Abstract
Objective
In patients with Alzheimer’s disease (AD) with psychosis or agitation that respond to haloperidol treatment, to evaluate the risk of relapse following discontinuation.
Methods
In outpatients with AD with symptoms of psychosis or agitation, responders to 20 weeks of haloperidol (0.5 to 5 mg daily) were randomized to a 24-week, double-blind pilot trial of discontinuation on placebo versus continuation haloperidol. Phase A response criteria were minimum 50% reduction in 3 target symptoms, and improvement on the Clinical Global Impression-Change (CGI-C) score for psychosis/agitation. Phase B relapse criteria required 50% worsening in target symptoms and on the CGI-C. Alpha=0.1 was the significance criterion in this pilot study.
Results
Of 44 patients, 22 patients responded in Phase A. The sum score of target symptoms, and Brief Psychiatric Rating Scale psychosis and hostile suspiciousness factor scores, decreased in Phase A (p’s < .001). Extrapyramidal signs increased in Phase A (p < .01). Of 22 responders, 21 patients entered Phase B, and 20 had at least one follow-up visit. Four of 10 patients (40%) on continuation haloperidol relapsed compared to 8 of 10 patients on placebo (80%, χ2=3.3, p=0.07). In survival analyses, time to relapse was shorter on placebo than haloperidol (χ2=4.1, p=0.04).
Conclusions
Haloperidol open treatment was efficacious, and relapse was greater on placebo than with haloperidol continuation. In patients with AD who have psychosis or agitation and respond to antipsychotic medication, the increased risk of relapse after discontinuation needs to be weighed against the side effects associated with continuing the medication.
Keywords: Antipsychotic, discontinuation, Alzheimer’s disease
INTRODUCTION
In patients with Alzheimer’s disease (AD), behavioral symptoms of agitation and aggression occur in one-third to one-half of patients with moderate to severe illness (Finkel et al., 1996). Psychotic symptoms of delusions or hallucinations occur in approximately 10–30% of patients and can develop throughout the course of illness (Devanand et al., 1997; Lyketsos et al., 2000). Untreated psychosis and agitation are distressing to patients and caregivers, may worsen prognosis and lead to institutionalization (Chui et al., 1994; Scarmeas et al., 2007). In these patients, the evidence supporting the efficacy for non-pharmacologic behavioral interventions has been inconclusive for most types of interventions (Holm et al., 1999; Rovner et al., 1996; Kong et al., 2009), and the optimal behavioral treatment approach remains uncertain (Ballard et al., 2009a). Among several psychotropic medications that have been studied, only antipsychotic medications consistently have shown superiority to placebo, albeit with low to moderate efficacy in randomized, double-blind, placebo-controlled clinical trials (Schneider et al 2006a; Katz et al., 1999; Katz et al., 2007).
Among the antipsychotic medications, dose comparison studies have helped to define the optimal dose range for haloperidol, risperidone and olanzapine in patients with dementia (Devanand et al., 1998; De Deyn et al., 1999; Katz et al., 1999; Street et al., 2000). The use of conventional or first generation antipsychotic medications, and to a lesser extent atypical or second generation antipsychotic medications, can lead to the adverse neurological effects of extrapyramidal (Parkinsonian) signs with short-term use and tardive dyskinesia with long-term use. Antipsychotic medication use is also associated with weight gain and the metabolic syndrome. In several clinical trials that utilized different antipsychotic medications in elderly patients with dementia, an increased mortality risk was reported with an odds ratio of 1.5 to 1.7 for antipsychotic medication compared to placebo (Schneider et al., 2005). However, other evidence suggests no increase, and possibly a decrease, in mortality with antipsychotic use in long term care residents (Simoni-Wastila et al., 2009). Antipsychotic medications are still widely used because of the clinical urgency presented by agitated, aggressive and psychotic patients diagnosed with dementia, and the lack of viable treatment alternatives with established efficacy.
