Abstract
Rapamycin has long been considered an immunosuppressive agent due to its anti-proliferative effects on immune cells, and is currently used as a component of anti-rejection regimens in transplantation. Despite the large number of mechanistic and clinical studies investigating the impact of rapamycin on cell-mediated immunity, several paradoxes concerning rapamycin immunobiology remain. In particular, emerging evidence suggests that under certain circumstances rapamycin can exert immunostimulatory effects, boosting T cell responses in the face of pathogen infections and vaccines. Here, we review recent findings concerning the contradictory outcomes of rapamycin induced mTOR inhibition on CD4+ and CD8+ T cell responses in transplantation and protective immunity. These studies suggest that the conditions under which T cells are stimulated can profoundly modify the impact of rapamycin on antigen-specific T cell responses. Thus, further investigation into the cellular and molecular pathways underlying the dichotomous effects of rapamycin in transplantation is required to harness the full potential of this immunomodulatory agent to promote graft survival and maximize protective immunity.
Keywords: immunosuppression, pathogen, T cell differentiation, dendritic cell maturation, fatty acid metabolism, survival
In the early 1970’s on Easter Island, the compound now known as rapamycin was isolated from Streptomyces hygroscopicus and subsequently found to potently inhibit cell proliferation (1). In the 40 years since its discovery, rapamycin has been shown to have myriad effects on many different cell types in mammals. It is now known that rapamycin mediates these effects by forming a complex with FK506-binding protein 12 (FKBP-12), which binds to and inactivates mammalian target of rapamycin (mTOR) (Figure 1A) (1). mTOR is a serine/threonine protein kinase which is widely expressed among many cell types, and is an important component of several intracellular signaling pathways in naïve, effector, and regulatory T cells, involving the PI3K/Akt pathway (Figure 1A) (2). While great strides have been made in the last few years to understand the impact of rapamycin on T cell metabolism, differentiation, and lineage commitment, our current understanding of the effects of rapamycin on T cell biology is encumbered by an unusually high number of paradoxical effects, the mechanisms underlying most of which have yet to be elucidated.
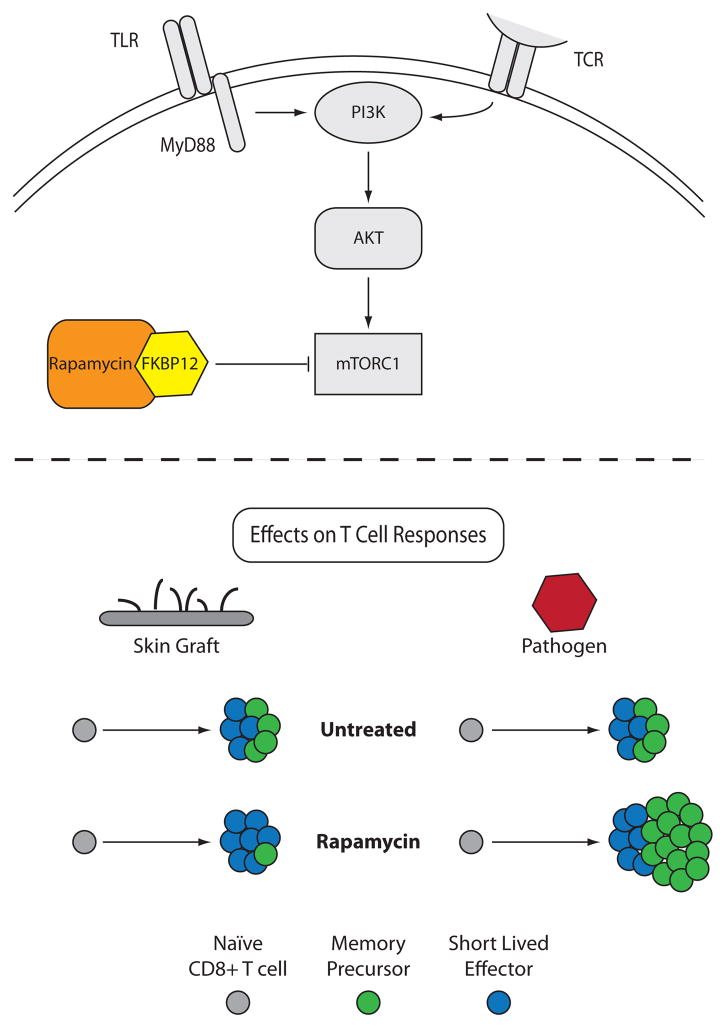
Figure 1. Differential effects of rapamycin therapy on antigen-specific CD8+ T cell responses following infection or transplantation.
