Abstract
Objectives
To identify clinical variables predictive of the risk of thromboembolism (TE), and to confirm the incidence of TE in primary and secondary childhood nephrotic syndrome (NS).
Study design
A comprehensive chart review identified 326 children with NS from any cause evaluated between 1999 and 2006. These patients had a total of 1472.8 patient-years of follow-up. Comparison statistics, survival analysis, and logistic regression were used to define TE epidemiology and clinical risk factors.
Results
We found that 9.2% of our cohort had experienced at least 1 TE. The overall incidence was 20.4 patients with TEs/1000 patient-years. The median time to the first TE was 70.5 days after diagnosis of NS. Deep venous thrombosis was the most common TE (76%) and was frequently associated with the use of a central venous catheter (45%). Significant independent predictors of TE included age ≥ 12 years at onset of NS (P < .0001), severity of proteinuria (P < .0001), and history of TE preceding diagnosis of NS (P < .0001). Life- or limb-threatening TEs represented 23.7% of the events.
Conclusions
Children with NS should be carefully followed for TE, particularly those who are age 12 years or older, have severe proteinuria, or have a previous history of TE.
Nephrotic syndrome (NS) is most commonly idiopathic in nature, resulting from any of several well-described primary glomerulopathies that are defined by histopathology and clinical criteria.1 Idiopathic NS has an incidence of 2 to 7 per 100 000 children and a prevalence of about 16 cases per 100 000 children per year.2 In addition, several well-recognized secondary glomerulopathies may result in nephrotic range proteinuria or NS.1 Regardless of the underlying etiology, common complications of NS and its treatment include infection, cardiovascular disease, bone mineral loss, acute renal failure, and thromboembolism (TE).2
TE has been reported in 1.8% to 5% of children with idiopathic NS, in contrast to the much higher incidence in adults (20% to 30%).2,3 NS-associated TE has been studied extensively in idiopathic NS, with the focus primarily on acquired hemostatic defects, such as urinary loss of natural anticoagulant proteins.4 Our goal in the present study was to better describe the spectrum and incidence of TE in a population of children with NS, including both primary and secondary glomerulopathies. We hypothesized that specific, identifiable clinical variables would predict the risk for TE in these children. To test this hypothesis, we performed a multicenter chart review of a large number of children diagnosed with NS in an attempt to identify these variables.
Methods
Patients with NS were identified from inpatient and outpatient records at Nationwide Children's Hospital, Columbus, Ohio and the University of Michigan Health System, C.S. Mott Children's Hospital, Ann Arbor, Michigan. Probable records were identified using an ICD-9-CM search (usingNS codes 581.0-581.9, 582.1, and 583.1) and reviewed for eligibility. All patients age ≤ 21 years at the onset of NS who were newly diagnosed or seen for follow-up between January 1, 1999 and March 31, 2006 (a period of 7.25 years) were eligible for inclusion. The resulting time range of NS diagnosis was October 1, 1983 to March 14, 2006 (a period of 22.5 years). NS was defined as serum albumin concentration ≤ 3.0 g/dL and nephrotic-range proteinuria (urine protein:creatinine ratio [uPr:Cr] ≥ 2.0 in a spot urine sample). uPr:Cr was not available for a few of the younger patients, but these patients were included anyway, based on the presence of both a urine protein of 4+ by dipstick and serum albumin ≤ 3.0 g/dL. The study protocol was approved by each institution's institutional review board.
Data collected from the medical records included demographic information, date of NS onset, type of NS, nadir serum albumin level, peak uPr:Cr, clinical or radiologic evidence of TE, date(s) of TE, anatomic site(s) of TE, and history of central venous access device (CVAD) use within any TE-affected vessel. At one of the institutions, all patients with TE were screened for prothrombotic risk factors according to institutional protocol. These studies included genetic evaluation for factor V Leiden (FVL) and prothrombin G20210A gene mutation; protein C, protein S (PS), and antithrombin III (AT3) activities; plasma homocysteine; and assessment for the presence of antiphospholipid antibodies or lupus anticoagulant (APL). Because of incomplete follow-up, confirmation of antiphospholipid syndrome in patients with APL using strict diagnostic criteria was not possible.5,6 Age at onset of NS and time from onset of NS to first TE were calculated. TE events occurring within 1 week before, simultaneous with, or after diagnosis of NS were considered to be associated with NS. TE occurring more than 1 week before diagnosis of NS were evaluated as a risk factor for TE but were not considered to be associated with NS.