Given the potential adverse consequences of long-term use of antipsychotic medications, uncontrolled (Fitz and Mallya, 1992; Horwitz et al., 1995) and placebo-controlled discontinuation trials have been conducted, primarily in nursing homes (Thapa et al., 1994; Cohen-Mansfield et al., 1999; Ballard et al., 2008). These studies generally showed little difference in relapse rates for continuation medication versus placebo, but all of them had the same three limitations: (1) most patients had received antipsychotics for long periods, often years, and many did not have target symptoms at study entry. In these patients, not surprisingly, discontinuation of these medications did not lead to emergent symptoms; (2) response to the psychotropic medication was typically not established before discontinuation; and (3) patients were often discontinued from different antipsychotics and other psychotropics, limiting the ability to assess relapse risk for specific antipsychotic medications. In a recent discontinuation trial, the primary outcome was the Severe Impairment Battery that assesses cognition (Ballard et al., 2008), a domain where significant changes would not be expected with antipsychotic discontinuation.
One approach to overcome these limitations is to conduct an open treatment trial with a single antipsychotic medication in patients with AD who have symptoms of psychosis or agitation of sufficient intensity to require treatment, after which responders to the treatment are randomized, double-blind, to continuation medication or placebo to compare the likelihood of relapse, and time to relapse. Using this study design, after an initial open haloperidol treatment phase of target behavioral and psychotic symptoms in outpatients with AD, we randomized clinical responders to a pilot, double-blind discontinuation trial of haloperidol versus placebo treatment.
METHODS
Outpatients with probable AD who presented with agitation, aggression or psychosis to a Memory Disorders Clinic or an affiliated Behavioral Neurology practice group participated in a clinical trial. The protocol was approved by the New York State Psychiatric Institute and Columbia University Institutional Review Boards. Informed consent was obtained for all subjects, with surrogate consent provided by family members in cases where the patient lacked capacity to consent, based on New York State regulations. The study is registered at clinical trials.gov, identifier NCT00009217.
Inclusion criteria were age 50–95 years and the clinical diagnoses of dementia by DSM-IV criteria and probable AD by NINCDS-ADRDA criteria (McKhann et al., 1984), based on history, physical examination and structural brain imaging. The Folstein Mini Mental State Exam (MMSE; Folstein et al., 1975) permitted range was 5–26. All psychiatric assessments were based on informant interview, typically the primary caregiver. Patients needed to have current symptoms of psychosis or agitation to enter the study. Criteria for “psychosis” required the presence of delusions and/or hallucinations identified by the Columbia University Scale for Psychopathology in Alzheimer’s Disease (CUSPAD, Devanand et al., 1992) and a minimum Brief Psychiatric Rating Scale (BPRS) psychosis factor score of at least 4 (moderate severity) on one of the following two items: hallucinatory behavior or unusual thought content, or a total score of greater than 6 on the sum score of these two items. These two items comprise the psychosis factor, excluding the item for conceptual disorganization because this symptom often occurs in AD in the absence of psychosis. Agitation was defined as a score of greater than 3 (present at least 10 days per month) on one or more of the CERAD Behavioral Rating Scale for Dementia items for agitation, purposeless wandering, verbal aggression or physical aggression (Tariot et al., 1995).
Exclusion criteria were acute unstable medical condition, delirium, alcohol or substance abuse or dependence during the prior year, clinical evidence of stroke, other dementias including vascular or Lewy body or frontotemporal dementia, multiple sclerosis, Parkinson’s disease, Huntington’s disease, tardive dyskinesia, diagnosis of a psychotic disorder antedating the onset of dementia, antipsychotic medication usage during the 4 weeks prior to study entry, and contraindication to the use of haloperidol.
Phase A open treatment (20 weeks)
Patients receiving psychotropic medications had a 1-week washout prior to entering Phase A. During Phase A, the study physician prescribed flexible doses of haloperidol 0.5 mg to 5 mg daily (primarily given at bedtime), individually titrated to maximize therapeutic response and minimize side effects (primarily extrapyramidal side effects). In Phase A, visits occurred at 0, 2, 4, 8, 12, 16 and 20 weeks (Figure 1).
Figure 1.
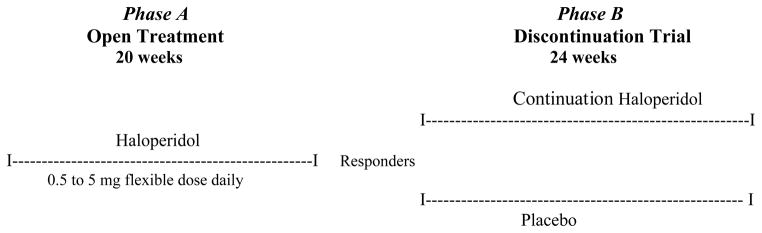
STUDY FLOW SHEET.
