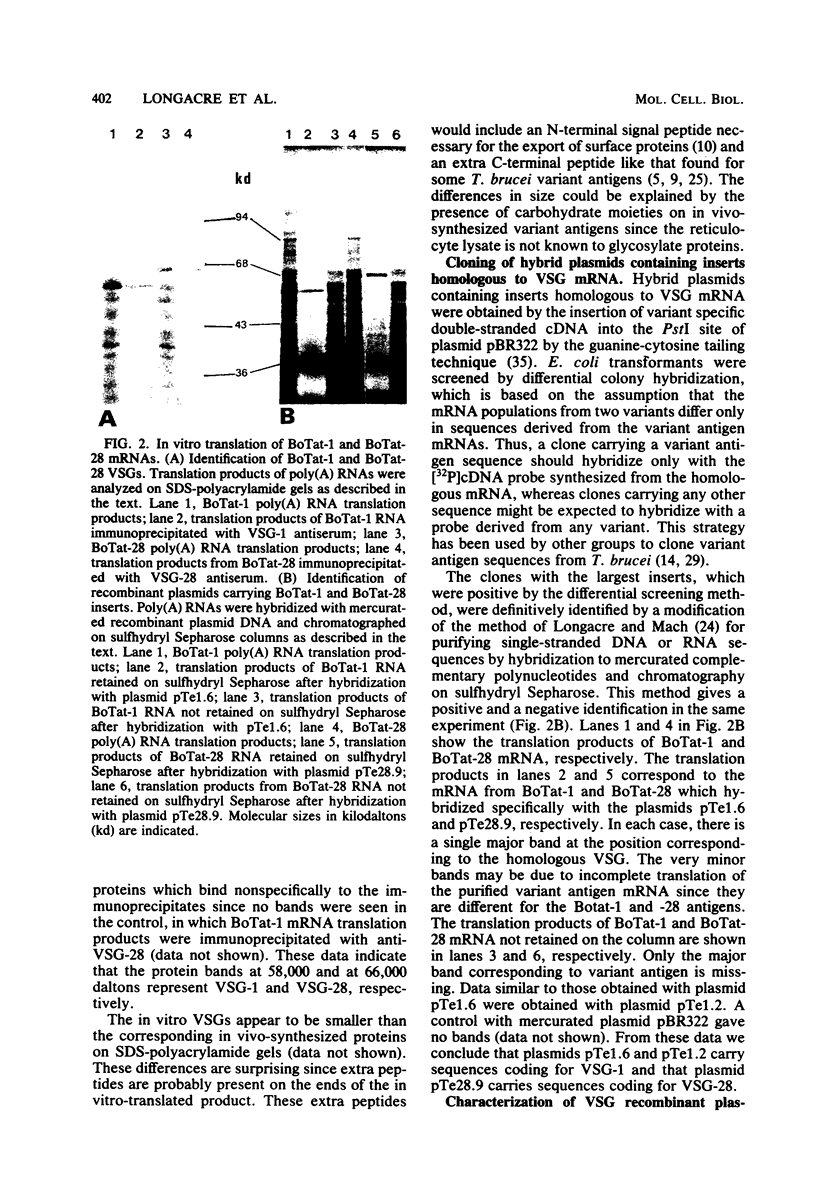
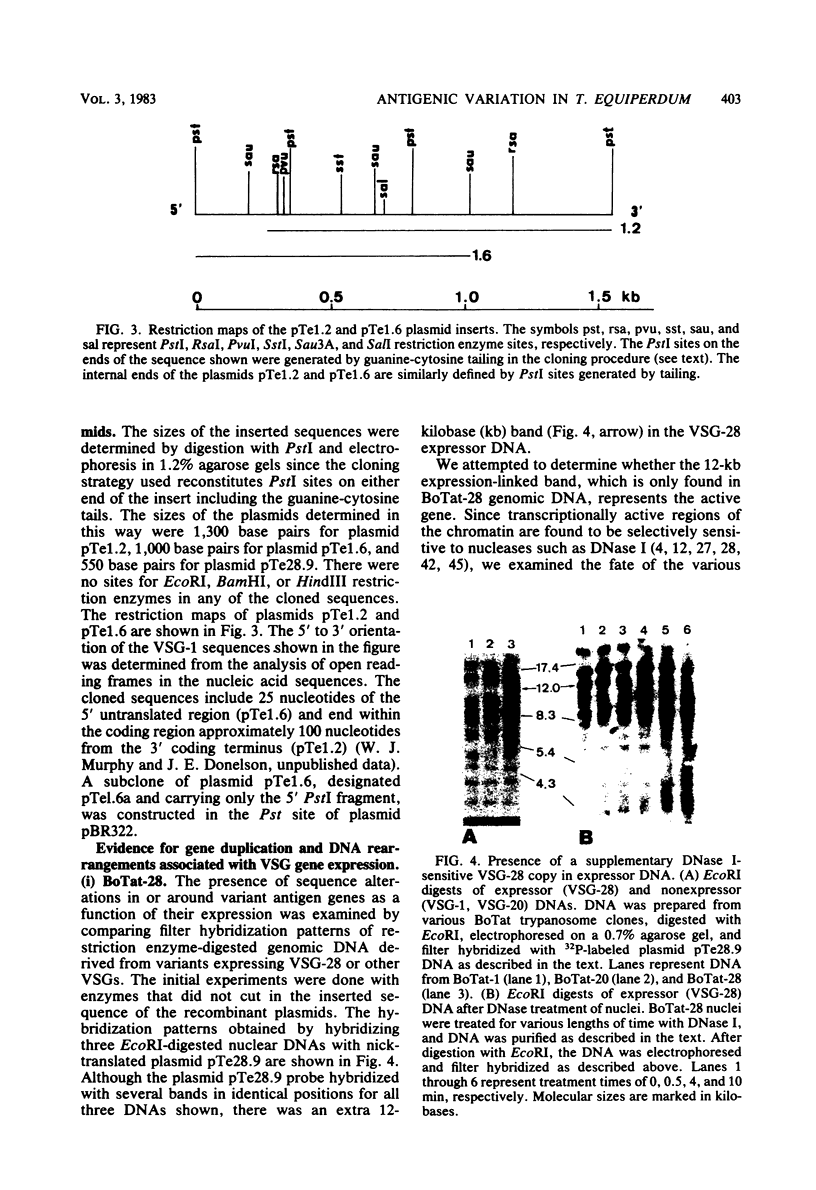
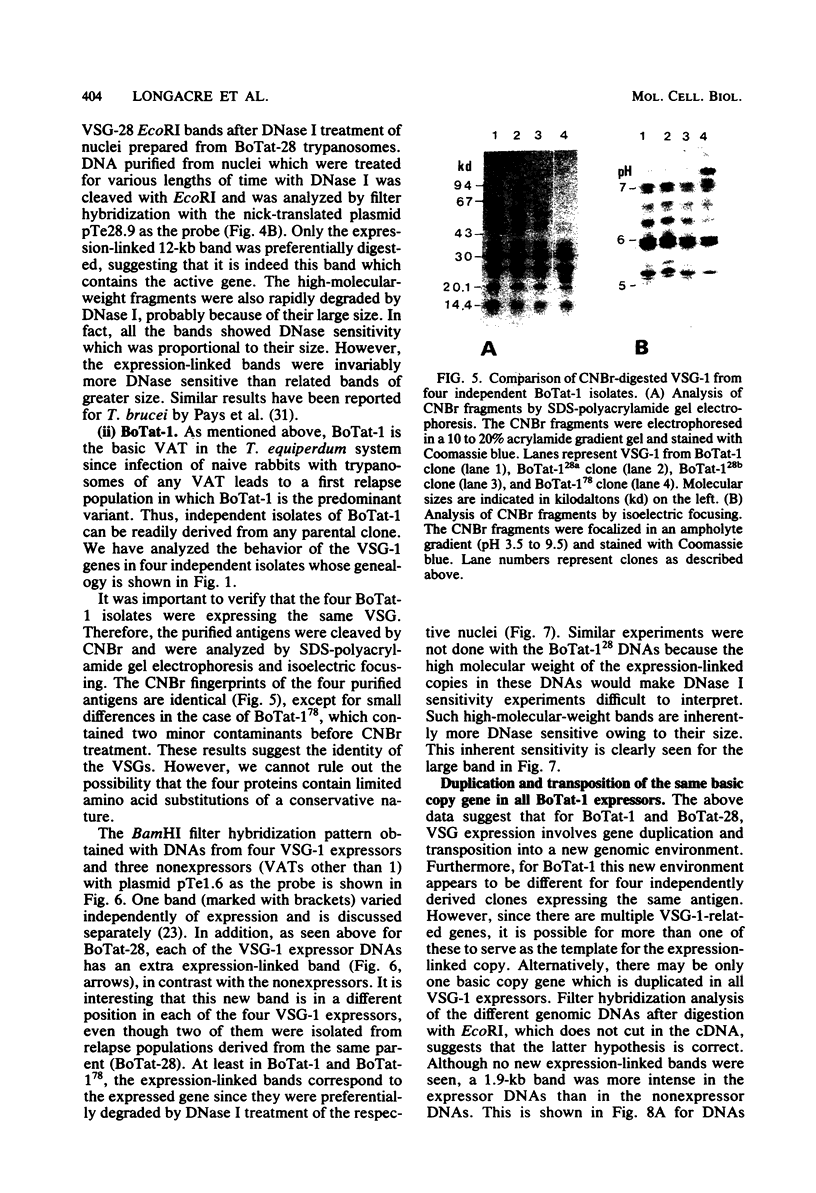
Abstract
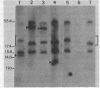
African trypanosomes resist the immune response of their mammalian hosts by varying the surface glycoprotein which constitutes their antigenic identity. The molecular mechanism of this antigenic variation involves the successive activation of a series of genes which code for different variant surface glycoproteins (VSGs). We have studied the expression of two VSG genes (those of VSG-1 and VSG-28) in Trypanosoma equiperdum, and we report the following findings. (i) The expression of both VSG genes is associated with the duplication and transposition of corresponding basic copy genes. (ii) The duplicated transposed copy appears to be the expressed copy. (iii) Although there are multiple genes which cross-hybridize with the VSG-1 cDNA probe, only one of these appears to be used as a template for the expression-linked copy in four independent BoTat-1 clones. (iv) Analysis of the genomic environments of the expressed VSG-1 genes from each of four independently derived BoTat-1 trypanosome clones revealed that there are at least three different sites into which the expression-linked copy can be inserted.
Full text
PDF

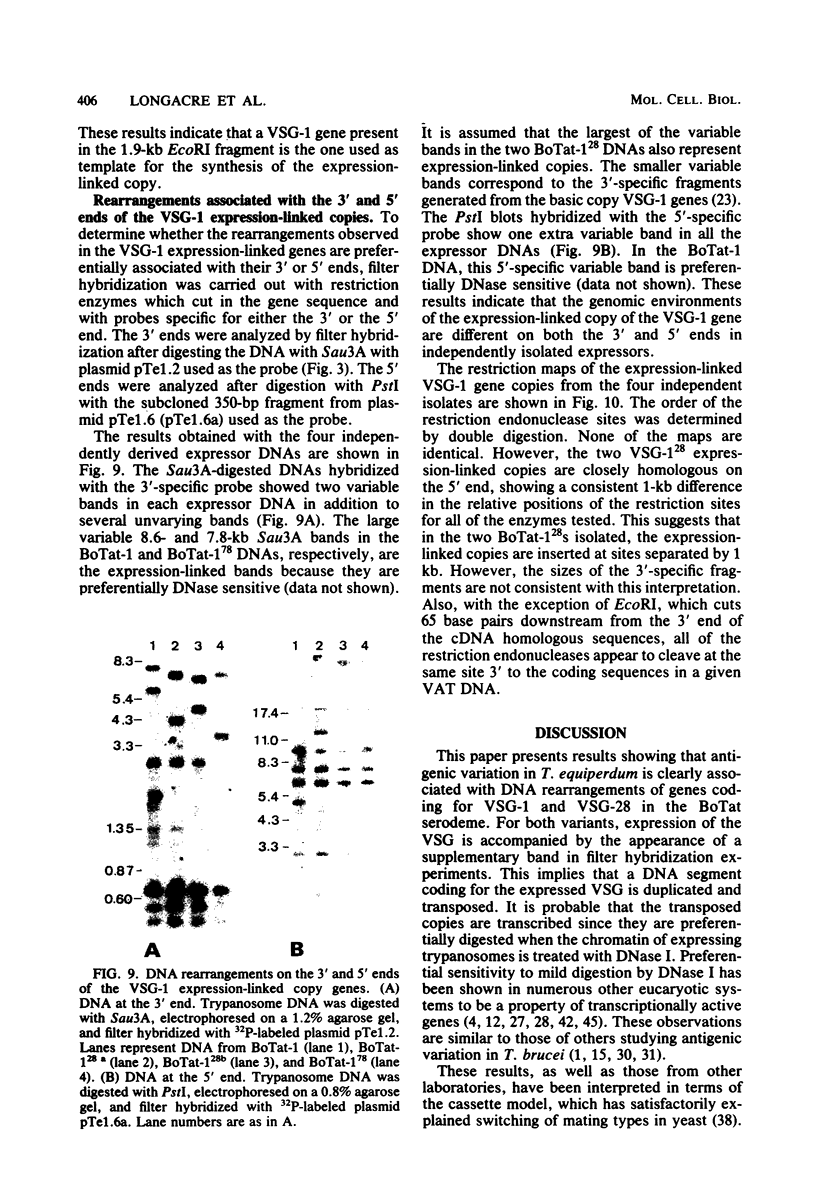
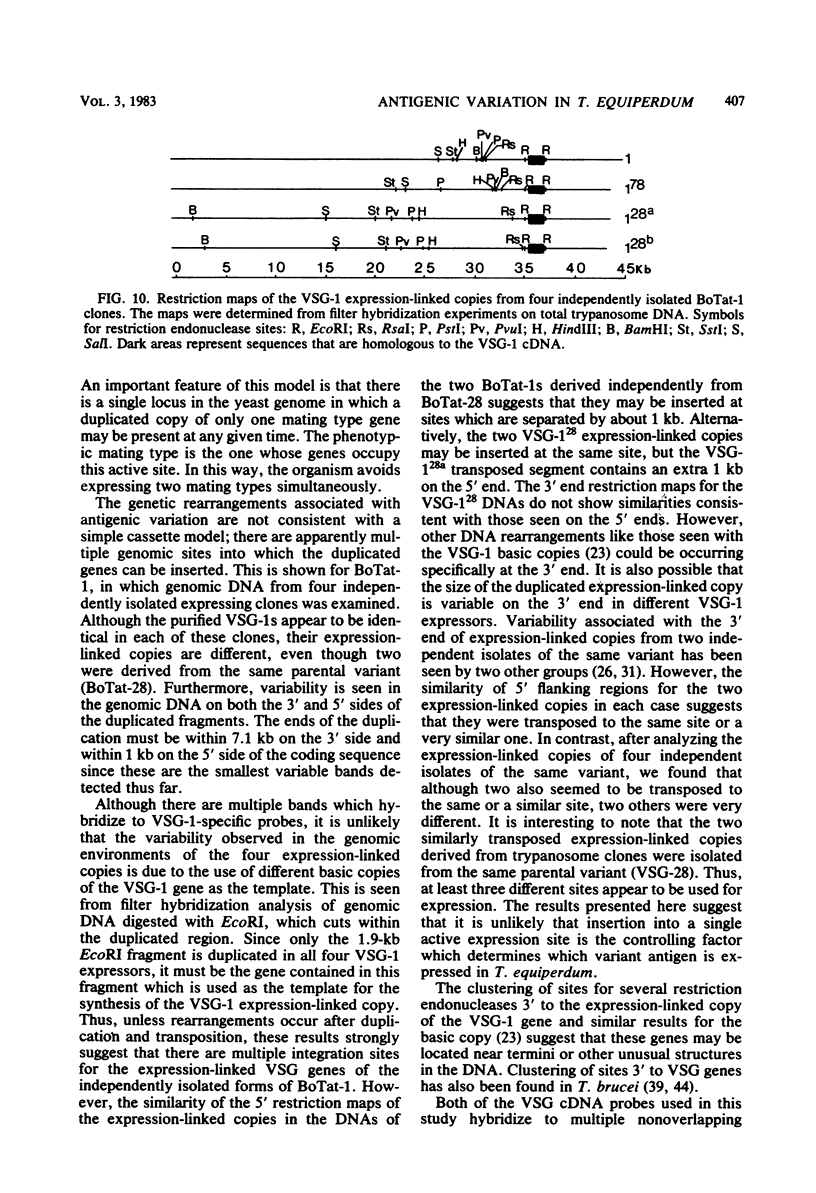