Histopathologic diagnoses were defined by the pathologists at each institution using standard criteria. For the purpose of analysis, the patients were divided into 2 groups based on histopathology and clinical course. The group designated “primary NS” comprised patients with primary glomerulopathies, including steroid-sensitive NS (SSNS) with no biopsy performed (n = 81), biopsy-proven minimal-change NS (n = 56), focal segmental glomerulosclerosis (n = 63), mesangial proliferative glomerulonephritis (n = 17), membranous nephropathy (MN; n = 10), membranoproliferative glomerulonephritis (n = 14), and congenital NS (n = 3). SSNS was defined as resolution of proteinuria (trace or negative protein by dipstick) on 3 consecutive days in response to oral prednisone 2 mg/kg/day.1 The “secondary NS” group comprised patients with NS due to secondary glomerulopathies caused by other illnesses, including systemic lupus erythematosis (SLE; n = 48), Henoch-Schonlein purpura nephritis (n = 6), crescentic glomerulonephritis (n = 6), IgA nephropathy (n = 5), and various others (n = 17). The patients with SLE all met the American College of Rheumatology criteria for SLE diagnosis and had renal biopsy findings consistent with SLE glomerulonephritis.7,8 In adults, MN is associated with increased risk for TE complications; the lower incidence of MN in children precluded a direct assessment of this risk factor.3 Consequently, a special category, “membranous histology,” including patients with either MN or class V SLE histology (which closely resembles MN histologically) was created as a surrogate to allow assessment of this possible risk factor.9 Pulmonary embolism (PE) was defined as any thrombus within a pulmonary artery, regardless of whether or not a primary deep venous thrombosis (DVT) was identifiable.
Basic demographic comparisons were performed between patients with TE and those without TE. Two-tailed Student t-tests or nonparametric Wilcoxon rank-sum tests were used for continuous variables, and χ2 tests or Fisher exact tests were used for categorical variables. The Holm step-down method was applied to control the type I error rate, using an overall level of significance of α = 0.05. Limited multivariate logistic regression modeling was performed using forward stepwise selection to estimate the relative significance of the various potential predictors. The presence of significant 2-way interactions was determined, and odds ratios (ORs) and 95% confidence intervals (CI) were reported. Model fit and discrimination were assessed using the Hosmer-Lemeshow goodness-of-fit test and the area under the receiver operating characteristic curve, respectively. Kaplan-Meier plots were produced for survival analysis using the Mantel-Cox log-rank test. For the univariate, multivariate, and survival analyses, TE occurring within 5 years after the diagnosis of NS was considered the primary outcome. All analyses were performed using SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina) and Stata/SE 9.2 (Stata-Corp, College Station, Texas).
Results
A total of 370 patient charts with the appropriate ICD-9-CM codes were identified within the specified time frame and screened for inclusion. Of these patients, 326 met all of the eligibility criteria for inclusion (Table I). The cohort was 52% male and 69% Caucasian, with a median age of 6.5 years at NS diagnosis. Median follow-up time was 3.7 years after the onset of NS, with a total of 1472.8 patient-years of follow-up for the cohort. Patients with primary NS represented 75% of the cohort; patients with SLE comprised 59% of the secondary NS group.
Table I. Patient demographics.
| Category | Total | Non-TE patients | TE patients |
|---|---|---|---|
| Number of patients | 326 | 296 | 30 |
| Sex, male, n (%) | 168 (52%) | 156 (53%) | 12 (40%) |
| Age at NS diagnosis, years, median (range)* | 6.5 (0-20.7) | 6.2 (0-20.7) | 13.8 (1-20.3) |
| Age ≥ 12 years at NS diagnosis, n (%)† | 99 (30%) | 80 (27%) | 19 (63%) |
| Age at TE diagnosis, years, median (range) | 15.2 (1-21.6) | ||
| Race, n (%) | |||
| African-American | 68 (21%) | 60 (20%) | 8 (27%) |
| Caucasian | 224 (69%) | 204 (69%) | 20 (67%) |
| Other | 34 (10%) | 32 (11%) | 2 (7%) |
| NS category, n (%)* | |||
| Primary NS | 244 (75%) | 228 (77%) | 16 (53%) |
| Secondary NS | 82 (25%) | 68 (23%) | 14 (47%) |
| Follow-up time, years, median (range) | 3.7 (0-20.8) | 3.7 (0-20.8) | 3.7 (0.3-14) |
| Peak uPr:Cr, median (range)* | 10.0 (2.1-307.7) | 9.8 (2.1-307.7) | 14.2 (3.9-294.7) |
| Serum albumin nadir, median (range) | 1.9 (0.6-3) | 1.9 (0.6-3) | 1.8 (1-2.7) |
| Previous history of TE, n (%)† | 5 (2%) | 1 (0.3%) | 4 (13%) |
| Membranous histology, n (%)*,s‡ | 32 (10%) | 24 (8%) | 8 (27%) |
Comparison between patients without and with TEs.