At baseline evaluation, the three most prominent target symptoms of psychosis or agitation were identified, scored on a 7-point scale (0=absent to 6=extreme), and tracked during the study. Any combination of psychotic or agitation/aggression symptoms was permitted, e.g., two separate delusions and one symptom of agitation, or three symptoms of agitation/aggression. These symptoms were not directly derived from the CUSPAD, BPRS or CERAD scales used to determine inclusion criteria for this study. Criteria for clinical response were minimum 50% reduction from baseline in the sum score of these 3 target symptoms, a sum score ≤ 6 on these 3 items (range 0–18), and minimal or greater improvement on the Clinical Global Impression-Change score (rated only for symptoms of psychosis or agitation).
Phase B discontinuation trial (24 weeks)
Responders by end-Phase A were eligible for Phase B, a 24-week, random assignment (1:1 assignment of haloperidol and placebo), double-blind, trial of continuation haloperidol (same dose as end-Phase A) versus switch to placebo (Figure 1).
Haloperidol and placebo were made up in identical-looking opaque white capsules. Haloperidol dosage was continued at the same dose as at end-Phase A. For patients randomized to placebo, there was a 2-week double-blind sequential placebo substitution tapering period: patients on 4 mg daily at end-Phase A switched to 2 mg daily for 1 week, 1 mg daily for the next week and then switched completely to placebo; patients on 2 or 3 mg daily switched to 1 mg daily for 2 weeks and then switched to placebo, and patients who received 0.5 mg or 1 mg were switched directly to placebo without a tapering period. Time-points of assessment during Phase B were 0 (same as end-Phase A), 2, 4, 8, 12, 16, 20, 24 weeks. Criteria for relapse were minimum 50% worsening from the sum score of three target symptoms at end Phase A, a sum score ≥ 6 on these 3 items (range 0–18), and minimal or greater worsening on the Clinical Global Impression-Change score (rated for psychosis/agitation). Criteria for relapse needed to be met at only a single time point during Phase B.
No concomitant psychotropic medications were permitted throughout the trial. Compliance with study medication was assessed with pill counts at all visits. Somatic side effects were assessed by the Treatment Emergent Symptom Scale (TESS), extrapyramidal signs by the Unified Parkinson’s Disease Rating Scale (UPDRS; Fahn et al., 1987), and tardive dyskinesia by the Rockland TD scale (Simpson et al., 1979).
At baseline, end-Phase A and end-Phase B, cognition was assessed by the Mini Mental State Exam (MMSE) and impairment in activities of daily living was assessed by the modified Blessed Functional Activity Scale (BFAS, Blessed et al., 1968; Stern et al., 1990). For all early terminations, the entire assessment battery was completed at the exit visit.
Statistical Analyses
Alpha=0.1 was the criterion for statistical significance because of the small sample size in this pilot study. For Phase A, baseline descriptive statistics were evaluated and intent-to-treat analyses were conducted with the last observation carried forward. Repeated measures ANOVA was conducted across the three major time-points (baseline, week 10, week 20) with the sum score of three target symptoms being the primary efficacy measure and BPRS psychosis and hostile suspiciousness factor scores being secondary measures. Significant effects in ANOVA were followed up by post hoc t-tests. This analytic strategy was used to evaluate changes from baseline to week 20 for measures of efficacy as well as side effects, global cognition and activities of daily living.
Among Phase A responders who were randomized to Phase B, patients who had at least one study visit after randomization had their last observation carried forward and were included in data analysis. In Phase B, survival analysis (Kaplan Meier) was conducted to compare time to relapse on continuation haloperidol versus placebo. Logistic regression analysis was conducted to evaluate severity of target symptoms at baseline and end-Phase A as predictors of relapse in Phase B. Age, sex, and baseline MMSE were covariates in relevant analyses.
