Abstract
Androgen receptor (AR) signaling persists in castration-resistant prostate carcinomas (CRPCs), due to several mechanisms that include increased AR expression and intratumoral androgen metabolism. We investigated the mechanisms underlying aberrant expression of transcripts involved in androgen metabolism in CRPC. We compared gene expression profiles and DNA copy number alteration (CNA) data from 29 normal prostate tissue samples, 127 primary prostate carcinomas (PCas) and 19 metastatic PCas. Steroidogenic enzyme transcripts were evaluated by qRT-PCR in PCa cell lines and circulating tumor cells (CTCs) from CRPC patients. Metastatic PCas expressed higher transcript levels for AR and several steroidogenic enzymes, including SRD5A1, SRD5A3, and AKR1C3, while expression of SRD5A2, CYP3A4, CYP3A5 and CYP3A7 was decreased. This aberrant expression was rarely associated with CNAs. Instead, our data suggest distinct patterns of coordinated aberrant enzyme expression. Inhibition of AR activity by itself stimulated AKR1C3 expression. The aberrant expression of the steroidogenic enzyme transcripts were detected in CTCs from CRPC patients. In conclusion, our findings identify substantial interpatient heterogeneity and distinct patterns of dysregulated expression of enzymes involved in intratumoral androgen metabolism in PCa. These steroidogenic enzymes represent targets for complete suppression of systemic and intratumoral androgen levels, an objective that is supported by the clinical efficacy of the CYP17 inhibitor abiraterone. A comprehensive AR axis targeting approach via simultaneous, frontline enzymatic blockade and/or transcriptional repression of several steroidogenic enzymes, in combination with GnRH analogs and potent anti-androgens, would represent a powerful future strategy for PCa management.
Keywords: Prostate cancer, androgen synthesis, testosterone, dihydrotestosterone, CYP17, AKR1C3, abiraterone, MDV3100 (enzalutamide)
INTRODUCTION
Gonadal androgen depletion and/or blockade have been the standard first-line systemic treatment for advanced prostate cancer (PCa) for the past 7 decades, producing declines in prostate specific antigen (PSA) and tumor regression. Despite peripheral androgen levels in the castrate range, eventual regrowth occurs as a castration-resistant PCa (CRPC) and is invariably lethal (1). The androgen receptor (AR) signaling axis remains active in most CRPCs, as evidenced by the frequent re-expression of AR target genes such as PSA and TMPRSS2. The AR axis thus represents an important therapeutic target, a concept that has been validated in recent clinical trials of second-line hormonal manipulations with abiraterone acetate, a CYP17 inhibitor that blocks steroid biosynthesis (2–7), and MDV3100 (enzalutamide), a new anti-androgen (5, 8–11). Several mechanisms that allow AR activation despite castrate levels of peripheral testosterone have been reported in CRPC, including the persistence of residual intratumoral androgens at concentrations sufficient to activate AR (12–19). Compared to primary prostate tumors or normal prostate tissue, CRPC displays up-regulated expression of several transcripts encoding for enzymes involved in androgen metabolism (18, 20–23).
The clinical relevance of the above findings is validated by the activity of the CYP17 inhibitor abiraterone (2–7), recently shown to prolong overall survival in chemotherapy-treated CRPC patients, and now FDA-approved for this indication. The responses, however, are incomplete and all tumors eventually progress with resumed PSA expression, an indication of re-activation of AR signaling. Preliminary evidence suggests that abiraterone-resistant PCas overexpress CYP17A1 and other steroidogenic transcripts (including STAR, CYP11A1, HSD3B1 and AKR1C3) (24), suggesting maintenance of capacity for in situ steroidogenesis as a potential mechanism of treatment failure. Additional data suggest that intratumor CYP11-dependent pregnenolone/progesterone synthesis can contribute to resistance to abiraterone (25) and strengthen the notion that CRPCs resistant to CYP17 inhibition may remain ligand-dependent and AR-dependent, and, therefore, responsive to therapies that can further suppress de novo intratumoral steroid synthesis (25). We hypothesized that the delineation of the mechanisms leading to dysregulated expression of androgen metabolism enzymes would provide important insight into possible mechanisms of resistance to abiraterone, and would help identify additional targets in this pathway and facilitate rational design of future drug combinations for clinical trials in CRPC as candidate components of a comprehensive AR axis targeting approach.
Towards that aim, we mined datasets from a recently reported comprehensive integrated oncogenomic analysis of banked tissue samples from primary and metastatic prostate PCas and normal prostate controls (26) in order to define the frequency of alterations in androgen metabolism pathways. We found aberrant expression for several of these steroidogenic enzymes and investigated mechanisms accounting for this phenomenon.
MATERIALS AND METHODS
PCa tissue specimens and oncogenomic profiling
The methodology for our integrated analysis of transcriptomes and CNAs in prostate cancer has been reported previously (26). Briefly, gene expression profiles of 29 normal prostate tissue samples, 131 primary PCas and 19 metastatic (8 non-castrate, 11 castrate) PCas were generated using Affymetrix Human Exon 1.0 ST arrays. Data from 4 primary tumor samples were excluded from analysis due to prior neoadjuvant hormonal or chemotherapy treatment. Expression outliers, defined as transcripts with significant up- or downregulation in that particular specimen compared to the distribution of expression for that transcript in normal prostate samples, were determined as previously (26–27). In this nonparametric approach, an empirical distribution function generated from transcript expression in the 29 normal prostate tissues was used to transform expression in the tumor samples, from which outliers were determined with the criteria described in the Benjamini and Hochberg algorithm (28) at an error rate (a) = 0.01 (26).
Copy-number alterations (CNAs) were assessed with Agilent 244K array comparative genomic hybridization (aCGH) microarrays (described in detail in (26)).
All patients provided informed consent. Samples were procured and the study was conducted under MSKCC Institutional Review Board (IRB) approval. Clinical and pathologic data were entered and maintained in a prospective prostate cancer database.