P < .01.
P < .0001.
Class V SLE or MN.
Of the 326 patients in the cohort, 30 (9.2%) had a total of 38 NS-associated TEs, for an incidence of 20.4 patients with TEs per 1000 patient-years of follow-up, or 25.8 TE events per 1000 patient-years. TEs occurred in 6.6% of the patients in the primary NS group and 17.1% of those in the secondary NS group (P < .01) (Table I). The raw incidence of TE was slightly higher in females than in males (25.3 vs 15.8/1000 patient-years) and slightly higher in African-Americans than in Caucasians (28.5 vs 19.1/1000 patient-years). The sex and ethnicity differences in TE incidence did not reach statistical significance, however (Table I).
In the patients with TE, the median age of NS onset was 13.8 years, the median age at first TE was 15.2 years (Table I), and the median time from NS diagnosis to first TE was 70.5 days (Table II; available at www.jpeds.com). Four patients (13.3%) presented with TE simultaneous to NS diagnosis. The 38 TE events included 29 DVTs, 8 PEs, 1 arterial thrombus, and 1 thrombosed arteriovenous fistula (1 patient had both a PE and a DVT). Nine of these events (23.7%; 8 PE and 1 arterial thrombus) were considered potentially life- or limb-threatening events, for an incidence of 6.1 life- or TEs per 1000 patient-years.
Table II. Details of patients with TE complications of NS.
| Patient | NS type* | Sex | Race | Age at NS onset, years | Follow-up, years | TE events/patient | TE type | Time from NS onset to first TE, days | CVAD | Thrombophilia |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Secondary | F | Caucasian | 11.3 | 6.1 | 1 | DVT | 1839 | Yes | NP |
| 2 | Secondary | F | Caucasian | 17.3 | 4.3 | 1 | DVT | 25 | No | NP |
| 3 | Secondary | F | African American | 6.5 | 1.7 | 1 | DVT | 209 | Yes | NP |
| 4 | Secondary | M | Asian | 1.3 | 0.5 | 1 | DVT | 39 | Yes | NP |
| 2 | DVT | Yes | ||||||||
| 5 | Secondary | M | Caucasian | 12.3 | 1.4 | 1 | DVT | 65 | Yes | FVL |
| 6 | FSGS | F | Hispanic | 14.4 | 5.5 | 1 | AVF | 728 | No | NP |
| 7 | FSGS | F | Caucasian | 1.0 | 0.3 | 1 | DVT | 30 | Yes | None |
| 8 | FSGS | M | Caucasian | 1.7 | 3.8 | 1 | DVT | 39 | Yes | NP |
| 2 | DVT | No | ||||||||
| 9 | FSGS | M | Caucasian | 5.8 | 4.3 | 1 | DVT | 899 | Yes | NP |
| 10 | FSGS | M | African American | 18.2 | 5.1 | 1 | DVT | 1226 | Yes | NP |
| 11 | FSGS | M | Caucasian | 4.1 | 10.5 | 1 | PE | 3832 | No | NP |
| 12 | MCNS | F | Caucasian | 5.2 | 7.9 | 1 | PE | 1344 | No | FVL; APL |
| 13 | MCNS | F | Caucasian | 4.6 | 14.0 | 1 | DVT | 3863 | No | FVL |
| 14 | MCNS | M | Caucasian | 17.2 | 2.2 | 1 | DVT | 25 | No | FVL; APL |
| 15 | MCNS | M | Caucasian | 1.3 | 11.7 | 1 | PE | 2825 | No | None |
| 16 | MesPGN | M | African American | 17.8 | 1.8 | 1 | DVT | 7 | No | NP |
| 2 | DVT | No | ||||||||
| 17 | MesPGN | M | Caucasian | 2.2 | 5.5 | 1 | DVT | 1963 | Yes | NP |
| 18 | MN | F | African American | 14.8 | 3.6 | 1 | DVT | 395 | No | APL |
| 19 | MN | F | Caucasian | 12.4 | 2.2 | 1 | DVT/PE | -1 | No | AT3; APL |
| 20 | MPGN | F | Caucasian | 17.2 | 0.7 | 1 | DVT | 65 | No | NP |
| 2 | DVT | No | ||||||||
| 3 | DVT | No | ||||||||
| 21 | MPGN | F | Caucasian | 12.4 | 5.2 | 1 | DVT | 63 | Yes | HH |
| 2 | DVT | No | ||||||||
| 22 | SLE I-IV | F | Caucasian | 13.8 | 6.6 | 1 | DVT | 1503 | No | NP |
