Abstract
Some copy number variants (CNVs) are strongly implicated in both schizophrenia and autism spectrum disorders (ASDs). Childhood-onset schizophrenia (COS) occurs rarely with 0.1 – 1% of all schizophrenia diagnoses manifesting before age 10. 3q29 deletions are associated with both autism and schizophrenia, and are rare - the frequency of the deletion estimated to be 1 in 1750 in developmental disorders. Only one patient with a 3q29 deletion was identified out of the first 1174 families with ASDs included in the Simons Simplex Collection (SSC). We report on detailed clinical findings for this patient with a de novo 3q29 deletion who, as a young child, developed a very rare overlap of symptoms of both autism and early onset psychosis. His ASD was first diagnosed at the age of 4 years and his psychotic symptoms began at 5 years old. This is only the second case reported thus far of this rare event of co-occurring autism and very early onset psychosis in a child with a 3q29 deletion. It is also the earliest case of a child with autism developing comorbid psychosis - manifesting by the age of 5 years.
Keywords: autism, 3q29, DLG1, PAK2, intellectual disability, psychosis
INTRODUCTION
Multiple rare de novo copy number variants (CNVs) contribute to genetic vulnerability to autism and schizophrenia [Cook and Scherer, 2008]. CNVs such as 1q21.1 deletion [Stone, 2008; Levinson et al., 2011; Sanders et al., 2011], 16p11.2 duplication [Cook and Scherer, 2008; McCarthy et al., 2009; Rapoport et al., 2009; Levinson et al., 2011; Sanders et al., 2011],17q12 deletion [Moreno-De-Luca et al., 2010], and 22q11.2 deletion [Vorstman et al., 2006; Cook and Scherer, 2008; Rapoport et al., 2009; Levinson et al., 2011], are found both in autism and schizophrenia. Interestingly, despite the overlap of associated CNVs, the rate of developing schizophrenia in ASDs is only approximately 1% [Volkmar and Cohen, 1991], with the vast majority of cases of schizophrenia having an age of onset in adolescence or young adulthood.
3q29 deletions are rare with the frequency estimated to be 1 in 1750 in developmental disorders [Kaminsky et al., 2011]. Sanders and colleagues [2011] identified only one patient, this one, with a 3q29 deletion out of the first 1174 families with ASDs included in the Simons Simplex Collection (SSC). Previously reported features associated with the 3q29 deletion include schizophrenia, autism, depression, speech delay, learning disabilities, delayed walking, long and narrow face, microcephaly, macrocephaly, long tapering fingers, joint laxity, high nasal bridge, recurrent middle ear infections, chest wall deformity (both pectus carinatum and pectus excavatum), ataxic gait, cleft lip and palate, high palate, horseshoe kidney, hypospadias, ligamentous laxity, joint contractures, abnormal skin pigmentation, crowded/dysplastic teeth, widely spaced teeth, adenoidectomy, removal of cholesteatoma, downslanting palpebral fissures, large low-set posteriorly rotated ears, and smooth philtrum [Willatt et al., 2005; Ballif et al., 2008; Clayton-Smith et al., 2010; Cobb et al., 2010; Mulle et al., 2010; Quintero-Rivera et al., 2010]. Willatt and colleagues [2005] found the most consistent feature of the 3q29 microdeletion syndrome to be mild to moderate intellectual disability, although Cobb and colleagues described a patient with a Full Scale IQ in the low average range [2010].
We present the detailed clinical findings of a patient with the very rare co-occurrence of autism and childhood-onset psychosis at the age of 5 years, and a de novo 3q29 deletion identified in the SSC genetic study. This is the second case reported with a documented early history of symptoms that confirms a co-occurrence of both autism and psychosis in a child with this deletion; the first was written by Quintero-Rivera and colleagues and described a girl who had an older age of onset of psychosis, by the age of 10 years [2010].