RESULTS
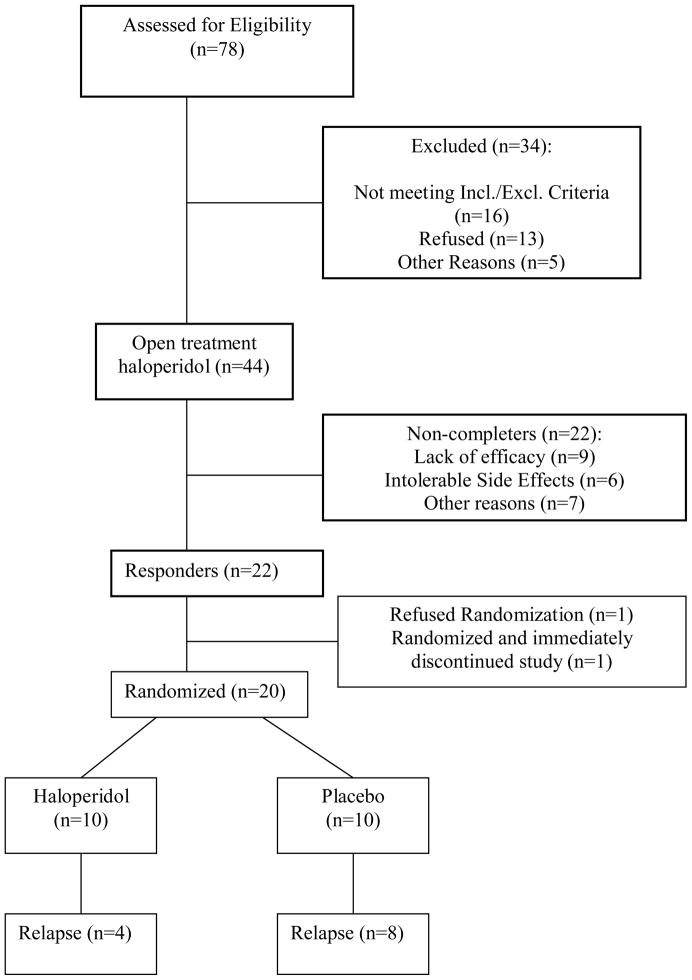
Figure 2 describes the number of subjects across the stages of recruitment and study participation.
Figure 2.
CONSORT diagram of recruitment and patient flow through the study.
The majority of study participants (n=44) were female (57%). The ethnic distribution was 56.8% White, 13.6% African American, and 29.6% Hispanic. Mean age was 75.0 (SD 8.0) years and mean education was 10.9 (SD 5.2) years. Baseline and end-Phase A rating scale scores in responders and non-responders are described in Table 1. By end-Phase A (or early termination), mean dose of haloperidol was 1.58 (SD 1.0) mg/day, and did not differ between responders (mean 1.53 SD 0.9) and non-responders (mean 1.61, SD 1.0). There were 15 Phase A non-completers (34%), with all early terminations attributed either to lack of efficacy (n=9) or side effects (n=6).
Table 1.
Baseline and end-Phase A (20 weeks) clinical assessment ratings in responders (n=22) and non-responders (n=22) to open label haloperidol treatment.
| Rating Scales | Responders | Non-responders | ||
|---|---|---|---|---|
| Baseline Mean (SD) | End- Phase A Mean (SD) | Baseline Mean (SD) | End- Phase A Mean | |
| Sum of three target symptoms | 14.5 (1.2) | 5.9 (0.9) | 14.6 (1.3) | 10.8 (3.3) |
| BPRS total | 26.6 (5.5) | 19.0 (2.7) | 28.8 (6.3) | 27.2 (6.9) |
| BPRS psychosis factor | 5.9 (2.5) | 3.6 (0.9) | 7.0 (3.0) | 6.2 (3.7) |
| BPRS hostile suspiciousness factor | 11.1 (3.0) | 6.0 (1.2) | 11.3 (2.6) | 8.4 (2.9) |
| UPDRS total | 0.8 (0.8) | 0.7 (1.0) | 0.5 (0.6) | 0.7 (0.8) |
| Mini Mental State Exam (0–30) | 12.9 (6.3) | 12.3 (6.3) | 12.5 (6.0) | 13.8 (5.3) |
Target symptoms were each score on a 0–6 scale (absent to extreme), sum score range 0–18.