| 23 | SLE I-IV | F | Caucasian | 20.3 | 4.3 | 1 | DVT | 65 | Yes | HH; APL |
| 24 | SLE I-IV | F | African American | 15.0 | 3.2 | 1 | DVT | 76 | Yes | APL |
| 25 | SLE I-IV | F | Caucasian | 15.0 | 1.5 | 1 | PE | 0 | No | PS; APL |
| 2 | DVT | No | ||||||||
| 26 | SLE I-IV | M | Caucasian | 19.7 | 1.9 | 1 | Arterial | 363 | No | NP |
| 27 | SLE V | F | Hispanic | 18.5 | 2.9 | 1 | DVT | 1 | No | NP |
| 28 | SLE V | F | African American | 15.6 | 6.6 | 1 | PE | 38 | No | NP |
| 2 | PE | No | ||||||||
| 29 | SLE V | F | African American | 13.8 | 2.3 | 1 | PE | 817 | No | NP |
| 30 | SLE V | M | Caucasian | 13.9 | 2.9 | 1 | DVT | 0 | No | NP |
| 13.8† | 3.7† | 38‡ | 70.5† | 13‡ |
AVF, arteriovenous fistula; FSGS, focal segmental glomerulosclerosis; HH, hyperhomocysteinemia; MCNS, minimal change nephrotic syndrome; MesPGN, mesangial proliferative glomerulone-phritis; MPGN, membranoproliferative glomerulonephritis; NP, not performed; PS, protein S deficiency.
Lupus nephritis classes I-IV (SLE I-IV), lupus nephritis class V (SLE V).
Median.
Total.
The anatomic distribution of the 29 DVT events was 10 (34.5%) in the lower extremities, 6 (20.7%) in the upper extremities, 6 (20.7%) intrathoracic, 5 (17.2%) intra-abdominal (including 3 renal vein thrombi), and 2 (6.9%) in the head/neck region. Three DVTs (10.3%) involved more than one anatomic region, and another 2 (6.9%) were bilateral events. Thirteen DVTs (44.8%) were associated with the use of a CVAD.
As shown in Table I, the median age at diagnosis of NS was older in the patients with TE compared with those without TE (13.8 vs 6.2 years; P < .01). Moreover, the likelihood of TE was significantly greater in patients who were diagnosed with NS after age 12 years than in those diagnosed earlier in life (19.2% vs. 4.9%; P < .0001). The children with TE also tended to have a higher peak uPr:Cr than those without TE (median, 14.2 vs 9.8; P < .01). Serum albumin nadir was not predictive of TE in our study population.
Five patients with NS had a history of TE that preceded their NS diagnosis. Four of these 5 patients had at least one NS-associated TE event. Thus, patients with previous TE represented 13% of the NS-associated TE group, compared with only 0.3% of the group without NS-associated TE (P < .0001) (Table I).
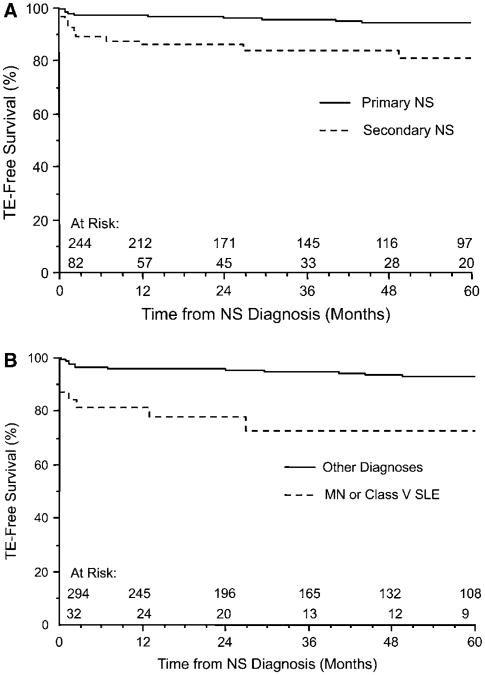
As suggested by the higher incidence of TE in the secondary NS group, the patients in this group had a lower 5-year TE-free survival compared with those in the primary NS group (84.1% vs 95.1%; P < .001, log-rank test) (Figure, A). This finding did not appear to be related to the predominance of SLE cases in the secondary NS group (data not shown). To assess the risk of TE associated with membranous histology, we combined patients with membranous histology of any cause (ie, class V SLE [n = 22] and MN [n = 10]) as a surrogate means of assessing a possible correlation. The patients with membranous histology had a higher incidence of TE than the patients with other histopathologic subtypes or SSNS (25% vs 7.5%; P < .01) (Table I). These patients also had a lower 5-year TE-free survival compared with the rest of the cohort (75% vs 94.2%; P < .0001, log-rank test) (Figure, B). TE frequency did not differ significantly between patients with class V SLE and those with non–class V SLE histology (27.3% vs 11.5%; P = .16).