The complete data is freely available through a web-based portal (29). The full raw data is available via GEO (accession no. GSE21032).
List of studied transcripts
We studied transcripts for enzymes participating in androgen synthesis and metabolism (Fig. 1A and Suppl. Table 1). We also used a previously published AR-dependent transcript signature (30) and applied it to our gene expression data to quantify AR axis signaling output.
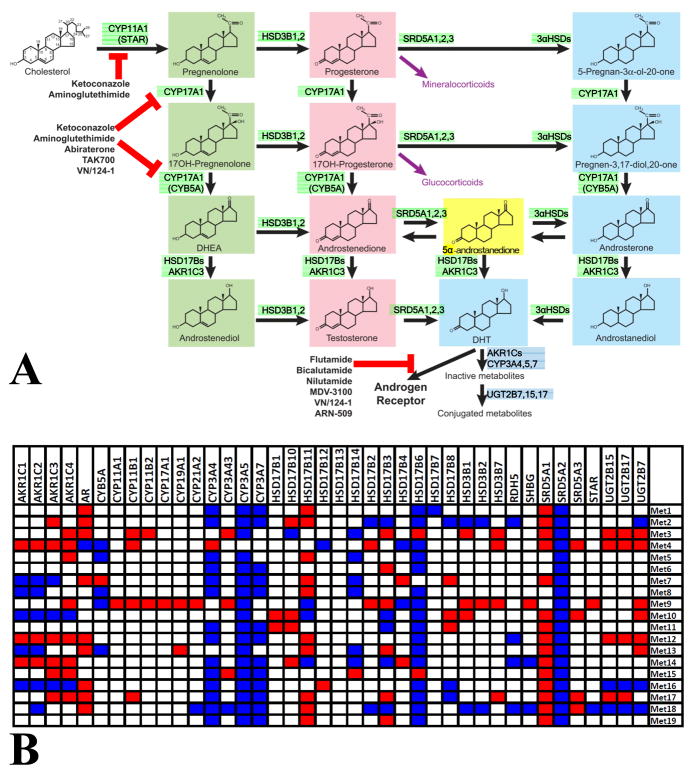
Fig. 1. Pathways of testosterone/DHT biosynthesis and metabolism, associated enzymes and their expression in metastatic PCa specimens.
A. Cholesterol, the precursor of all steroidogenesis, is converted to DHT via several enzymatic steps: In the Δ5 pathway (named after the presence of a double carbon bond in the C5 position of the A steroid ring; steroids highlighted in green) and the Δ4 pathway (steroids highlighted in light red), testosterone is synthesized and then reduced by 5α-reductases to DHT, that has a ~5–10-fold higher affinity for AR. Androgen precursors can also be reduced before testosterone synthesis, generating an alternate pathway (“backdoor pathway”, steroids highlighted in light blue) that bypasses testosterone and leads to DHT. This pathway has been proposed to be active in prostate tissue, in particular prostate cancer (17). Recently, it was demonstrated that the dominant route of DHT synthesis in CRPC bypasses testosterone (23), and instead requires 5α-reduction of androstenedione by SRD5A1 to 5α-androstanedione (highlighted in yellow), which is then converted to DHT. Testosterone and DHT are oxidized (via cytochrome P450 3A oxidases) followed by conjugation to glucuronides (via uridine diphospho-glucuronosyl transferases UGT2B7, UGT2B15 and UGT2B17), that are then excreted. Enzymes involved in promoting testosterone/DHT synthesis are highlighted in green, while enzymes promoting their metabolism/inactivation are highlighted in dark blue. The target sites of clinically relevant inhibitors are also shown (Figure modified from (19)).
B. Heatmap of outliers (red: overexpressed transcript, blue: underexpressed transcript) for AR and transcripts involved in androgen metabolism in the metastatic PCa specimens (outlier expression compared to the distribution of expression in normal prostate samples, see Methods and (26)).
In vitro treatment of PCa cells
PCa LNCaP cells (purchased from American Type Culture Collection, Manassas, VA, and passaged for fewer than 6 months) were grown in RPMI-1640 medium supplemented with 10% FBS (Omega Scientific, Tarzana, CA). For androgen deprivation, the cells were incubated in RPMI-1640 medium supplemented with 10% charcoal-stripped FBS (CSS, Omega Scientific) for 48 hrs. R1881 (NEN Life Science Products, Boston, MA) was used at 1 nM. The novel antiandrogen MDV3100 (enzalutamide; Medivation, San Francisco, CA) (8–9) was used at 10 μM. Quantitative RT-PCR analysis for steroidogenic enzyme expression was performed using a StepOne Plus instrument and Taqman probes (both from Applied Biosystems, Foster City, CA).
Quantitative RT-PCR analysis of CTCs from CRPC patients for expression of AR, KLK3 (PSA) and steroidogenic enzymes
Circulating tumor cells, defined as EpCaM(+), CD45(−) events, were collected by Fluorescence-activated cell sorting (FACS, MoFlo2; Beckman Coulter, Brea CA), using empirically defined gates based on healthy volunteer samples spiked with (positive control) or without (negative control) prostate cancer cells (LNCaP cells). Ten ml of blood from CRPC patients, obtained with the patients’ informed consent under a MSKCC IRB-approved protocol were collected into an EDTA tube (BD Biosciences, Bedford, MA) and processed within 5 hours of blood draw. Mononuclear cells were isolated via density gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare Biosciences, Piscataway, NJ) and labeled with conjugated antibodies EpCaM-PE and CD45-APC (Miltenyi Biotec Inc., Auburn, CA). EpCaM(+)/CD45(−)/DAPI(−) events were sorted into 1-Step RT-PCR mix (Invitrogen, Carlsbad, CA) for primer-specific multiplex reverse transcription (using Universal PCR Master Mix, Applied Biosystems) and 14 cycle “pre-amplification” PCR using Taqman probes (Applied Biosystems). Standard 40-cycle quantitative PCR was then performed on the 96×96 BioMark™ chip (Fluidigm, South San Francisco, CA).