A) PI3K activity results in Akt phosphorylation and subsequently mTOR complex 1 activation, which is blocked by the complex of rapamycin bound to FKBP12. B) In blocking the mTOR pathway in a transplantation model, rapamycin minimally impacts expansion of donor-reactive CD8+ T cells, while attenuating differentiation of these cells into CD62L-expressing central memory-like cells. In contrast, mTOR blockade with rapamycin leads to enhanced activation and expansion of antigen-specific CD8+ T cells during the course of an infection, while promoting the development of CD62Lhi CD8+ central memory-like cells.
Rapamycin as an immunosuppressant: what we thought we knew
Rapamycin has long been appreciated as an inhibitor of cell growth, shown to differentially affect the different mTOR complexes (1). Inhibition of the mTOR complex further mediates immunosuppressive function through an anti-proliferative effect and by attenuating signaling through IL-2R and other cytokine receptors (the so-called signal three of T cell activation), thereby preventing full activation of the T cell (2). Studies in human cells revealed that the impact of rapamycin on T cell proliferation was dependent on both the strength of signal through the TCR as well as the degree of costimulation provided, such that in the presence of strong TCR signals and high costimulation, rapamycin failed to inhibit proliferation (3). However, even in the presence of costimulation, inhibition of mTOR signaling with rapamycin has been shown to induce anergy in antigen-specific T cells (4). More recent studies have demonstrated that the immunosuppressive properties of rapamycin are even broader, impacting the generation and expansion of regulatory T cells (Tregs) as well as the maturation and function of dendritic cells (DC) (both discussed further below) (2). Despite these pluripotent immunosuppressive effects, rapamycin proved to be a relatively weak inhibitor of graft rejection when used as a monotherapy. For example, in experimental murine models of fully MHC disparate skin transplantation, rapamycin treatment resulted in a very modest prolongation of graft survival of only a few days (5). Likewise, rapamycin (sirolimus) monotherapy is not often used in clinical transplantation; instead, rapamycin is frequently administered as part of multi-drug regimens (6). Specifically, it can be used in combination with mycophenolate mofetil, often with favorable outcomes in terms of graft survival in transplant recipients (6). In addition, rapamycin has been combined with lymphodepleting agents prior to transplantation in order to prolong graft survival (7). Studies designed to elucidate the mechanisms underlying the observed synergism between rapamycin and lymphocyte depletion revealed that the addition of rapamycin attenuated the generation of effector memory T cells (TEM) and increased the relative proportion of Treg following depletional therapy, resulting in a more favorable TEM/Treg balance (5). Thus, the immunosuppressive effects of rapamycin during transplantation are well-documented; however, the ubiquitous nature of its target, mTOR, makes it difficult to pinpoint the precise pathways involved in rapamycin’s salutary effect following transplantation.