Figure.
TE-free survival in A, patients with primary versus secondary NS (P < .001, log-rank test) and B, patients with membranous histology (MN or SLE class V) versus other diagnoses (P < .0001, log-rank test).
Twelve of the 30 patients with TE were subjected to laboratory investigation for prothrombotic risk factors. Ten of these 12 patients (83.3%) exhibited evidence of risk factors (Table II). Five patients had only 1 risk factor, and 5 had 2 risk factors; no patient had more than 2 identifiable risk factors. The most common risk factors were heterozygous FVL (n = 4) and APL (n = 7); other risk factors are listed in Table II. Of the 7 patients with APL, only 3 (42.9%) had SLE.
Univariate ORs for all potential TE predictors were calculated using a primary outcome measure of TE within 5 years from diagnosis of NS (Table III). Age at diagnosis was considered as both a continuous and a categorical (dichotomized at age 12) variable. Because of the skewness of the peak uPr:Cr data, the raw values for this variable were natural log (ln)-transformed, to normalize the data for extreme outliers. These crude ORs were then used to build a multivariate logistic regression model.
Table III. Univariate and multivariate ORs for TE risk.
| Univariate ORs | |||||
|---|---|---|---|---|---|
|
| |||||
| Potential predictor | Crude OR | 95% CI | P value | ||
| Age at NS onset | 1.16 | (1.08,1.25) | .0001 | ||
| Age, ≥ 12 years versus < 12 years | 8.59 | (3.31, 22.28) | < .0001 | ||
| Follow-up time, years | 0.87* | (0.77,1.03) | .10 | ||
| Serum albumin nadir | 0.63* | (0.25,1.54) | .31 | ||
| ln-peak uPr:Cr | 1.63* | (1.07, 2.47) | .02 | ||
| Diagnosis category, primary NS versus secondary NS | 0.28 | (0.12, 0.64) | < .01 | ||
| SLE, yes versus no | 3.72 | (1.54, 9.00) | < .01 | ||
| Race/ethnicity | |||||
| African American versus Caucasian | 1.95 | (0.79, 4.82) | .32 | ||
| Other versus Caucasian | 0.87 | (0.19, 3.97) | |||
| Sex, female versus male | 2.02 | (0.87, 4.72) | .10 | ||
|
| |||||
| Multivariate ORs | |||||
|
| |||||
| Parameter | Estimate | SE | Estimated OR | 95% CI | P value |
|
| |||||
| Intercept | −6.53 | 1.11 | – | – | < .0001 |
| Age at NS onset, ≥ 12 years versus < 12 years | 2.94 | 0.61 | 18.89 | (5.70, 63.17) | < .0001 |
| ln-peak uPr:Cr | 1.09 | 0.27 | 2.98* | (1.74, 5.10) | < .0001 |
| Follow-up time, years | −0.18 | 0.09 | 0.92 | (0.72,1.11) | .39 |
OR for a 1-unit increase in the predictor/parameter.
Table III gives parameter estimates and ORs from the multivariate modeling procedure. Age at diagnosis (dichotomized at age 12 years), as well as ln-peak uPr:Cr, were found to be significant predictors of TE. From this model, the likelihood of TE is 18.89 times greater in patients diagnosed with NS at age ≥12 years compared with those diagnosed earlier (95% CI = 5.70 to 63.17). In addition, the likelihood of TE is 2.98 times greater for every ∼2.7-fold (1 ln unit) increase in the peak uPr:Cr (95% CI = 1.74 to 5.10). After adjusting for age and ln-peak uPr:Cr, the following variables were not significant predictors of TE: follow-up time (P = .39), NS category (P = .34), and SLE (P = .80). The interaction between age and ln-peak uPr:Cr was not significant (P = .74). Because only 5 patients had a history of TE before the onset of NS, this factor could not be considered in the logistic regression model due to computational issues.