BPRS: Brief Psychiatric Rating Scale.
BPRS psychosis factor excluded the item for conceptual disorganization because this symptom often occurs in AD in the absence of psychosis.
UPDRS: Unified Parkinson’s Disease Rating Scale.
In intent-to-treat analyses with the last observation carried forward (n=44), repeated measures ANOVA on the sum score of target symptoms at baseline, week 12 and week 20 showed a significant effect of time (p < .001). The sum score of three target symptoms decreased from baseline to end Phase A (t=16.5, p < .001), as did the BPRS psychosis factor (t=5.2, p < .001) and hostile suspiciousness factor (t=9.7, p < .001).
Of the 44 patients, 22 patients (50%) met criteria for response by end-Phase A. In logistic regression analyses on responder status as the outcome, age, sex, baseline MMSE and baseline severity of target symptoms were not significant. Over this 20-week time frame, there were no significant increases in somatic side effects (TESS scores). There was an increase in extrapyramidal signs (t=2.9, p < .01). One patient developed minimal to mild tardive dyskinesia (global score=1, 0–5 range) on the Rockland tardive dyskinesia scale by end-Phase A.
Twenty of the 21 patients randomized in Phase B to continuation haloperidol or placebo had at least one follow-up visit after randomization and were included in the Phase B analysis. Among patients who did not relapse, reasons for early study termination prior to 24 weeks in Phase B were side effects (n=2), moving out of the area (n=1), medical illness (n=1), and noncompliance (n=1). All data from these patients were included in the intent-to-treat, last observation carried forward, analyses. Mean haloperidol daily dose (kept constant during Phase B) was 1.35 mg (SD 0.83).
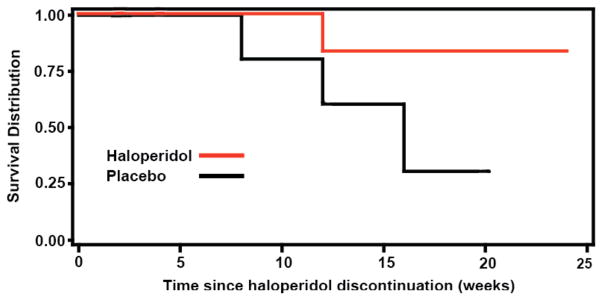
During Phase B, 4 of 10 patients (40%) on continuation haloperidol relapsed compared to 8 of 10 patients on placebo (80%, χ2=3.3, p=0.07). In Kaplan Meier survival analyses (Figure 3), time to relapse was shorter on placebo compared to haloperidol (log rank test, χ2=4.1, p=0.04). Among patients who relapsed, patients on haloperidol reached the end-point of relapse by mean 8.0 (SD 10.7) weeks and patients on placebo reached the end-point of relapse by mean 5.8 (SD 6.7) weeks. In logistic regression analyses, neither baseline severity of target symptoms at start-Phase A nor severity of residual target symptoms at end-Phase A significantly predicted the likelihood of relapse in Phase B. Type of target symptom, i.e., psychosis or agitation, did not predict relapse. In these analyses, age, sex and baseline MMSE were not significant covariates. The change in MMSE and BFAS scores did not differ between haloperidol and placebo in Phase B. No patient died during the course of the study.
Figure 3.
DISCUSSION
In Phase A, target behavioral and psychotic symptoms decreased significantly with open haloperidol treatment with an average daily dose in the 1–2 mg range, consistent with findings from a placebo-controlled dose comparison trial (Devanand et al., 1998). The results in Phase B demonstrated an increased risk of relapse upon discontinuation of antipsychotics after improvement in target symptoms with treatment. Severity of target symptoms at end-Phase A was not associated with relapse in Phase B, but these analyses were limited by small sample size. Increased severity of psychosis, agitation or aggression has been associated with a greater probability of relapse after antipsychotic discontinuation in patients who had been receiving these medications, typically for years, in nursing homes and similar facilities (Ballard et al., 2004). In a subsequent study, the same research group found a small, non-significant advantage for continuation antipsychotic medication (several antipsychotics including atypicals were used) compared to placebo in decreasing relapse in behavioral and psychotic symptoms in the short-term, and this difference became larger and significant by 12-month follow-up (Ballard et al., 2008). These published results are in the same direction, though with smaller group differences, than in our trial. Earlier reports of antipsychotic (Thapa et al., 1994; Bridges-Parlet et al., 1997; Cohen-Mansfield et al., 1999) and benzodiazepine (Cohen-Mansfield et al., 1999) discontinuation showed that withdrawal of these psychotropic medications did not significantly increase the risk of relapse compared to placebo.