MATERIALS and METHODS
Genetic Results
Fragile X testing was negative for the patient. As part of the IRB approved Simons Simplex study, DNA from whole blood from the patient and both parents was examined using the Human 1M DNA Analysis BeadChip (Illumina Inc., San Diego, CA, USA) microarray revealing a de novo deletion of 3q29 (chr3:197,161,073-198,851,029); hg18/NCBI build 36.1). The deletion was confirmed by fluorescence in situ hybridization (FISH) analysis of the lymphoblastoid cell line from the SSC repository using BAC clone RP11-481O2 from the abnormal region (See Fig 1). The deletion was subsequently confirmed in a clinical laboratory using the EmArray Cyto60K custom oligonucleotide array (Emory Genetics Laboratory, Atlanta, GA) with a separately drawn sample to allow reporting of clinical results back to the family. The deletion determined using EmArray was chr3:197,224,799-198,801,500 (hg18/NCBI build 36.1) (See Fig 1).
Figure 1. Results from Microarray and Confirmatory FISH Analyses Showing the 3q29 Deletion.
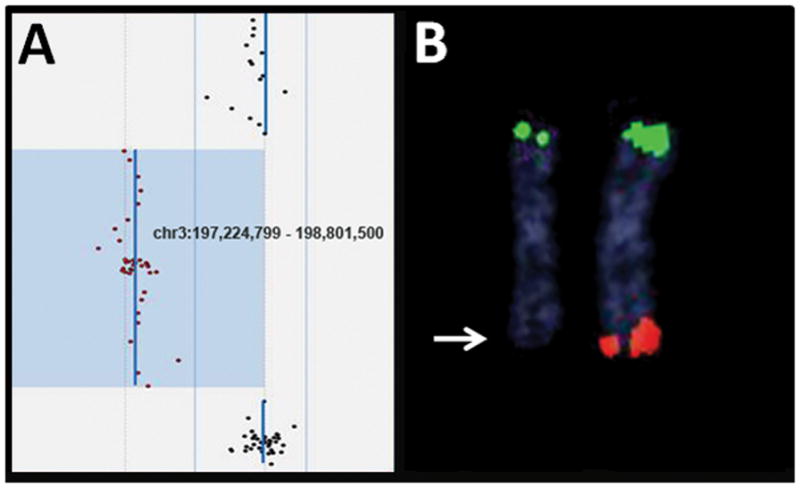
A) The deleted region, identified by EmArray microarray analysis, is indicated by the abnormal oligonucleotide probes (red) shifted to the left from the normal oligonucleotide probes (black).
B) FISH analysis with probes for the 3p (green)(BAC clone CTC-228K22) and 3q (red)(BAC clone CTB-56H22) telomere regions confirmed the deletion (arrow)
Research Evaluation
At age 12 years the patient was enrolled in the SSC study according to a protocol approved by the University of Illinois at Chicago IRB. He was administered the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview – Revised (ADI-R) and met criteria for classification of autism on both instruments. The Differential Abilities Scale (DAS) second edition demonstrated a full scale IQ of 53 (mean 100, standard deviation (SD) 15). The Peabody Picture Vocabulary Test 4 (PPVT4) was administered to assess receptive language (standard score 87; mean 100, SD 15). His Child Behavior Check List (CBCL) Thought Problem syndrome score was elevated with a T score of 84, greater than the 97th percentile (Tscore distribution mean 50, SD 10). His Somatic Complaints syndrome score was also clinically significant with a T score of 72. On the DSM oriented scales his score for Affective Problems was also elevated with a T score of 78 and Anxiety Problems was elevated at T score of 71. His overall adaptive behavior composite standard score on Vineland – II was 64 (mean 100, SD 15).
CLINICAL REPORT
The patient was born at term to nonconsanguineous healthy parents via vaginal birth after an uncomplicated pregnancy. His gestational age was 40 weeks, he weighed 3402 g (50th centile) and had a birth length of 53 cm (95th centile). He had some difficulties regulating temperature at birth. At 6 – 9 months of age, he had hypotonia and difficulty bearing his own weight. He began demonstrating abnormal movements of his arms and legs and was diagnosed with possible phenylketonuria and possible Tourette’s syndrome. As an infant, he also had gastroesophageal reflux.