Discussion
In this large retrospective cohort, TE was identified in children with NS due to either primary or secondary glomerulopathies. Nearly 10% of the children developed symptomatic TEs during treatment, > 20% of which were life- or limbthreatening. More than half of the TEs developed within months of NS diagnosis, and nearly half were associated with the use of a CVAD. The risk of TE was correlated with the severity of proteinuria and was increased in patients with secondary glomerulopathy, membranous histology, or SLE; age ≥12 years; or a previous history of thromboembolic disease. Multivariate analysis identified age (≥12 years) and severity of proteinuria as the most significant independent risk factors for TE.
In this cohort, TE was found in 9.2% of the patients with NS, a notably higher prevalence than reported previously (1.8% to 5%).2,3 But those previous reports focused only on patients with primary glomerulopathies.2 In our study, we found a similar prevalence of TE (6.6%) in patients primary NS; thus, the higher prevalence in our cohort may be due to the inclusion of patients with secondary NS. The secondary NS group comprised predominantly patients with SLE (58.5%). SLE is a known risk factor for thrombosis; thus, including these patients in studies of NS-associated TE may inflate the prevalence of this complication.10 The increased risk of TE in patients with SLE has been attributed mainly to the development of APL.10 All 3 of our patients with SLE and TE who were evaluated for APL were positive.
Conversely, the present study actually may underestimate the prevalence of TE complications. A previous retrospective study of children with frequently relapsing and steroid-resistant NS found that 26.9% exhibited evidence of previous TE on ventilation-perfusion scintigraphy, suggesting that clinically silent TE may be much more common than previously estimated by studies designed to collect data only from symptomatic events.11 In children with congenital NS, the incidence of TE may be as high as 10%, but only 0.9% of our cohort had congenital NS.12 Furthermore, our inclusion criteria were designed to capture patients with consistent, documented follow-up, to improve the likelihood of capturing any late TE events. It is possible that this strategy missed some TEs in patients who were not followed at our institutions throughout their entire course of NS, resulting in an underestimation of this complication.
The median time from NS diagnosis to first TE event was relatively brief (70.5 days), suggesting that TE is more likely to occur close to the time of diagnosis than during subsequent relapses. Previous studies also have found that TE tends to occur early in the course of NS.13 But 7 patients in our cohort had multiple TEs, and 1 patient did not develop clinical signs of TE until 10.6 years after diagnosis of NS. Relapsing NS is common in childhood; however, data regarding relapse/remission status at the time of TE events were not available for our cohort, hindering assessment of risk factors present at differing time points in the course of NS.2
The majority of TE events (76.3%) were DVTs, and CVAD use was associated with nearly half (44.8%) of these events. CVAD use is a well-known risk factor for TE in children.14 Our findings suggest that CVADs should be avoided whenever possible, and particular vigilance should be maintained when CVAD use is deemed necessary for supportive care of patients with NS.
The incidence of TE was greater in our patients with NS age ≥ 12 years. This finding is similar to previous epidemiology studies of childhood TE. For instance, in children, the incidence of TE appears to peak in infancy, with a secondary adolescent peak beginning at approximately age 12 years.14,15 Just why age affects TE risk remains unclear, but puberty may play a role. SLE is also more common during adolescence, raising the possibility that age and SLE status may be confounding.16 But in our multivariate analysis, increasing age was found to increase the risk of TE independent of SLE status. This could be a reflection of inadequate power in our sample to detect an SLE impact on TE risk; however, a recent analysis of TE risk in patients with SLE found that younger age was similarly protective of TE.17 Likewise, previous studies of hospital discharge data have suggested a higher incidence of TE in African-American children, and, as in other studies, SLE was more common in African-Americans than in other races in this cohort (26.5% vs 18.6%; P< .01).16,18 There was no detectable racial predilection for TE in this study, however.