These negative reports may be related to many patients having received these medications for months and often years without clear evidence of target symptoms requiring such medications. In contrast, our study was restricted to patients who had target symptoms of psychosis or agitation of moderate or greater severity that were likely to improve with haloperidol treatment, and therefore they may have been more likely to relapse upon discontinuation during the six months of Phase B. Another possible explanation for the greater differences observed in our study is that all patients were treated with a single antipsychotic medication, haloperidol, whereas prior published placebo-controlled studies involved discontinuation off a variety of antipsychotics and other psychotropic medications which limited the ability to estimate relapse risk for a specific antipsychotic medication.
Antipsychotic use has been shown to be associated with increased mortality with an odds ratio of 1.5 to 1.7 in elderly patients with dementia (Schneider et al., 2005). Increased mortality risk was also demonstrated in a recent antipsychotic discontinuation trial in nursing homes (Ballard et al., 2009b), though there are other reports showing either no increase (Raivio et al., 2007; Suh and Shah, 2005) or a decrease in mortality risk associated with antipsychotic use in patients with dementia (Simoni-Wastila et al., 2009). No patient died during our outpatient study but the small sample size limited estimation of mortality risk. Haloperidol usage was associated with a significant increase in extrapyramidal signs. Tardive dyskinesia was rare in this sample, possibly because of the low doses used and the relatively short duration of the study. Global measures of cognitive change and activities of daily living did not differ by treatment group in Phase B.
The strengths of this study included identification of target symptoms followed by systematic open antipsychotic treatment with only responders randomized in the discontinuation trial, and the use of a single antipsychotic medication, haloperidol. The main limitations of this study were the small sample size and the moderately high dropout rate, though the latter is comparable to that observed in other studies of similar patient populations followed for an extended period of time (Ballard et al., 2009b; Schneider et al., 2006b). For alpha of 0.05 (two-tailed), to obtain adequate power (80%) for the observed relapse rates of 80% on placebo and 40% on antipsychotic, a sample size of 56 patients (28 in each group) is required. For 80% power using a more conservative difference in relapse rates of 75% on placebo and 45% on antipsychotic, a sample size of 96 patients (48 in each group) is required. These estimates assume a 1:1 treatment assignment ratio and do not account for dropout.
The mean baseline values for measures of behavioral complications, e.g., BPRS scores, and cognitive status, e.g. MMSE scores, were comparable to other studies in the literature including CATIE (Schneider et al., 2006a), thereby enhancing generalizability. The CATIE study results suggested that some patients benefit appreciably from antipsychotic treatment but that others cannot tolerate these medications, leading to few differences between treatments on the outcome measure of all-cause discontinuation (Schneider et al., 2006a; Sultzer et al., 2007). The results of this pilot trial, which need confirmation in a larger multicenter study involving a broader sample with greater medical comorbidities, suggest that the need for effective treatment of difficult-to-manage behavioral complications and the potential increased risk of relapse with discontinuation should be weighed against the neurological and metabolic side effects, and mortality risks, of using antipsychotic medications in patients with dementia.
Key points.
Open treatment with antipsychotic medications can be effective in patients with Alzheimer’s disease who have symptoms of psychosis or agitation/aggression.
There was an increased risk of relapse in target symptoms of psychosis and agitation following placebo-controlled discontinuation of haloperidol.
This increased risk of relapse following antipsychotic discontinuation needs to be weighed against the propensity to neurological and other side effects of antipsychotic medications.
The findings in this pilot study need confirmation in a larger clinical trial.
Acknowledgments
Supported by NIH grants R01MH55735 and R01 AG17761.