Statistical analysis
The average expression of each transcript of interest was compared between normal prostate tissue, primary PrCa and metastatic PrCa using one-way analysis of variance (ANOVA) and the LSD, Bonferroni and Dunnett C post-hoc tests. Two-sided t-tests were used to compare the number of “pro-androgenic” or “anti-androgenic” transcripts that are differentially expressed in metastastic vs. primary carcinomas. Chi-square tests were used to compare the number of cases of metastastic vs. primary carcinomas that had differential expression of at least one “pro-androgenic” or “anti-androgenic” transcript. The linear correlation between various individual (or groups of) mRNAs was evaluated by calculation of the Pearson correlation coefficient.
RESULTS
High interpatient variability of dysregulated expression of individual transcripts involved in androgen metabolism in PCa
Expression of our panel of transcripts encoding for enzymes involved in androgen synthesis and metabolism (Fig. 1A and Suppl. Table 1) was analyzed for outliers (over-expressors or under-expressors) and revealed high interpatient variability, with several specific transcripts highly over- or under-expressed in nearly all samples and others altered only in a minority of tumors (results are presented in Fig. 1B and 2, for metastatic and primary tumors respectively). Average mRNA levels for each transcript in each group are presented in Suppl. Table 2. Results from one-way ANOVA comparing average expression of each transcript between groups (normal, primary PrCa and metastatic PrCa), as well as the respective P values, are presented in Suppl. Table 3.
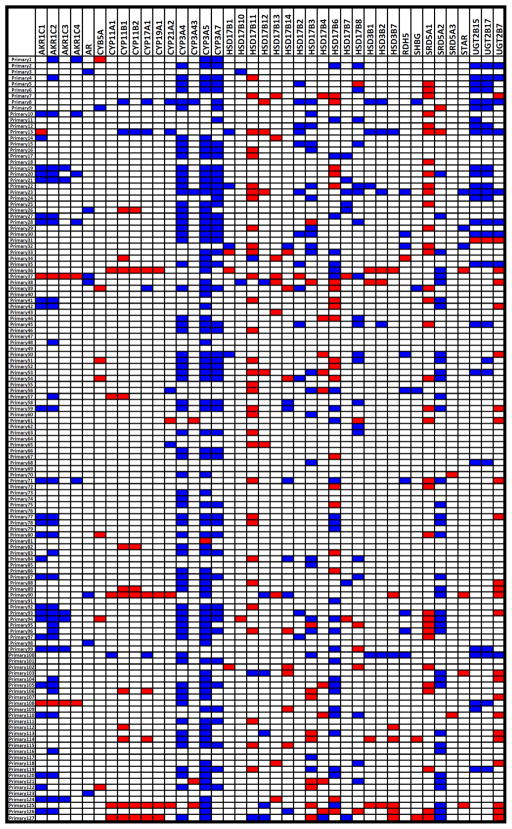
Fig. 2.
Heatmap of outliers (red: overexpressed transcript, blue: underexpressed transcript) for AR and transcripts involved in androgen metabolism in the primary PCa specimens (outlier expression compared to the distribution of expression in normal prostate samples, see Methods and (26)).
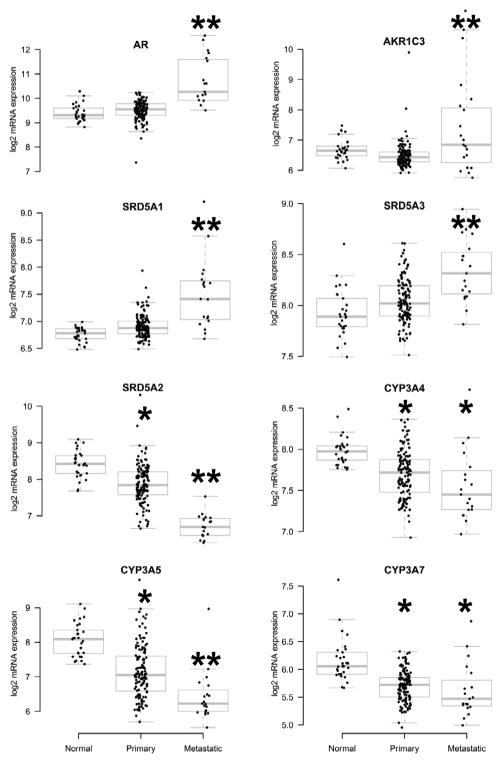
We found increased average expression of AR, AKR1C3, SRD5A1, and SRD5A3, and decreased average expression of SRD5A2, CYP3A4, CYP3A5 and CYP3A7 in metastatic PrCa (boxplots for log2-based mRNA expression are shown in Fig. 3). Importantly, several other transcripts were dysregulated in smaller subsets of tumors (suggesting potential contribution to activation of the androgen-AR axis in those particular tumors), while not reaching statistical significance on average among all tumors in our panel. This key finding raises the hypothesis that increased intratumoral androgens may be caused by dysregulation of different enzymes in different tumors. For the transcripts that are expected to have a “pro-androgenic” effect (i.e. increase ligand availability and, thus, AR activity: Group A in Suppl. Table 1), we found that metastatic carcinomas overexpressed, on average, 4.7 transcripts ((range 1–13, SD 2.7), compared to 1.7 transcripts in the primary carcinomas (range 0–11, SD 2.0, 2-sided t-test P=0.00024). All (19/19) metastastic carcinomas overexpressed at least one such transcript, compared to 92/127 for primary carcinomas (Chi-square P<0.01). For the “anti-androgenic” transcripts (i.e. enzymes overall associated with androgen degradation/inactivation and, thus, expected to decrease AR activity: Group B in Suppl. Table 1), the metastatic carcinomas under-expressed, on average, 3.5 transcripts (range 0–9, SD 2.2), compared to 2.6 transcripts for the primary carcinomas (range 0–9, SD 2.0, 2-sided t-test P=0.128). Moreover, 18/19 metastatic carcinomas under-expressed at least one such transcript, compared to 102/127 for primary carcinomas (Chi-square P=0.125). Thus, consistent with the data in Figure 1B, metastatic tissues exhibited significantly more variable expression patterns than primary carcinoma or normal prostate tissue.