Impact of rapamycin on CD8+ T cell differentiation in response to pathogens
Given this history as an immunosuppressive and anti-proliferative agent, it came as a great surprise when investigators began to interrogate the impact of rapamycin monotherapy on antigen-specific T cell responses during the course of viral or bacterial infection. Clinical observations published over the last decade hinted at the idea that patients treated with rapamycin demonstrated better outcomes with regard to cytomegalovirus (CMV) disease and were better able to control CMV viremia than patients treated with standard calcineurin inhibitor-based immunosuppression following transplantation (8). The authors of the study speculated that the underlying mechanism involved rapamycin-mediated attenuation of viral replication, as these viruses co-opt host machinery in order to replicate (8). Recently, however, in two studies published in Nature in 2009, Araki et al. and Pearce et al. demonstrated a paradoxical immunostimulatory effect of rapamycin on the CD8+ memory T cell response following pathogen infection (9, 10). Administration of rapamycin during the priming phase was found to increase the number of virus-specific memory T cells in LCMV-infected animals. Furthermore, when rapamycin was administered only during the contraction phase of the response, the antigen-specific T cells did not increase in quantity, but rather increased in quality, acquiring a more central memory-like phenotype (CD62Lhi KLRG-1lo CD27hi Bcl-2hi), and increased proliferative capacity upon rechallenge (Figure 1). In a series of elegant RNAi knock-down experiments, the authors showed that this enhancement in the antigen-specific response was a T cell-intrinsic effect. These results were further corroborated in a rhesus macaque model in which animals receiving rapamycin exhibited increased recall responses and enhanced differentiation of memory T cells following vaccination with modified vaccinia Ankara ((9)(11)). Since both the macaque and murine studies involved non-replicative viruses (or virus-like particles), these data provide further evidence that the direct impact of mTOR inhibition on viral replication is likely not the mechanism by which viral load might be attenuated following treatment with rapamycin. Instead, unlike the previously described effects of mTOR inhibition on dendritic cells, Treg, or other immune compartments (2), both studies point to a direct effect of rapamycin on CD8+ T cells to enhance both the quantity and quality of memory T cell differentiation in response to pathogen exposure in vivo.
What is the mechanism underlying this effect? The explanation could be linked to the ability of rapamycin to enhance fatty acid oxidation (FAO) in responding T cells. Studies in other cell types, including smooth muscle cells and hepatocytes, have demonstrated that inhibition of mTOR with rapamycin results in increased FAO along with reduced glucose utilization (12, 13). Importantly, the transition from glycolysis to FAO was recently shown to be critical for effector to memory transition in CD8+ T cells (10). In particular, T cells lacking TRAF6 were shown to be deficient in their ability to undergo FAO following growth factor withdrawal, and also exhibited a reduced ability to differentiate into memory T cells. However, treatment with the diabetes drug metformin restored FAO and enhanced differentiation of memory T cells, both in TRAF6-deficient as well as wild-type T cells (10). Similar results were observed in CD8+ T cells following treatment with rapamycin; thus, rapamycin may act on effector T cells to facilitate the metabolic switch from glycolysis to FAO, thereby enhancing memory T cell differentiation. Another possible mechanism by which rapamycin may augment the generation of T cell memory is by impacting the relative balance of expression of the transcription factors T-bet and Eomesodermin in CD8+ T cells (14). Specifically, blocking mTOR through rapamycin decreases the expression of T-bet, which is highly expressed in effector T cells, and promotes expression of Eomesodermin, which is highly expressed in memory T cells. Taken together, these data suggest possible mechanism(s) underlying the immunostimulatory effects of rapamycin on pathogen-specific T cell responses, and imply that exposure to rapamycin may produce different outcomes depending on the cell cycle and metabolic state of a given cell or population.
Fundamental differences in the effect of rapamycin on graft versus pathogen specific T cells
Given these findings on the effects of rapamycin on pathogen-specific T cell responses, the question of whether rapamycin also augmented antigen-specific CD8+ T cell response during transplantation arose. Was this enhancing effect of rapamycin being masked by the presence of other immunosuppressants following transplantation? Or was there something fundamentally different about the way rapamycin affects T cells responding to cognate antigen in the context of a graft versus a pathogen?