Peak uPr:Cr ratio also was independently predictive of TE risk, with increasing values being associated with increased TE risk. These data should be interpreted with caution, however, because of the highly skewed data set. In this cohort, the median uPr:Cr was 9.96 (95% CI = 2.94 to 79.94), with a significant number of outliers. Although no published data are available regarding the upper limit of pathophysiologic proteinuria, we suspect that some of these values may have been iatrogenically influenced by therapy with intravenous albumin and/or diuretics. Possible alternative explanations may be that these values may represent patients with NS of greater severity, or that albumin or diuretic therapy may increase the risk of TE.19 In addition, relapse status in relation to the timing of TE events and the uPr:Cr at the time of these events was not available for our cohort, further limiting our understanding of how to interpret this variable. In contrast to similar studies in adults, serum albumin nadir was not predictive of TE in our cohort.3
Other, less common clinical factors likely influence TE risk. Although rare, a previous history of TE in newly diagnosed patients with NS appears to be a particularly strong risk factor for additional TE after the development of NS; thus, these patients should be carefully considered for preventive strategies. Similar to the findings in adult studies, membranous histology (class V SLE or MN in this cohort) appears to increase the risk of NS-associated TE. TE occurring in children with MN has been reported previously.20
In previous studies, the molecular physiology of NS-associated prothrombotic risk has been attributed to urinary loss of anticoagulant proteins (primarily AT3 and PS), as well as to platelet hyperaggregability, altered fibrin polymerization, urinary loss of fibrinolytic proteins, and, most recently, low protein Z level.4,21-24 Other proposed mechanisms indirectly related to hemostasis include hyperviscosity, hyperlipidemia, and red blood cell hyperaggregation.3,25,26 It is well known that certain medications (including steroids and diuretics), as well as immobilization, may increase the risk of TE in children with NS.2,19 Because of the retrospective design of this study, these factors could not be adequately examined.
In the patients with TE in this cohort studied for congenital or acquired prothrombotic risk, the most commonly identified risk factors were APL and FVL. The incidence of APL and FVL in the patients without TE was not available, however; thus, the relative risk of TE due to these markers cannot be estimated. Notably, most of the patients with TE and APL did not have comorbid SLE (4 of 7 had primary NS), suggesting that either NS or its treatment may predispose children to APL. In fact, APL has been detected in children without SLE.27,28 Another possible explanation for the detection of APL in these patients includes intercurrent viral illness. It is well known that viral illnesses can induce transient APL in children, and that they also are associated with NS relapse.29,30 Thus, these actually may be serendipitous results rather than independent predictors of TE risk. Interestingly, acquired prothrombotic risk due to urinary protein loss (ie, AT3 and PS deficiencies) was not prominent in our patients with TE; however, this may be related to the timing of these assays in relation to NS remission status, which was not clearly identifiable in this retrospective cohort. Prospective longitudinal cohort studies are needed to confirm these findings and assess for other potential TE risk factors.
Glossary
- APL
Antiphospholipid antibodies/lupus anticoagulant
- AT3
Antithrombin III
- CI
Confidence interval
- CVAD
Central venous access device
- DVT
Deep venous thrombosis
- FVL
Factor V Leiden
- MN
Membranous nephropathy
- NS
Nephrotic syndrome
- OR
Odds ratio
- PE
Pulmonary embolism
- PS
Protein S
- SLE
Systemic lupus erythematosis
- SSNS
Steroid-sensitive nephrotic syndrome
- TE
Thromboembolism
- uPr:Cr
Urine protein:creatinine ratio
Footnotes
Presented in part at the 2007 Pediatric Academic Societies Meeting, Toronto, Canada and the 2007 American Societyof Nephrology Meeting, San Francisco, California.
The authors declare no conflicts of interest.
References
- 1.Valentini RP, Smoyer WE. Nephrotic syndrome. In: Kher KK, Schnaper HW, Makker SP, editors. Clinical Pediatric Nephrology. 2nd. London: Informa Healthcare; 2006. pp. 155–94. [Google Scholar]
- 2.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–39. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 3.Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338:1202–11. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- 4.Zaffanello M, Franchini M. Thromboembolism in childhood nephrotic syndrome: a rare but serious complication. Hematology. 2007;12:69–73. doi: 10.1080/10245330600940048. [DOI] [PubMed] [Google Scholar]