Footnotes
DISCLOSURE:
Dr. Devanand has received grants from the National Institutes of Health, research support from Novartis and Eli Lilly, and has served as a consultant to GSK, Bristol Myers Squibb, and Sanofi-Aventis. Dr. Pelton has received research support from GSK, Novartis and Forest and has served as a consultant to Bristol Myers Squibb and Pfizer. Dr. Sackeim has received grants from the National Institutes of Health and served as a consultant to MECTA, Cyberonics, and Neuronetics. Dr. Marder has received grants from the National Institutes of Health, the Parkinson Disease Foundation and research support from Amarin Neuroscience LTD, Boehringer-Ingelheim and Neurosearch.
References
- Ballard CG, Thomas A, Fossey J, Lee L, Jacoby R, et al. A 3-month, randomized, placebo-controlled, neuroleptic discontinuation study in 100 people with dementia: the neuropsychiatric inventory median cutoff is a predictor of clinical outcome. J Clin Psych. 2004;65:114–119. doi: 10.4088/jcp.v65n0120. [DOI] [PubMed] [Google Scholar]
- Ballard C, Margallo Lana M, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing to take or discontinued from treatment with neuroleptics (the DARTAD trial) PLoS Med. 2008;5:e76. doi: 10.1371/journal.pmed.0050076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Brown R, Fossey J, Douglas S, Bradley P, Hancock J, James IA, Juszczak E, Bentham P, Burns A, Lindesay J, Jacoby R, O’Brien J, Bullock R, Johnson T, Holmes C, Howard R. Brief psychosocial therapy for the treatment of agitation in Alzheimer disease (The CALM-AD trial) Am J Geriatr Psychiatry. 2009a;17(9):726–33. doi: 10.1097/JGP.0b013e3181b0f8c0. [DOI] [PubMed] [Google Scholar]
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu LM, Jacoby R DART-AD investigators. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009b;8(2):151–7. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bridges-Parlet S, Knopman D, Steffes S. Withdrawal of neuroleptic medications from institutionalised dementia patients, results of a doubleblind baseline-treatment-controlled pilot study. J Geriatric Psych Neurol. 1997;10:119–126. doi: 10.1177/089198879701000306. [DOI] [PubMed] [Google Scholar]
- Chui HC, Lyness SA, Sobel E, et al. Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer’s disease. Arch Neurol. 1994;51(7):676–81. doi: 10.1001/archneur.1994.00540190056015. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Lipson S, Werner P, Billig N, Taylor L, Woosley R. Withdrawal of haloperidol, thioridazine, and lorazepam in the nursing home: a controlled, double-blind study. Arch Intern Med. 1999;159(15):1733–40. doi: 10.1001/archinte.159.15.1733. [DOI] [PubMed] [Google Scholar]
- DeDeyn, Rabheru, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53:946–955. doi: 10.1212/wnl.53.5.946. [DOI] [PubMed] [Google Scholar]
- Devanand D, Miller L, Richards, et al. The Columbia University Scale for Psychopathology in Alzheimer’s Disease. Arch Neurol. 1992;49:371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Miller L, Richards M, Marder K, Bell K, Mayeux R, Stern Y. The Columbia University Scale for Psychopathology in Alzheimer’s disease. Arch Neurol. 1992b;49:371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Jacobs DM, Tang M-X, Castillo-Castaneda CD, Sano M, Marder K, Bell K, Bylsma FW, Brandt J, Albert M, Stern Y. The course of psychopathologic symptoms in mild to moderate Alzheimer’s disease. Arch Gen Psychiatry. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Marder K, Michaels KS, Sackeim HA, Bell K, Sullivan MA, Cooper TB, Pelton GH, Mayeux R. A randomized, placebo-controlled, dose-comparison trial of haloperidol treatment for psychosis and disruptive behaviors in Alzheimer’s disease. Am J Psychiatry. 1998;155:1512–1520. doi: 10.1176/ajp.155.11.1512. [DOI] [PubMed] [Google Scholar]
- Fahn S, Marsden CD, Calne D. Recent developments in Parkinson’s disease. Macmillan Healthcare Information; Florham Park, NJ: 1987. [Google Scholar]
- Finkel SI, Costa e Silva J, Cohen G, et al. Behavioral and psychological signs and symptoms of dementia: a consensus statement of current knowledge and implications for research and treatment. Int Psychogeriatr. 1996;8(Suppl 3):497–500. doi: 10.1017/s1041610297003943. [DOI] [PubMed] [Google Scholar]