To address these questions, a recent study from our group directly compared the impact of rapamycin on CD8+ T cells responding to a graft versus a pathogen, using a system in which the same epitope was presented to a monoclonal T cell population in both circumstances (15). We observed that while treatment with rapamycin dramatically increased the antigen-specific CD8+ T cell response to the pathogen, the identical T cell population responding to antigen in the context of a graft failed to be enhanced in the presence of rapamycin (Figure 1). As observed by Araki et al., antigen-specific T cells responding to the pathogen also exhibited enhanced quality, by increased expression of CD62L, which is associated with enhanced development into a central memory phenotype (9, 16), while antigen-specific T cells responding to a graft failed to exhibit this change. These results suggested that the environment in which an antigen is presented alters the influence of rapamycin on antigen-specific T cell expansion, and highlight a fundamental difference between antigen presented by an infectious agent as compared to an allograft. What is not known is why or how mTOR inhibition through rapamycin treatment exerts such disparate effects on T cells responses. Interestingly, concomitant infection of skin graft recipients with a bacterial pathogen did not restore the enhancing effects of rapamycin on the graft-specific T cells, demonstrating that TLR- or other pathogen-associated inflammatory signals provided in trans were not sufficient to precipitate the rapamycin-induced augmentation (15). These data suggested a principal difference in the way rapamycin impacts T cells responding to antigen presented in the context of a pathogen versus that of a graft. We speculate that antigen persistence may be playing a role in the difference in T cell responses. In the context of an infection with Listeria monocytogenes, antigen persists for approximately five days. Conversely, in the setting of an allograft, antigen is continuously being expressed and presented until full rejection, potentially cooperating with rapamycin to exhaust or attenuate graft-specific T cell responses. The mechanism underlying this phenomenon remains to be determined, and is a critically important issue for the field of transplantation.
Taken together, the implications of these results are far-reaching. First, donor-reactive memory T cells have been shown to pose a potent barrier to graft survival following transplantation, as evidenced by basic studies in mouse models and the fact that clinical studies revealed a correlation between pre-transplant frequency of donor-reactive memory T cells and incidence of acute rejection within the first year (17, 18). These memory T cells can arise in unsensitized individuals due to molecular mimicry between pathogen-derived and alloantigens via prior infections or exposure to environmental antigens (19). Molecular mimicry between pathogen- and allo-antigens is now known to be more common than previously appreciated: recent evidence indicated that 45% of virus-specific memory T cell clones obtained from normal healthy humans possessed cross-reactivity with an alloantigen (20). It would appear from this study that in the presence of rapamycin, presentation of cognate or cross-reactive antigen delivered either by a graft or a pathogen to the same T cell could evoke markedly different responses. Further studies to determine the effect of rapamycin on pathogen-elicted allo-crossreactive CD8+ T cell responses are therefore required.
In addition, the finding that rapamycin enhances the antigen-specific CD8+ T cell response to pathogens suggests that treatment of transplant recipients with rapamycin monotherapy may increase immunity to viruses or vaccines (9). However, the ability of rapamycin to augment pathogen-specific CD8+ T cell responses in the context of other immunosuppressive agents is completely unknown. Currently, the standard of care in most instances is to reduce the intensity of immunosuppression if patients experience infectious complications. As such, further studies are required to inform and direct clinical use of rapamycin as an adjunct therapy for boosting protective immunity to both live infections as well as vaccines in transplant recipients.