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N., Thomashow L., Milhausen M., Stuart K. Structural analysis of variant and invariant genes in trypanosomes. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1043–1049. doi: 10.4269/ajtmh.1980.29.1043. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baltz T., Baltz D., Pautrizel R. Affinité de la concanavaline A pour Trypanosoma equiperdum applications a l'isolement de la fraction glycoprotéique spécifique du type antigénique. Ann Immunol (Paris) 1976 Sep-Oct;127(5):761–774. [PubMed] [Google Scholar]
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Cross G. A., Hoeijmakers J. H., Borst P. A variant surface glycoprotein of Trypanosoma brucei synthesized with a C-terminal hydrophobic 'tail' absent from purified glycoprotein. Nature. 1980 Dec 11;288(5791):624–626. doi: 10.1038/288624a0. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):55–72. doi: 10.1098/rspb.1978.0057. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D. M., White R. L., Finnegan D. J., Hogness D. S. Characterization of six cloned DNAs from Drosophila melanogaster, including one that contains the genes for rRNA. Cell. 1975 Jun;5(2):149–157. doi: 10.1016/0092-8674(75)90023-9. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Frasch A. C., Bernards A., Borst P., Cross G. A. Novel expression-linked copies of the genes for variant surface antigens in trypanosomes. Nature. 1980 Mar 6;284(5751):78–80. doi: 10.1038/284078a0. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kourilsky P., Mercereau O., Gros D., Tremblay G. Hybridization of filters with competitor DNA in the liquid phase in a standard and a micro-assay. Biochimie. 