Fig. 3.
Boxplots of average mRNA expression (log2-based) for AR, AKR1C3, SRD5A1, SRD5A3, SRD5A2, CYP3A4, CYP3A5, and CYP3A7 in normal prostate tissue, primary PCas and metastatic PCas. We found increased expression of AR, AKR1C3, SRD5A1, and SRD5A3, and decreased expression of SRD5A2, CYP3A4, CYP3A5 and CYP3A7 in metastatic PrCa. **: P<0.01 vs both normal tissue and primary carcinomas; *: P<0.01 vs normal tissue. Complete results are presented in Suppl. Table 3.
Association of expression of transcripts involved in androgen metabolism with AR transcriptional output
We investigated whether the variability in expression of these steroid-related transcripts leads to enhanced AR signaling output. We calculated a “composite steroid enzyme expression” as the sum of the “pro-androgenic” transcripts (that overall are associated with steroid synthesis and increased AR activity) minus the sum of the “anti-androgenic” transcripts (that overall are involved in androgen degradation/inactivation and, thus, expected to decrease AR activity), and found that there was a positive correlation with the AR transcriptional output signature in our metastatic specimen panel (Pearson correlation coefficient R2=0.43, P=0.0022, Suppl. Fig. 1A). The statistical significance persisted even when the AR transcript itself was removed from the analysis (R2=0.36, P=0.0061, Suppl. Fig. 1B).
Gene copy-number alterations appear not to be the cause of dysregulated expression of transcripts involved in androgen metabolism in PCa
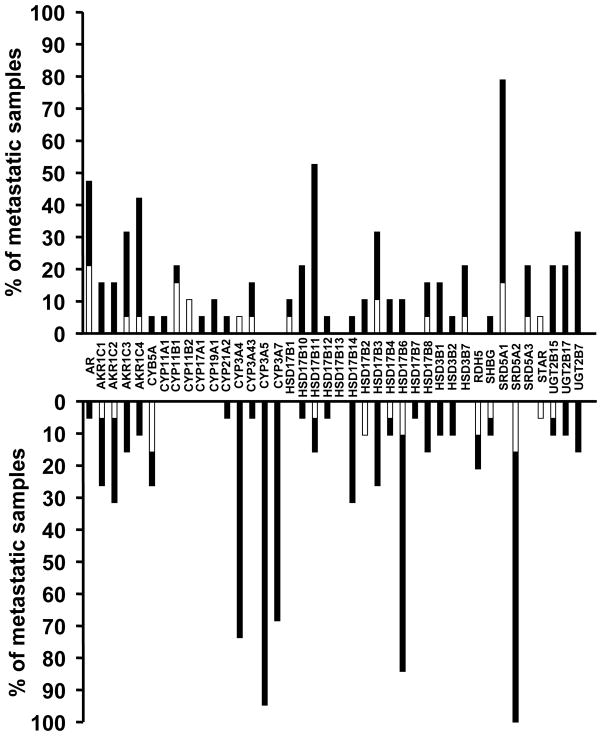
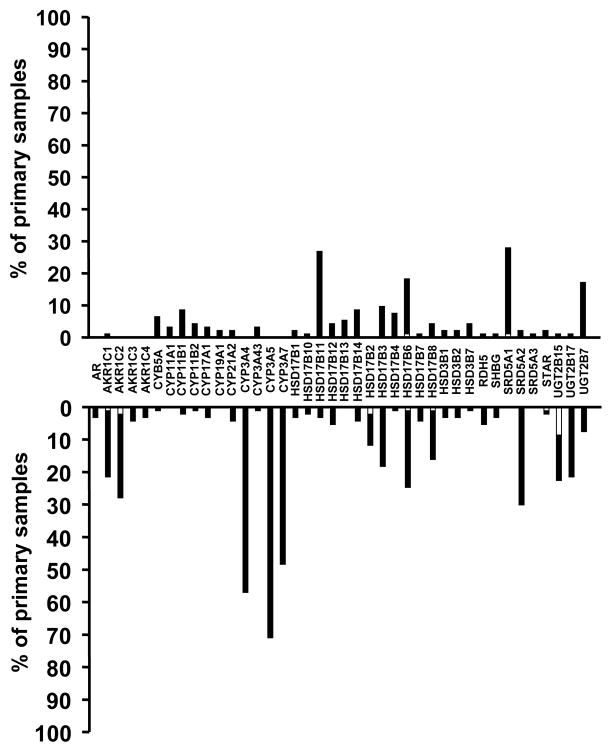
We integrated the copy-number alterations (CNAs) identified in (26) with our transcriptome data, to assess the role of genomic alterations on the steroid metabolism axis in our PCa specimens. The histograms in Figures 4 (metastatic carcinomas) and 5 (primary carcinomas) demonstrate the fraction of outliers for each transcript superimposed with the fraction of samples exhibiting CNA. With the exception of very few transcripts (e.g. CYP11B1 in Fig. 4), only a minority of the specimens with altered mRNA expression (over- or under-expressor outliers) had corresponding gene copy gains or losses that could account for the dysregulated mRNA levels. Thus, for most of these genes, transcriptional regulation, rather than altered gene copy number, is the likely cause of dysregulated expression.
Fig. 4.