Another fork in the road: mTOR inhibition inhibits Th cell differentiation
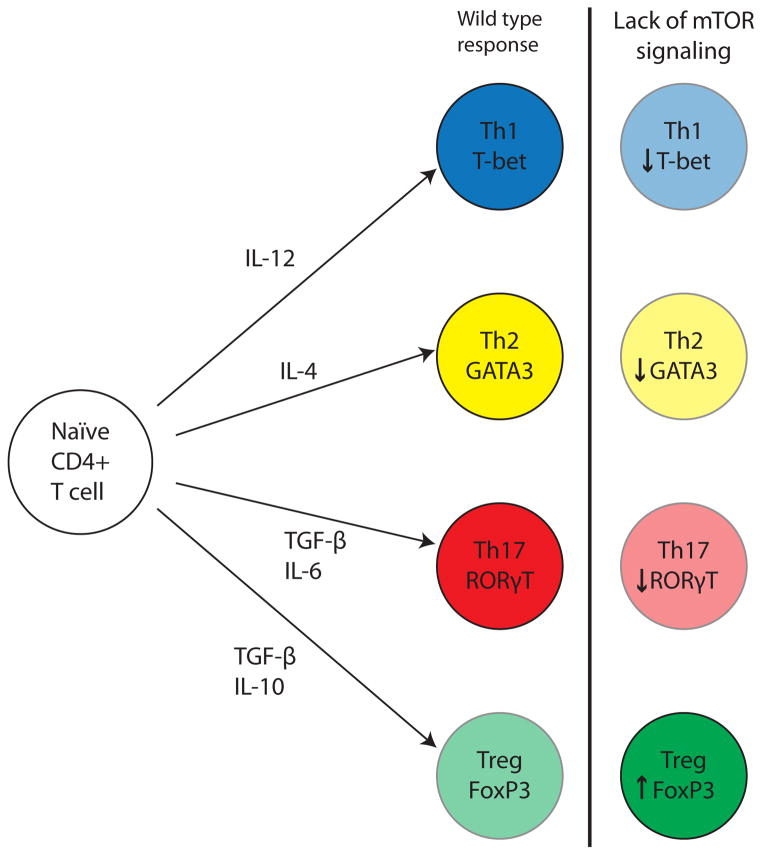
The studies discussed above demonstrated that inhibition of mTOR through rapamycin results in an increase in the magnitude and functionality of CD8+ pathogen-specific memory T cell responses. Conversely, studies on CD4+ pathogen-specific T cells failed to demonstrate an enhancement in the function and magnitude of the response. Specifically, Delgoffe and colleagues used a cre-lox system to ablate the mTOR gene in T cells only (21). Their work demonstrated that in response to an acute viral infection, CD4+ T cells deficient in mTOR failed to express the Th transcription factors T-bet, GATA3, and RORγt, and therefore failed to polarize into Th1, Th2, and Th17 subsets following activation, despite overall normal activation (i.e. expression of activation markers and IL-2 production) (21) (Figure 2). The authors attributed this to an attenuation of STAT-mediated signaling downstream of the respective cytokine receptors required for Th polarization. Thus, inhibition of mTOR may have disparate effects on CD4+ versus CD8+ T cell populations, enhancing antigen-specific CD8+ populations while inhibiting differentiation of CD4+ T cells. Again, the precise mechanisms underlying this dichotomy remain unknown.
Figure 2. Reduced signaling through the mTOR complex alters differentiation of CD4+ T cell responses.
The lack of signaling through mTOR in antigen-specific CD4+ T cells leads to reduced polarization into either Th1, Th2, or Th17 T cells. In contrast, mTOR inhibition by rapamycin leads to enhanced development of regulatory T cells.
Rapamycin and regulation: An important facet of its immunosuppressive effects
Another intriguing aspect of rapamycin is that it has consistently been found to augment rather than suppress Treg generation, functionality, and survival (2) (Figure 2). For example, studies have shown that Tregs cultured in vitro with rapamycin exhibited increased levels of expansion as compared to untreated controls (22).
Although prolonged rapamycin treatment has been associated with reduced peripheral T cell numbers due to impaired thymic output, this effect seems to be reversed in the Treg compartment (23). Specifically, rapamycin has been shown to increase the proportion of FoxP3-expressing CD4+ T cells among recent thymic emigrants. This effect is compounded by the fact that rapamycin promotes survival of natural Tregs while increasing the upregulation of FoxP3 in conventional T cells and enhancing Treg function (24). One purported mechanism underlying this effect is the fact that mTOR inhibition can result in reduced Akt phosphorylation and hence increased Smad3 activity, which has been shown to lead to an increase in FoxP3 expression (25).
Effects on antigen presenting cells: immunosuppressive and immunostimulatory
Consistent with its effects on T cell differentiation, rapamycin may also impart paradoxical effects on dendritic cell differentiation and maturation. For a number of years, mTOR inhibition in DCs has been associated with inhibited activation and upregulation of costimulatory molecules on the DC cell surface, and reduced IL-12 and TNF secretion after exposure to IL-4 in in vivo and in vitro models (26). Furthermore, the effects of mTOR inhibition in plasmacytoid DCs, which are typically associated with the host response to viral infections, resulted in reduced secretion of type I IFNs by these antigen presenting cells (27).