1974;56(9):1215–1221. doi: 10.1016/s0300-9084(74)80014-3. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Labastie M. C., Baltz T., Richet C., Giroud C., Duvillier G., Pautrizel R., Degand P. Variant specific glycoproteins of Trypanosoma equiperdum: cross reacting determinants and chemical studies. Biochem Biophys Res Commun. 1981 Mar 31;99(2):729–736. doi: 10.1016/0006-291x(81)91804-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Longacre S. S., Mach B. Purification of specific DNA sequences by sulfhydryl-Sepharose chromatography of mercurated polynucleotides. J Biol Chem. 1978 Oct 25;253(20):7500–7507. [PubMed] [Google Scholar]
- Longacre S., Raibaud A., Hibner U., Buck G., Eisen H., Baltz T., Giroud C., Baltz D. DNA rearrangements and antigenic variation in Trypanosoma equiperdum: expression-independent DNA rearrangements in the basic copy of a variant surface glycoprotein gene. Mol Cell Biol. 1983 Mar;3(3):410–414. doi: 10.1128/mcb.3.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthyssens G., Michiels F., Hamers R., Pays E., Steinert M. Two variant surface glycoproteins of Trypanosoma brucei have a conserved C-terminus. Nature. 1981 Sep 17;293(5829):230–233. doi: 10.1038/293230a0. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Bernards A., Van der Ploeg L. H., Borst P. Characterization of the expression-linked gene copies of variant surface glycoprotein 118 in two independently isolated clones of Trypanosoma brucei. Nucleic Acids Res. 1982 Apr 10;10(7):2353–2366. doi: 10.1093/nar/10.7.