At age 2 years he had tics, obsessive compulsive behaviors, choreoathetoid, and stereotyped movements. He was evaluated by a pediatric neurologist, and was noted to have mild asymmetries on the right including an abnormal gait, brisker reflexes on the right, upgoing toe on the right, a tendency to circumduct the right foot during gait testing, subtle decrease of nasal labial fold on the right, and some delay of right compared to left sided smile. An MRI done at age 2 was normal.
He was given a diagnosis of pervasive developmental disorder not otherwise specified (PDD NOS) around the age of 4 years. Also around 4 years of age, a diagnosis of Sydenham chorea and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS) was considered in the context of worsening of abnormal movements and tics with pharyngitis.
Over the course of his childhood development, his movement disorder was so severe that he experienced fractures of the ulna and of at least one toe before the age of 5 years.
At age 5 years he had chicken pox, and his mother reported that it led to the onset of an “odd psychotic state” – he covered his ears, saw things on the wall moving, and even looked at his mother and did not recognize her.
His history of psychotic symptoms included auditory hallucinations of a clock ticking inside his head and visual hallucinations. He misinterpreted noises and was unable to leave the house because of his hallucinations.
On examination at age 16 years, he had dysmorphic pinnae, wide (11.25 mm) and high nasal bridge, pectus excavatum, and an unusually severe presentation of antipsychotic withdrawal dyskinesia – characterized by involuntary facial grimacing, new-onset posturing and fanning of his fingers when talking, lateral tongue movements, toe curling, involuntary lateral eye movements, his neck was being pulled to his chest, and he had cogwheel rigidity of arms. His Abnormal Involuntary Movements Scale (AIMS) score was 25 (maximum score 40).
His family history is significant for bipolar disorder in maternal aunt.
Medication Trials
Prior to his enrollment in the SSC, the patient was prescribed multiple medications for his neuropsychiatric symptoms. The duration and doses of many of these treatments are unknown but included a long list of typical and atypical antipsychotics, antidepressants, and mood stabilizers.
After many medication trials, he improved on loxapine. At age 16 he experienced an exacerbation in his symptoms of perseveration, obsessions, and compulsions. As loxapine no longer controlled his symptoms he was tapered off of it over the course of 3–4 weeks while simultaneously being up-titrated on quetiapine to 150mg per day. Approximately 1 week after completely discontinuing loxapine, he experienced severe symptoms consistent with a withdrawal dyskinesia as described above.
DISCUSSION
We describe a patient with the very rare co-occurrence of autism and childhood onset psychosis, obsessive compulsive disorder, and severe withdrawal dyskinesia, with an approximately 1.58 Mb deletion of 3q29. He presented with psychotic symptoms including auditory hallucinations that responded to antipsychotic medications. Childhood-onset psychosis is rare; 0.1 – 1% of all schizophrenia diagnoses manifest before age 10 years [Remschmidt et al., 1994]. While there are a few reports of autism and 3q29 deletion, and other reports of schizophrenia and 3q29 deletion, this is only the second report of autism co-occurring with early onset psychotic symptoms in a patient with this deletion and with even rarer onset of psychotic symptoms before the age of 6 years.
This patient had several additional clinical features that may have resulted from the 3q29 microdeletion including mild mental retardation, ataxic gait, pectus excavatum, recurrent middle ear infections, dysmorphic pinnae, and high nasal bridge.