Integration of expression outlier data with CNA analysis for AR and genes involved in androgen metabolism reveals that only a small subset of metastastic carcinoma specimens with altered mRNA expression (over- or under-expressor outliers) have gene copy gains or losses, respectively, that can account for the dysregulated mRNA levels. The majority of cases with dysregulated expression of transcripts involved in androgen metabolism are not associated with respective CNAs. Bars represent the percentage of metastastic carcinomas with outlier expression for each transcript involved in androgen metabolism (bars pointing up indicate overexpressor outliers, while bars pointing down indicate underexpressor outliers for each transcript). The white part of each bar indicates specimens with outlier level of expression that also exhibited DNA copy gain (for overexpressors) or loss (for underexpressors), respectively.
Patterns of coordinated expression of transcripts involved in androgen metabolism in PCas suggest distinct regulatory mechanisms
As our results suggested that the dysregulation of androgen-related transcripts in PCas occurs at the transcriptional level, we assessed for similarities in the pattern of their expression that might indicate the existence of common regulatory mechanisms. Using linear correlation analysis of the log2-based mRNA levels in primary and metastatic carcinomas, we identified 4 distinct groups of transcripts with highly co-regulated patterns of expression (Suppl. Table 4): Group 1: CYP11A1, CYP11B1, CYP11B2, CYP17A1, CYP19A1, CYP21A2, HSD3B1, HSD3B2, HSD3B7, RDH5, SHBG and STAR; Group 2: AKR1C1, AKR1C2, AKR1C3 and AKR1C4; Group 3: CYP3A4, CYP3A5 and CYP3A7; and Group 4: UGT2B15 and UGT2B17. These 4 groups suggest respective distinct patterns of (dys)regulation of expression of enzymes involved in androgen metabolism in PCas. Group 1 includes most enzymes expressed in the adrenals and necessary for conversion of cholesterol to adrenal androgen precursors (DHEA and androstenedione). Group 2 is the AKR1C family of enzymes, which, among other functions, can convert adrenal androgens to testosterone. The Group 3 enzymes are involved in Phase I of DHT inactivation (oxidation), while Group 4 enzymes catalyze Phase II of DHT inactivation (glucuronidation). For more details on the role of these enzymes in androgen metabolism, please see Fig. 1A.
The enzymes of the AKR1C family are negatively regulated by androgen
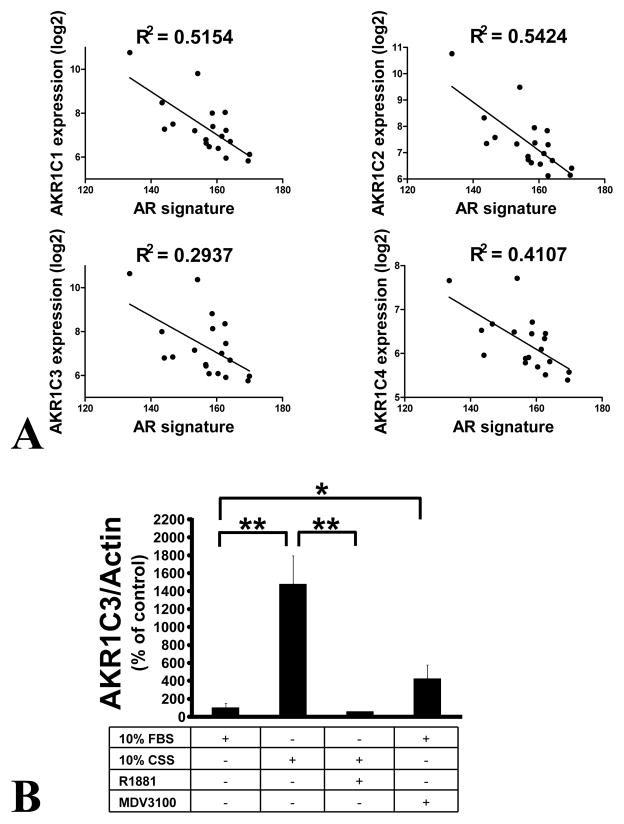
We next investigated the regulation of the Group 2 transcripts, i.e. the AKR1C family enzyme transcripts, because AKR1C3 plays a crucial role in conversion of DHEA and androstenedione to testosterone. We mined our transcriptome data (from primary and metastatic tumors) for transcripts highly co-regulated with AKR1C3. Not surprisingly, we found the other family members, AKR1C1, AKR1C2 and AKR1C4, to be co-regulated (Suppl. Table 5). Transcripts highly negatively associated with AKR1C3 were KLK3, ACPP, ABCC4, KLK2 and other AR-driven transcripts (Suppl. Table 5). These findings suggested that high AKR1C family enzyme expression is inversely associated with AR activity. This was confirmed in our transcriptome data from metastatic specimens, where the AR transcriptional output (quantified using an AR-dependent gene signature previously derived by treating the LNCaP prostate cancer cell line with androgen for 24 hrs (30)), was inversely associated with expression of each individual AKR1C family enzyme (Fig. 6A).
Fig. 6. Expression of the AKR1C family members is inversely related to androgen signaling.
(A) Inverse correlation between expression levels of AKR1C1, AKR1C2, AKR1C3 and AKR1C4 vs an AR-regulated gene signature (indicative of AR signaling output) in metastatic PCas. All P values<0.001.
(B) Exposure of LNCaP cells to androgen deprivation (medium supplemented with 10% charcoal-stripped serum, CSS) for 48 hrs potently upregulates expression of AKR1C3. This upregulation is suppressed by addition of the synthetic androgen R1881 (1 nM). Moreover, treatment of LNCaP cells (growing in medium supplemented with 10% regular FBS) with the anti-androgen enzalutamide (MDV3100) upregulates AKR1C3 expression. AKR1C3 mRNA levels were quantified by qRT-PCR, normalized to actin mRNA levels, and expressed as a % over values of control wells (grown in medium supplemented with 10% regular FBS)±SD (*=P<0.05, **=P<0.005).