- 5.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–11. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 9.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–30. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 10.Calvo-Alen J, Toloza SM, Fernandez M, Bastian HM, Fessler BJ, Roseman JM, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA), XXV: smoking, older age, disease activity, lupus anticoagulant, and glucocorticoid dose as risk factors for the occurrence of venous thrombosis in lupus patients. Arthritis Rheum. 2005;52:2060–8. doi: 10.1002/art.21149. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer PF, Gonda S, Barthels M, Krohn HP, Brodehl J. Thromboembolic complications in children with nephrotic syndrome: risk and incidence. Acta Paediatr Scand. 1986;75:804–10. doi: 10.1111/j.1651-2227.1986.tb10294.x. [DOI] [PubMed] [Google Scholar]
- 12.Mahan JD, Mauer SM, Sibley RK, Vernier RL. Congenital nephrotic syndrome: evolution of medical management and results of renal transplantation. J Pediatr. 1984;105:549–57. doi: 10.1016/s0022-3476(84)80418-7. [DOI] [PubMed] [Google Scholar]
- 13.Andrew M, Brooker LA. Hemostatic complications in renal disorders of the young. Pediatr Nephrol. 1996;10:88–99. doi: 10.1007/BF00863459. [DOI] [PubMed] [Google Scholar]
- 14.Andrew M, Monagle PT, Brooker L. Thromboembolic Complications During Infancy and Childhood. Hamilton, Ontario, Canada: BC Decker; 2000. [Google Scholar]
- 15.Andrew M, David M, Adams M, Ali K, Anderson R, Barnard D, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83:1251–7. [PubMed] [Google Scholar]
- 16.Gottlieb BS, Ilowite NT. Systemic lupus erythematosus in children and adolescents. Pediatr Rev. 2006;27:323–30. doi: 10.1542/pir.27-9-323. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis. 2009;68:238–41. doi: 10.1136/ard.2008.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004;145:563–5. doi: 10.1016/j.jpeds.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Lilova MI, Velkovski IG, Topalov IB. Thromboembolic complications in children with nephrotic syndrome in Bulgaria (1974-1996) Pediatr Nephrol. 2000;(15):74–8. doi: 10.1007/s004679900253. [DOI] [PubMed] [Google Scholar]
- 20.Louis CU, Morgenstern BZ, Butani L. Thrombotic complications in childhood-onset idiopathic membranous nephropathy. Pediatr Nephrol. 2003;18:1298–300. doi: 10.1007/s00467-003-1320-0. [DOI] [PubMed] [Google Scholar]
- 21.Collet JP, Mishal Z, Lesty C, Mirshahi M, Peyne J, Baumelou A, et al. Abnormal fibrin clot architecture in nephrotic patients is related to hypofibrinolysis. Influenceof plasma biochemical modifications:a possible mechanism for the high thrombotic tendency? Thromb Haemost. 1999;82:1482–9. [PubMed] [Google Scholar]
- 22.Lau SO, Tkachuck JY, Hasegawa DK, Edson JR. Plasminogen and anti-thrombin III deficiencies in the childhood nephrotic syndrome associated with plasminogenuria and antithrombinuria. J Pediatr. 1980;96:390–2. doi: 10.1016/s0022-3476(80)80678-0. [DOI] [PubMed] [Google Scholar]
- 23.Schlegel N. Thromboembolic risks and complications in nephrotic children. Semin Thromb Hemost. 1997;23:271–80. doi: 10.1055/s-2007-996100. [DOI] [PubMed] [Google Scholar]
- 24.Ozkaya O, Bek K, Fisgin T, Aliyazicioglu Y, Sultansuyu S, Acikgoz Y, et al. Low protein Z levels in children with nephrotic syndrome. Pediatr Nephrol. 2006;21:1122–6. doi: 10.1007/s00467-006-0167-6. [DOI] [PubMed] [Google Scholar]
- 25.Bohler T, Linderkamp O, Leo A, Wingen AM, Scharer K. Increased aggregation with normal surface charge and deformability of red blood cells in children with nephrotic syndrome. Clin Nephrol. 1992;38:119–24. [PubMed] [Google Scholar]
- 26.Querfeld U, Lang M, Friedrich JB, Kohl B, Fiehn W, Scharer K. Lipoprotein (a) serum levels and apolipoprotein (a) phenotypes in children with chronic renal disease. Pediatr Res. 1993;34:772–6. doi: 10.1203/00006450-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Franchini M, Veneri D. The antiphospholipid syndrome. Hematology. 2005;10:265–9. doi: 10.1080/10245330500141309. [DOI] [PubMed] [Google Scholar]
- 28.Kratz C, Mauz-Korholz C, Kruck H, Korholz D, Gobel U. Detection of antiphospholipid antibodies in children and adolescents. Pediatr Hematol Oncol. 1998;15:325–32. doi: 10.3109/08880019809014016. [DOI] [PubMed] [Google Scholar]
- 29.Male C, Lechner K, Eichinger S, Kyrle PA, Kapiotis S, Wank H, et al. Clinical significance of lupus anticoagulants in children. J Pediatr. 1999;134:199–205. doi: 10.1016/s0022-3476(99)70416-6. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi S, Wada N, Murakami H, Funaki S, Inagaki T, Harada K, et al. Triggers of relapse in steroid-dependent and frequently relapsing nephrotic syndrome. Pediatr Nephrol. 2007;22:232–6. doi: 10.1007/s00467-006-0316-y. [DOI] [PubMed] [Google Scholar]