The recurrent deletion in 3q29 mediated by segmental duplications in this patient is approximately 1.58 Mb in size and encompasses many genes including ones implicated in intellectual disability such as PAK2 and DLG1 [Willatt et al., 2005; Mulle et al., 2010; Dasouki et al., 2011], and a gene involved in neuronal migration, FBXO45 [Willatt et al., 2005; Saiga et al., 2009; Mulle et al., 2010; Dasouki et al., 2011]. Previous cases reported with deletions of 3q29 have ranged in size from 0.8 Mb [Mulle et al., 2010] to 2.1 Mb [Quintero-Rivera et al., 2010]. DLG1 is part of the of Neurexin/Neuroligin/DLG/SAPAP/Shank core transsynaptic complex. Mutations of genes in this complex may confer susceptibility to ASDs [Peca and Feng, 2012]. DLG1 is a paralogue of the X linked gene, DLG3, which when disrupted may cause intellectual disability [Tarpey et al., 2004]. In a study of post-mortem brain tissue it was found that DLG1 had decreased protein levels in the prefrontal cortex of patients with schizophrenia when compared to controls [Toyooka et al., 2002]. Toyooka and colleagues hypothesized that the reduction of DLG1 protein in the prefrontal cortex likely contributed to glutamatergic dysfunction [2002].
The relationship of DLG1 haploinsufficiency to dyskinesias, psychotic symptoms, and obsessive-compulsive disorder (OCD) is unclear. Carroll and colleagues could not find evidence for any amino acid changing genetic variants in PAK2 or an excess of DLG1 rare and singleton non-synonymous genetic variations in cases with schizophrenia when compared to control; however they noted that they may have had an underpowered study [2011]. DLG1 interacts with the GluA1 AMPA receptor subunit; AMPA receptors bind to glutamate [Howard et al., 2010]. Increased glutamatergic activity is implicated in the development and maintenance of levodopa induced dyskinesias [Ouattara et al., 2011]. It is possible that a DLG1 deletion affected glutamate receptor function and contributed to our patient’s dyskinesia. SAP90/PSD95-associated proteins (SAPAPs) induce the translocation of DLG4 from the cytosol to the plasma membrane [Takeuchi et al., 1997]. SAPAP3 is a scaffolding protein which leads to anxiety and OCD symptoms when its gene is deleted in mice [Welch et al., 2007]. Based on these observations, it is possible that a deletion of one copy of DLG1 resulted in impaired binding of SAPAPs which may have contributed to the patient’s OCD symptoms.
FBXO45, also on 3q29, is a gene with a role in neurotransmitter regulation [Tada et al., 2010] that may also contribute to autism or schizophrenia when disrupted. FBXO45, when disrupted, may lead to dysfunctional neurotransmission and disruptions in neuronal communication [Tada et al., 2010]. Saija and colleagues [2009] found FBXO45 is required for normal neuromuscular synaptogenesis, axon path-finding, and neuronal migration. Quintero-Rivera and colleagues proposed that FBXO45 haploinsufficiency may lead to alterations in synaptic glutamate release via accumulation of synaptic proteins normally degraded [2010].
Discontinuation of the antipsychotic loxapine in the patient resulted in an extremely severe withdrawal dyskinesia, characterized by grimacing, posturing, involuntarily deviated gaze and tongue movements, increased tone, and worsening of gait ataxia. The severity of the withdrawal dyskinesia (AIMS=25) is greatly beyond that normally observed in clinical practice, particularly for a relatively lower dose of loxapine (16% of the maximum recommended dose) and only 4 years of treatment. It is unknown if the patient’s previously described movement disorders and/or neurotransmitter dysfunction that resulted from his 3q29 deletion influenced this presentation.
Quintero-Rivera and colleagues [2010] described the first case of a child with autism and co-occurrence of psychosis: a girl with a 1.6 Mb copy number loss of 3q29 who at the age of 5 years had been diagnosed with autism and by the age of 10 years suffered with symptoms of auditory hallucinations and violence towards others. Her differential diagnosis was bipolar disorder with psychotic features versus schizophrenia and her deletion was paternal in origin; her father, who was also diagnosed with bipolar disorder, did not carry the deletion [Quintero-Rivera et al., 2010]. Mulle and colleagues [2010] described an individual they felt strengthened the link with schizophrenia, autism and intellectual disability, who had schizophrenia, a 836 kb 3q29 deletion, and a childhood history of mild learning disability and impaired social interaction. They found deletions in 3q29 to be statistically significantly associated with schizophrenia, including 6 cases with the deletion and none of the controls having this deletion [Mulle et al., 2010].