We tested this hypothesis in vitro by measuring AKR1C3 transcript expression in LNCaP cells deprived of androgen. Incubation in medium supplemented with steroid-depleted serum resulted in potent upregulation of AKR1C3 (Fig. 6B). This effect was reversed by addition of the synthetic androgen R1881, confirming the negative impact of androgen on AKR1C family enzyme expression (Fig. 6B). Furthermore, the novel anti-androgen enzalutamide stimulated AKR1C3 expression (Fig. 6B), confirming that both AR antagonism and androgen deprivation can upregulate AKR1C3.
Analysis of human CTCs from CRPC patients for expression of AR, PSA and steroidogenic enzymes
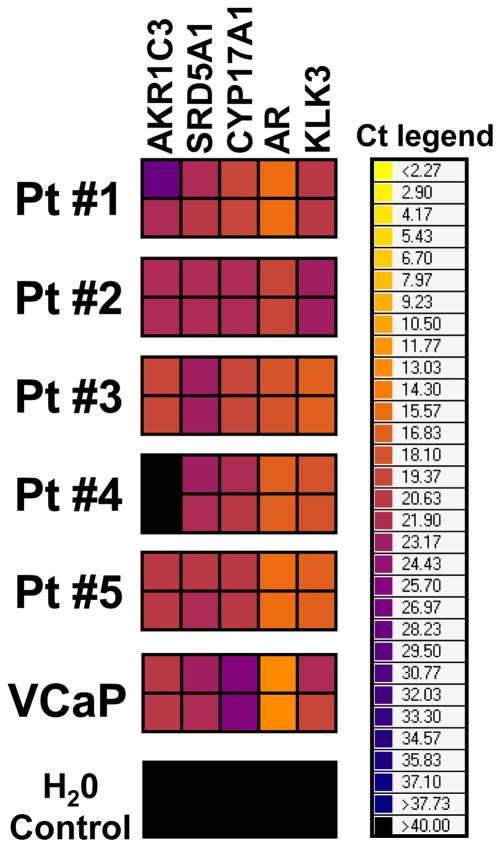
Multiplex qRT-PCR analysis for AKR1C3, SRD5A1, CYP17A1, AR, and KLK3 (PSA) transcripts revealed positivity in CTCs purified from the peripheral blood of CRPC patients (Fig. 7). This provides proof-of-principle that these steroidogenic enzymes can be detected in CTCs, and further confirms that they are expressed by the EpCaM(+) epithelial component of the tumor.
Fig. 7.
Multiplex qRT-PCR analysis of CTCs from CRPC patients for AKR1C3, SRD5A1, CYP17A1, AR, and KLK3 (PSA) transcripts reveals positivity in several CTC samples, confirming that these transcripts are expressed in the cancer cells in these tumors and providing a non-invasive method for monitoring of their expression. Results are presented as Ct (cycle threshold) values (i.e. the number of cycles required for the fluorescent signal to cross a previously defined threshold) in a heatmap. Ct values are inversely proportional to the amount of target nucleic acid in the sample. Therefore, low Ct values (orange or even yellow color) indicate strong expression of the target mRNA, while high Ct values (e.g. dark blue color) indicate weak expression. Each sample was run in duplicate. VCaP cells served as a positive control.
DISCUSSION
Suppression of gonadal androgen synthesis does not achieve complete ablation of androgen signaling in the prostate microenvironment. Even when circulating testosterone is confirmed to be at castrate levels, intratumoral androgens persist at levels sufficient to activate AR (12–17). CRPC can locally convert adrenal precursors to more active androgens (testosterone and DHT) (31–32). Moreover, de novo steroidogenesis in CRPC, using cholesterol as a precursor, has been supported by some (18, 22, 33), but not all studies (21). In the present study, we investigated the mechanism(s) leading to aberrant expression of enzymes involved in steroid metabolism in CRPC. Using data from an integrated oncogenomic analysis of primary and metastatic specimens (26), we documented that metastatic PCas express higher average transcript levels for AR and several steroidogenic enzymes, including SRD5A1, SRD5A3, and AKR1C3, while expression of SRD5A2, CYP3A4, CYP3A5 and CYP3A7 is decreased, compared to normal prostate tissue or primary prostate carcinoma. Collectively, these data demonstrate that CRPC cells have increased expression of AR and steroidogenic enzymes, and decreased expression of enzymes that can inactivate DHT (CYP3A4, CYP3A5 or CYP3A7), a state that is predicted to increase in situ androgen levels and enhance AR activation. This was supported by the finding of positive correlation between the composite enzyme expression and the AR transcriptional signaling output signature (a measure of AR activation) in our samples.
Moreover, we found high interpatient variability of expression of individual transcripts in primary and metastatic PCas, suggesting that, within individual tumors, activation of the androgen synthesis axis may occur at various levels and by various routes, but with a predicted common end result, i.e. increased tissue androgen levels and stimulation of AR. Such a result, which validates the androgen synthesis pathway en bloc as a mechanism of CRPC cell survival and resistance to androgen deprivation, should not be surprising, considering the vast heterogeneity observed in other oncogenic signaling pathways even within the same tumor (34), but may complicate targeting at the individual patient level. For example, while the predominant form of 5α-reductase in normal prostate is the type-2 (SRD5A2), in most PCas the relative expression pattern of the 2 enzymes is inverted, with increased expression of the type 1 (SRD5A1) and decreased expression of the type 2 enzyme. In clinical practice, this suggests that dutasteride, a dual 5α-reductase inhibitor (35), should be the preferred agent to target this enzymatic step in CRPC, rather than finasteride which is relatively selective for the type 2 enzyme.