- Fitz D, Mallya A. Discontinuation of a psychogeriatric program for nursing home residents: psychotropic medication changes and behavioral reactions. J Appl Gerontol. 1992;11(1):50–63. doi: 10.1177/073346489201100105. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Holm A, Michel M, Stern GA, Hung TM, Klein T, Flaherty L, Michel S, Maletta G. The outcomes of an inpatient treatment program for geriatric patients with dementia and dysfunctional behaviors. Gerontologist. 1999;39(6):668–76. doi: 10.1093/geront/39.6.668. [DOI] [PubMed] [Google Scholar]
- Horwitz GJ, Tariot PN, Mead K, Cox C. Discontinuation of antipsychotics in nursing home patients with dementia. Am J Geriatr Psychiatry. 1995;3:290–299. doi: 10.1097/00019442-199503040-00003. [DOI] [PubMed] [Google Scholar]
- Raivio MM, Laurie JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15:416–424. doi: 10.1097/JGP.0b013e31802d0b00. [DOI] [PubMed] [Google Scholar]
- Simoni-Wastila L, Ryder PT, Qian J, Zuckerman IH, Shaffer T, Zhao L. Association of antipsychotic use with hospital events and mortality among Medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry. 2009;17:417–427. doi: 10.1097/JGP.0b013e31819b8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GH, Shah A. Effects of antipsychotics on mortality in elderly patients with dementia: a 1-year prospective study in a nursing home. Int Psychogeriatr. 2005;17:429–441. doi: 10.1017/s1041610205002243. [DOI] [PubMed] [Google Scholar]
- Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry. 1999;60:107–15. doi: 10.4088/jcp.v60n0207. [DOI] [PubMed] [Google Scholar]
- Katz I, de Deyn PP, Mintzer J, Greenspan A, Zhu Y, Brodaty H. The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer’s disease and mixed dementia: a meta-analysis of 4 placebo-controlled clinical trials. Int J Geriatr Psychiatry. 2007;22(5):475–84. doi: 10.1002/gps.1792. [DOI] [PubMed] [Google Scholar]
- Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512–520. doi: 10.1080/13607860902774394. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Steinberg M, Tschanz, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Steele CD, Shmuely Y, Folstein MF. A randomized trial of dementia care in nursing homes. J Am Geriatr Soc. 1996;44(1):7–13. doi: 10.1111/j.1532-5415.1996.tb05631.x. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Blacker D, Albert M, Hadjigeorgiou G, Dubois B, Devanand D, Honig L, Stern Y. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol. 2007;64(12):1755–61. doi: 10.1001/archneur.64.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006a;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, et al. CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New Engl J Med. 2006b;355:1525–38. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Lee JH, Zoubok B, et al. A rating scale for tardive dyskinesia. Psychopharmacology. 1979;64:171–179. doi: 10.1007/BF00496058. [DOI] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Hesdorfer D, Sano M. Measurement and prediction of functional change in Alzheimer’s disease. Neurology. 1990;40:8–14. doi: 10.1212/wnl.40.1.8. [DOI] [PubMed] [Google Scholar]
- Street JW, Clark WS, Gannon KS, et al. Olanzapine Treatment of Psychotic and Behavioral Symptoms in Patients with Alzheimer Disease in Nursing Care Facilities. Arch Gen Psychiatry. 2000;57:2269–2276. doi: 10.1001/archpsyc.57.10.968. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, Rosenheck RA, Hsiao JK, Lieberman JA, Schneider LS CATIE-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165(7):844–54. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, Blazina L, Teri L, Rubin E, Mortimer JA, Stern Y. The Behavior Rating Scale for Dementia (BRSD) of the Consortium to establish a registry for Alzheimer’s disease (CERAD) Am J Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- Thapa PB, Meador KG, Gideon P, Fought RL, Ray WA. Effects of antipsychotic withdrawal in elderly nursing home residents. J Am Geriatr Soc. 1994;42(3):280–6. doi: 10.1111/j.1532-5415.1994.tb01752.x. [DOI] [PubMed] [Google Scholar]