This patient had a de novo 3q29 deletion, autism with very rare co-occurring early onset psychotic symptoms, and several physical features associated with his deletion. This is the second patient (with the youngest age of onset of psychotic symptoms) reported in the literature of this extremely rare event of co-occurring pre-pubertal psychotic symptoms and autism being associated with a 3q29 deletion.
Acknowledgments
We thank the patient and his family for their cooperation, assistance, and support in this project. This work was supported in part by 5T32MH067631-07 Training in the Neuroscience of Mental Health (AS), K08MH083888 (JRB), and grant support from the Simons Foundation (CLM, EC).
Footnotes
The authors have no conflicts of interest to disclose.
References
- Ballif BC, Theisen A, Coppinger J, Gowans GC, Hersh JH, Madan-Khetarpal S, Schmidt KR, Tervo R, Escobar LF, Friedrich CA, McDonald M, Campbell L, Ming JE, Zackai EH, Bejjani BA, Shaffer LG. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplication. Mol Cytogenet. 2008;1:8. doi: 10.1186/1755-8166-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LS, Williams HJ, Walters J, Kirov G, O’Donovan MC, Owen MJ. Mutation screening of the 3q29 microdeletion syndrome candidate genes DLG1 and PAK2 in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:844–849. doi: 10.1002/ajmg.b.31231. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, Giblin C, Smith RA, Dunn C, Willatt L. Familial 3q29 microdeletion syndrome providing further evidence of involvement of the 3q29 region in bipolar disorder. Clin Dysmorphol. 2010;19:128–132. doi: 10.1097/MCD.0b013e32833a1e3c. [DOI] [PubMed] [Google Scholar]
- Cobb W, Anderson A, Turner C, Hoffman RD, Schonberg S, Levin SW. 1.3 Mb de novo deletion in chromosome band 3q29 associated with normal intelligence in a child. Eur J Med Genet. 2010;53:415–418. doi: 10.1016/j.ejmg.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455:919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- Dasouki MJ, Lushington GH, Hovanes K, Casey J, Gorre M. The 3q29 microdeletion syndrome: report of three new unrelated patients and in silico “RNA binding” analysis of the 3q29 region. Am J Med Genet A. 2011;155A:1654–1660. doi: 10.1002/ajmg.a.34080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MA, Elias GM, Elias LA, Swat W, Nicoll RA. The role of SAP97 in synaptic glutamate receptor dynamics. Proc Natl Acad Sci U S A. 2010;107:3805–3810. doi: 10.1073/pnas.0914422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky EB, Kaul V, Paschall J, Church DM, Bunke B, Kunig D, Moreno-De-Luca D, Moreno-De-Luca A, Mulle JG, Warren ST, Richard G, Compton JG, Fuller AE, Gliem TJ, Huang S, Collinson MN, Beal SJ, Ackley T, Pickering DL, Golden DM, Aston E, Whitby H, Shetty S, Rossi MR, Rudd MK, South ST, Brothman AR, Sanger WG, Iyer RK, Crolla JA, Thorland EC, Aradhya S, Ledbetter DH, Martin CL. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med. 2011;13:777–784. doi: 10.1097/GIM.0b013e31822c79f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, Zhang N, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Kendler KS, Freedman R, Dudbridge F, Pe’er I, Hakonarson H, Bergen SE, Fanous AH, Holmans PA, Gejman PV. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, Perkins DO, Dickel DE, Kusenda M, Krastoshevsky O, Krause V, Kumar RA, Grozeva D, Malhotra D, Walsh T, Zackai EH, Kaplan P, Ganesh J, Krantz ID, Spinner NB, Roccanova P, Bhandari A, Pavon K, Lakshmi B, Leotta A, Kendall J, Lee YH, Vacic V, Gary S, Iakoucheva LM, Crow TJ, Christian SL, Lieberman JA, Stroup TS, Lehtimaki T, Puura K, Haldeman-Englert C, Pearl J, Goodell M, Willour VL, Derosse P, Steele J, Kassem L, Wolff J, Chitkara N, McMahon FJ, Malhotra AK, Potash JB, Schulze TG, Nothen MM, Cichon S, Rietschel M, Leibenluft E, Kustanovich V, Lajonchere CM, Sutcliffe JS, Skuse D, Gill M, Gallagher L, Mendell NR, Craddock N, Owen MJ, O’Donovan MC, Shaikh TH, Susser E, Delisi LE, Sullivan PF, Deutsch CK, Rapoport J, Levy DL, King MC, Sebat J. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–1227. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-De-Luca D, Mulle JG, Kaminsky EB, Sanders SJ, Myers SM, Adam MP, Pakula AT, Eisenhauer NJ, Uhas K, Weik L, Guy L, Care ME, Morel CF, Boni C, Salbert BA, Chandrareddy A, Demmer LA, Chow EW, Surti U, Aradhya S, Pickering DL, Golden DM, Sanger WG, Aston E, Brothman AR, Gliem TJ, Thorland EC, Ackley T, Iyer R, Huang S, Barber JC, Crolla JA, Warren ST, Martin CL, Ledbetter DH. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet. 2010;87:618–630. doi: 10.1016/j.ajhg.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle JG, Dodd AF, McGrath JA, Wolyniec PS, Mitchell AA, Shetty AC, Sobreira NL, Valle D, Rudd MK, Satten G, Cutler DJ, Pulver AE, Warren ST. Microdeletions of 3q29 confer high risk for schizophrenia. Am J Hum Genet. 2010;87:229–236. doi: 10.1016/j.ajhg.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara B, Gregoire L, Morissette M, Gasparini F, Vranesic I, Bilbe G, Johns DR, Rajput A, Hornykiewicz O, Rajput AH, Gomez-Mancilla B, Di Paolo T. Metabotropic glutamate receptor type 5 in levodopa-induced motor complications. Neurobiol Aging. 2011;32:1286–1295. doi: 10.1016/j.neurobiolaging.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Peca J, Feng G. Cellular and synaptic network defects in autism. Curr Opin Neurobiol. 2012 doi: 10.1016/j.conb.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Rivera F, Sharifi-Hannauer P, Martinez-Agosto JA. Autistic and psychiatric findings associated with the 3q29 microdeletion syndrome: case report and review. Am J Med Genet A. 2010;152A:2459–2467. doi: 10.1002/ajmg.a.33573. [DOI] [PubMed] [Google Scholar]
- Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48:10–18. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remschmidt HE, Schulz E, Martin M, Warnke A, Trott GE. Childhood-onset schizophrenia: history of the concept and recent studies. Schizophr Bull. 1994;20:727–745. doi: 10.1093/schbul/20.4.727. [DOI] [PubMed] [Google Scholar]
- Saiga T, Fukuda T, Matsumoto M, Tada H, Okano HJ, Okano H, Nakayama KI. Fbxo45 forms a novel ubiquitin ligase complex and is required for neuronal development. Mol Cell Biol. 2009;29:3529–3543. doi: 10.1128/MCB.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, Chu SH, Moreau MP, Gupta AR, Thomson SA, Mason CE, Bilguvar K, Celestino-Soper PBS, Choi M, Crawford EL, Davis L, Wright NRD, Dhodapkar RM, DiCola M, DiLullo NM, Fernandez TV, Fielding-Singh V, Fishman DO, Frahm S, Garagaloyan R, Goh GS, Kammela S, Klei L, Lowe JK, Lund SC, McGrew AD, Meyer KA, Moffat WJ, Murdoch JD, O’Roak BJ, Ober GT, Pottenger RS, Raubeson MJ, Song Y, Wang Q, Yaspan BL, Yu TW, Yurkiewicz LR, Beaudet AL, Cantor RM, Curland M, Grice DE, Gunel M, Lifton RP, Mane SM, Martin DM, Shaw CA, Sheldon M, Tischfield JA, Walsh CA, Morrow EM, Ledbetter DH, Fombonne E, Lord C, Martin CL, Brooks AI, Sutcliffe JS, Cook EH, Geschwind D, Roeder K, Devlin B, State MW. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JLODM, Gurling H, Kirov GK, Blackwood DH, Corvin A, Craddock NJ, Gill M, Hultman CM, Lichtenstein P, McQuillin A, Pato CN, Ruderfer DM, Owen MJ, St Clair D, Sullivan PF, Sklar P, Purcell SM, Stone JL, Ruderfer DM, Korn J, Kirov GK, Macgregor S, McQuillin A, Morris DW, O’Dushlaine CT, Daly MJ, Visscher PM, Holmans PA, O’Donovan MC, Sullivan PF, Sklar P, Purcell SM, Gurling H, Corvin A, Blackwood DH, Craddock NJ, Gill M, Hultman CM, Kirov GK, Lichtenstein P, McQuillin A, O’Donovan MC, Owen MJ, Pato CN, Purcell SM, Scolnick EM, St Clair D, Stone JL, Sullivan PF, Sklar P, O’Donovan MC, Kirov GK, Craddock NJ, Holmans PA, Williams NM, Georgieva L, Nikolov I, Norton N, Williams H, Toncheva D, Milanova V, Owen MJ, Hultman CM, Lichtenstein P, Thelander EF, Sullivan P, Morris DW, O’Dushlaine CT, Kenny E, Waddington JL, Gill M, Corvin A, McQuillin A, Choudhury K, Datta S, Pimm J, Thirumalai S, Puri V, Krasucki R, Lawrence J, Quested D, Bass N, Curtis D, Gurling H, Crombie C, Fraser G, Kwan SL, Walker N, St Clair D, Blackwood DH, Muir WJ, McGhee KA, Pickard B, Malloy P, Maclean AW, Van Beck M, Visscher PM, Macgregor S, Pato MT, Medeiros H, Middleton F, Carvalho C, Morley C, Fanous A, Conti D, Knowles JA, Ferreira CP, Macedo A, Azevedo MH, Pato CN, Stone JL, Ruderfer DM, Korn J, McCarroll SA, Daly M, Purcell SM, Sklar P, Purcell SM, Stone JL, Chambert K, Ruderfer DM, Korn J, McCarroll SA, Gates C, Daly MJ, Scolnick EM, Sklar P. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Okano HJ, Takagi H, Shibata S, Yao I, Matsumoto M, Saiga T, Nakayama KI, Kashima H, Takahashi T, Setou M, Okano H. Fbxo45, a novel ubiquitin ligase, regulates synaptic activity. J Biol Chem. 2010;285:3840–3849. doi: 10.1074/jbc.M109.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- Tarpey P, Parnau J, Blow M, Woffendin H, Bignell G, Cox C, Cox J, Davies H, Edkins S, Holden S, Korny A, Mallya U, Moon J, O’Meara S, Parker A, Stephens P, Stevens C, Teague J, Donnelly A, Mangelsdorf M, Mulley J, Partington M, Turner G, Stevenson R, Schwartz C, Young I, Easton D, Bobrow M, Futreal PA, Stratton MR, Gecz J, Wooster R, Raymond FL. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:318–324. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Iritani S, Makifuchi T, Shirakawa O, Kitamura N, Maeda K, Nakamura R, Niizato K, Watanabe M, Kakita A, Takahashi H, Someya T, Nawa H. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J Neurochem. 2002;83:797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Cohen DJ. Comorbid association of autism and schizophrenia. Am J Psychiatry. 1991;148:1705–1707. doi: 10.1176/ajp.148.12.1705. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, Swaab H, Kahn RS, van Engeland H. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willatt L, Cox J, Barber J, Cabanas ED, Collins A, Donnai D, FitzPatrick DR, Maher E, Martin H, Parnau J, Pindar L, Ramsay J, Shaw-Smith C, Sistermans EA, Tettenborn M, Trump D, de Vries BB, Walker K, Raymond FL. 3q29 microdeletion syndrome: clinical and molecular characterization of a new syndrome. Am J Hum Genet. 2005;77:154–160. doi: 10.1086/431653. [DOI] [PMC free article] [PubMed] [Google Scholar]