Furthermore, in the era of personalized medicine, this interpatient heterogeneity in intracrine metabolic pathways raises the question whether real-time profiling of a patient’s tumor cells may provide predictive biomarkers of sensitivity to androgen synthesis inhibitors and even guide a more focused treatment approach by targeting the specific overexpressed enzyme. Although we have not performed a conclusive study, our preliminary data demonstrate that expression of these steroidogenic enzymes is detectable in circulating tumor cells (CTCs) from CRPC patients. This confirms that these enzymes are expressed by the PCa cells and opens the possibility of serially monitoring their expression using CTCs as a non-invasive source of material (“liquid biopsy”). Such approach could be supplemented by measurement of mRNA expression for AR (both full-length and alternatively spliced), as well as sequencing for AR mutations (36). In the setting of the clinical availability of novel AR antagonists (enzalutamide) (8–9, 11) and inhibitors of CYP17 (abiraterone) (2, 4, 6–7), AKR1C3 (37–40) and SRD5A1 (dutasteride) (35), CTC profiling for the respective targets provides a platform for identification and exploration of biomarkers that may guide patient eligibility for clinical trial enrollment and may serve as a potential basis for individualized therapy, possibly predicting drug efficacy and evaluating mechanism(s) of resistance.
The aberrant expression patterns of androgen axis transcripts were only rarely associated with respective CNAs in our cohort, suggesting that this dysregulation occurs mainly at the mRNA level. Analysis of these expression patterns identified distinct groups with highly co-regulated expression. One group of transcripts, comprising aldo-keto reductase family 1 (AKR1C1 through 4), was found to be inversely correlated to AR transcriptional activity, as reflected by an AR-dependent gene signature (30). This suggested that the expression of the AKR1Cs is suppressed by androgen. We confirmed that both androgen deprivation and an AR antagonist induce AKR1C3 expression. The AKR1C1-4 genes are located on chromosome 10p15 in tandem, sharing > 86% amino acid sequence identity (37). Our findings suggest that androgen deprivation triggers a feedback loop that enhances the ability of PCa cells to metabolize adrenal precursors into testosterone and DHT, thus sustaining tissue androgen levels. Evidence for such a feedback loop was recently reported in CRPC patients, where treatment with the AR antagonist enzalutamide resulted in increased bone marrow testosterone levels (41). Moreover, abiraterone-resistant PCa xenografts overexpress several steroidogenic enzymes, including AKR1C3 (24). This proposed adaptation/survival mechanism is also supported by the finding that, after gonadal androgen suppressive therapy, intraprostatic androgen levels persist at ~25% of baseline (while serum androgen levels decrease to ~7.5% of baseline) and are no longer correlated with the serum level of testosterone, but with serum levels of the adrenal precursors DHEA and DHEA-S (12–13, 42). This suboptimal suppression of intratumoral androgens may allow for the survival of cancer cells that will eventually lead to CRPC. Indeed, the rate of pathologic complete response in prostatectomy specimens removed after 3 to 8 months of neoadjuvant androgen deprivation therapy is < 3% (43). Collectively, these findings support our hypothesis that the almost universal persistence of PCa cells after gonadal androgen suppression, and the eventual emergence of CRPC, are facilitated by adaptive cellular changes that occur very early after initiation of gonadal suppression and allow PCa cells to maintain adequate intratumoral androgen levels and survive despite peripheral castrate androgen levels. A more comprehensive AR axis targeting at multiple levels (androgen synthesis, metabolism and action) and at all relevant sites (gonadal, adrenal, intratumoral) simultaneously at the time of initiation of endocrine therapy, aiming at maximal frontline inhibition of the AR axis, is warranted, instead of the current treatment paradigm of sequentially adding agents at the time of disease progression (44). Clinically, our hypothesis can be tested in trials incorporating abiraterone and/or enzalutamide at the time of initiation of GnRH analog therapy in the neoadjuvant or metastatic setting. Preliminary observations support the promise of this approach (45). Furthermore, AKR1C3 inhibitors (37–40) would also be interesting choices to be tested concurrently with GnRH analogs. A recently reported bifunctional inhibitor of both AKR1C3 and AR represents an intriguing paradigm (46).
An obvious limitation of our study is that, due to the retrospective nature of the analysis, direct measurement of androgen levels in these tissues could not be performed. Consequently, the correlation between mRNA levels and enzymatic activity cannot be confirmed in this study.
In summary, our comprehensive integrated oncogenomic approach identified aberrant expression of enzymes involved in androgen synthesis and metabolism that may lead to increased transcriptional output of the AR axis in CRPC. It is likely that the interpatient variations in these intracrine pathways of steroid metabolism can be evaluated by non-invasive, real-time monitoring of expression in CTCs and could serve as potential basis for individualized therapy. Collectively, these findings further support the notion that the AR axis is still a very important target in CRPC, and that, despite gonadal suppression, prostate tumors may not encounter (yet) a completely androgen-free microenvironment (47). The clinical activity of the CYP17 inhibitor abiraterone (2, 6–7) validates the importance of this pathway in CRPC. As inhibitors of AR (enzalutamide, ARN509) (8–9, 11, 48), CYP17 (abiraterone) (4), AKR1C3 (37–40, 46) and SRD5A1 (dutasteride) (35) are already available or in clinical development, we propose that frontline maximal suppression of the AR axis with combination therapy targeting simultaneously multiple components of this axis may enhance antitumor activity (44).
Supplementary Material
Fig. 5.
Integration of expression outlier data with CNA analysis for AR and genes involved in androgen metabolism reveals that only a small subset of primary carcinoma specimens with altered mRNA expression (over- or under-expressor outliers) have gene copy gains or losses, respectively, that can account for the dysregulated mRNA levels. The majority of cases with dysregulated expression of transcripts involved in androgen metabolism are not associated with respective CNAs. Bars represent the percentage of primary carcinomas with outlier expression for each transcript involved in androgen metabolism (bars pointing up indicate overexpressor outliers, while bars pointing down indicate underexpressor outliers for each transcript). The white part of each bar indicates specimens with outlier level of expression that also exhibited DNA copy gain (for overexpressors) or loss (for underexpressors), respectively.