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Panet A., Cedar H. Selective degradation of integrated murine leukemia proviral DNA by deoxyribonucleases. Cell. 1977 Aug;11(4):933–940. doi: 10.1016/0092-8674(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Pays E., Delronche M., Lheureux M., Vervoort T., Bloch J., Gannon F., Steinert M. Cloning and characterization of DNA sequences complementary to messenger ribonucleic acids coding for the synthesis of two surface antigens of Trypanosoma brucei. Nucleic Acids Res. 1980 Dec 20;8(24):5965–5981. doi: 10.1093/nar/8.24.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Lheureux M., Steinert M. The expression-linked copy of a surface antigen gene in Trypanosoma is probably the one transcribed. Nature. 1981 Jul 16;292(5820):265–267. doi: 10.1038/292265a0. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Meirvenne N., Le Ray D., Steinert M. Gene duplication and transposition linked to antigenic variation in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1981 May;78(5):2673–2677. doi: 10.1073/pnas.78.5.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., August T. The use of immunoprecipitation to study the synthesis and cleavage processing of viral proteins. J Immunol Methods. 1976;13(2):153–159. doi: 10.1016/0022-1759(76)90153-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Newlon C. S., Herskowitz I., Hicks J. B. Isolation of a circular derivative of yeast chromosome III: implications for the mechanism of mating type interconversion. Cell. 1979 Oct;18(2):309–319. doi: 10.1016/0092-8674(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Van Meirvenne N., Janssens P. G., Magnus E. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. 1. Rationalization of the experimental approach. Ann Soc Belg Med Trop. 1975;55(1):1–23. [PubMed] [Google Scholar]
- Van der Ploeg L. H., Bernards A., Rijsewijk F. A., Borst P. Characterization of the DNA duplication-transposition that controls the expression of two genes for variant surface glycoproteins in Trypanosoma brucei. Nucleic Acids Res. 1982 Jan 22;10(2):593–609. doi: 10.1093/nar/10.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Young J. R., Majiwa P. A., Doyle J. J., Shapiro S. Z. Contextural genomic rearrangements of variable-antigen genes in Trypanosoma brucei. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):945–949. doi: 10.1101/sqb.1981.045.01.111. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Young J. R., Majiwa P. A. Genomic rearrangements correlated with antigenic variation in Trypanosoma brucei. Nature. 1979 Dec 20;282(5741):847–849. doi: 10.1038/282847a0. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Camerini-Otero R. D. Limited DNase I nicking as a probe of gene conformation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1907–1911. doi: 10.1073/pnas.77.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]