Acknowledgments
Funding/Support: The authors acknowledge the joint participation by Adrienne Helis Malvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine. This work was also supported by the National Cancer Institute SPORE in Prostate Cancer (P50CA92629); the Prostate Cancer Foundation (N.M. and H.I.S.); the Department of Defense Prostate Cancer Research Program Physician Research Award (W81XWH-09-1-0307, to D.C.D), the Conquer Cancer Foundation of the American Society of Clinical Oncology Young Investigator and Career Development Awards (both to N.M.) and a Pilot/Feasibility Program of the Diabetes & Endocrinology Research Center (P30-DK079638) at Baylor College of Medicine (to N.M.). N.M. is a Dan L. Duncan Scholar, a Caroline Wiess Law Scholar and a member of the Dan L. Duncan Cancer Center (supported by the NCI Cancer Center Support Grant P30CA125123) at Baylor College of Medicine.
Footnotes
The authors have no conflict of interest to declare.
References
- 1.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol. 2009;10:981–91. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–8. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonarakis ES, Eisenberger MA. Expanding treatment options for metastatic prostate cancer. N Engl J Med. 2011;364:2055–8. doi: 10.1056/NEJMe1102758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 7.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt C. Abiraterone and MVD3100 take androgen deprivation to a new level. J Natl Cancer Inst. 2011;103:175–6. doi: 10.1093/jnci/djr014. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. ASCO Meeting Abstracts; 2012. p. LBA1. [Google Scholar]
- 12.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–6. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 14.Geller J, Albert JD, Nachtsheim DA, Loza D. Comparison of prostatic cancer tissue dihydrotestosterone levels at the time of relapse following orchiectomy or estrogen therapy. J Urol. 1984;132:693–6. doi: 10.1016/s0022-5347(17)49829-6. [DOI] [PubMed] [Google Scholar]
- 15.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 16.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–8. [PubMed] [Google Scholar]
- 17.Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22:243–58. doi: 10.1016/j.beem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsiades N, Chen Y, Scher HI. The AR axis as a pathogenetic mechanism and therapeutic target throughout the clinical states of prostate cancer: Opportunities for second-line hormonal manipulations in castration-resistant prostate cancer. In: Scardino Peter T, Marston Linehan W, Zelefsky Michael J, et al., editors. Comprehensive Textbook of Genitourinary Oncology. 4. 2011. pp. 262–73. [Google Scholar]
- 20.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 21.Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 70:1256–64. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 22.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108:13728–33. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostaghel EA, Marck B, Plymate S, Vessella RL, Balk SP, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, et al. Intratumoral De Novo Steroid Synthesis Activates Androgen Receptor in Castration Resistant Prostate Cancer and is Upregulated by Treatment with CYP17A1 Inhibitors. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh D, Chinnaiyan AM. Genomic outlier profile analysis: mixture models, null hypotheses, and nonparametric estimation. Biostatistics. 2009;10:60–9. doi: 10.1093/biostatistics/kxn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 29.http://www.cbioportal.org/public-portal/
- 30.Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–30. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Luu-The V, Belanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:207–21. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:223–8. doi: 10.1016/j.beem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 34.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Dalrymple SL, Becker RE, Denmeade SR, Isaacs JT. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res. 2006;12:4072–9. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 36.Danila DC, Anand A, Yao J, Gierszewska M, Kramer M, Muller S, et al. Predictive biomarkers in circulating tumor cells (CTC) from patients with castration-resistant prostate cancer (CRPC) through genomic analysis. ASCO Meeting Abstracts. 2012;30:4562. [Google Scholar]
- 37.Penning TM, Byrns MC. Steroid hormone transforming aldo-keto reductases and cancer. Ann N Y Acad Sci. 2009;1155:33–42. doi: 10.1111/j.1749-6632.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adeniji AO, Twenter BM, Byrns MC, Jin Y, Chen M, Winkler JD, et al. Development of potent and selective inhibitors of aldo-keto reductase 1C3 (type 5 17beta-hydroxysteroid dehydrogenase) based on N-phenyl-aminobenzoates and their structure-activity relationships. J Med Chem. 2012;55:2311–23. doi: 10.1021/jm201547v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrns MC, Jin Y, Penning TM. Inhibitors of type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3): overview and structural insights. J Steroid Biochem Mol Biol. 2011;125:95–104. doi: 10.1016/j.jsbmb.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol. 2008;75:484–93. doi: 10.1016/j.bcp.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efstathiou E, Titus MA, Tsavachidou D, Hoang A, Karlou M, Wen S, et al. MDV3100 effects on androgen receptor (AR) signaling and bone marrow testosterone concentration modulation: Apreliminary report. ASCO Meeting Abstracts. 2011;29:4501. [Google Scholar]
- 42.Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–6. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 43.Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–6. discussion 6–7. [PubMed] [Google Scholar]
- 44.Bedoya DJ, Mitsiades N. Abiraterone acetate, a first-in-class CYP17 inhibitor, establishes a new treatment paradigm in castration-resistant prostate cancer. Expert Rev Anticancer Ther. 2012;12:1–3. doi: 10.1586/era.11.196. [DOI] [PubMed] [Google Scholar]
- 45.Taplin M-E, Montgomery RB, Logothetis C, Bubley GJ, Richie JP, Dalkin BL, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): Results of a randomized phase II study. ASCO Meeting Abstracts. 2012;30:4521. [Google Scholar]
- 46.Chen M, Adeniji AO, Twenter BM, Winkler JD, Christianson DW, Penning TM. Crystal structures of AKR1C3 containing an N-(aryl)amino-benzoate inhibitor and a bifunctional AKR1C3 inhibitor and androgen receptor antagonist. Therapeutic leads for castrate resistant prostate cancer. Bioorg Med Chem Lett. 2012;22:3492–7. doi: 10.1016/j.bmcl.2012.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer. 2004;11:459–76. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- 48.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.