In contrast to these well-appreciated inhibitory effects of rapamycin on DC differentiation and maturation, recent studies have demonstrated paradoxical effects of rapamycin on macrophages and myeloid DC (28). Specifically, mTOR inhibition with rapamycin led to both enhanced production of IL-12 and reduced production of IL-10 in macrophages and myeloid DCs following TLR or bacterial stimulation. Furthermore, rapamycin was shown to increase autophagy by antigen presenting cells, leading to enhanced antigen presentation of M. tuberculosis. This increase in autophagy in the presence of rapamycin promoted increased Th1 type responses and protected mice from a lethal bacterial challenge (29). Thus, rapamycin appears have the capacity to exert immunosuppressive or immunostimulatory effects on DC populations; again, the conditions and parameters modifying this impact remain unknown.
Rapamycin as a fountain of youth: impact on longevity and survival
In perhaps the most surprising development in rapamycin biology is the unanticipated role it plays in increasing longevity and decreasing incidence of death from all causes. While experience in clinical transplantation has demonstrated an association of rapamycin with hyperlipidemia, thrombocytopenia, and impaired wound healing (6), inactivation of mTOR had previously been shown to increase lifespan in invertebrates including yeast, nematodes, and fruit flies. Recently, a study assessed the impact of administration of oral rapamycin on lifespan in both male and female mice of different strains and at different test sites across the country. Although the study targeted a much higher level of rapamycin in the blood than currently used in transplantation (60–70 ng/mL vs. 8–12 ng/mL, respectively), results demonstrated that whether initiated at either middle or old age (270 and 600 days, respectively), rapamycin increased the median and maximum lifespan in both genders (30). Distribution of disease phenotypes was not different between the groups. The authors speculated that mTOR inhibition may increase survival by postponing deaths from cancer or by mimicking the anti-aging effects of caloric restriction. Furthermore, the immunostimulatory effects of rapamycin on pathogen-specific CD8+ T cell responses, discussed above, would certainly suggest that treatment with rapamycin might decrease or delay death from infectious etiologies (9, 10).
Conclusion
Despite the large number of mechanistic and clinical studies investigating the impact of rapamycin on cell-mediated immunity, several unresolved questions concerning rapamycin immunobiology in transplantation and protective immunity remain (Table I). It is clear that further research into the cellular and molecular pathways underlying these apparent paradoxes is required to resolve these issues. For example, rigorous analysis of the conditions under which rapamycin may augment versus dampen antigen-specific T cell responses may allow us to harness these properties to selectively modulate both graft- and pathogen-specific immune responses in order to minimize infectious morbidities and maximize patient health following transplantation.
Table I.
Dichotomous effects of rapamycin in immunobiology
| Immunosuppressive | Immunostimulatory |
|---|---|
| ↓CD4+ T cell differentiation | ↑CD8+ T cell memory differentiation |
| ↑Treg development | ↑CD8+ T cell activation |
| ↓Response to skin graft | ↑Response to pathogen |
| ↓DC maturation | ↑IL-12 production by DCs |
| Additional Contradictory Effects | |
| ↑Survival | ↑Hyperlipidemia ↑Thrombocytopenia ↓Wound healing |
Acknowledgments
The authors were supported in part by NIAID awards AI073707 and AI81789 (to M.L.F)
Abbreviations
- mTOR
mammalian target of rapamycin
- DC
dendritic cell
- TEM
effector memory T cell
- Treg
regulatory T cell
- Th
T helper
- TLR
toll-like receptor
- CMV
Cytomegalovirus
- RNAi
RNA interference
- TRAF6
TNF receptor associated factor 6
- FAO
fatty acid oxidation
- GATA3
GATA binding protein 3
- RORγt
RAR-related orphan receptor γt
- FoxP3
forkhead box P3
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–68. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavik JM, Lim DG, Burakoff SJ, Hafler DA. Rapamycin-resistant proliferation of CD8+ T cells correlates with p27kip1 down-regulation and bcl-xL induction, and is prevented by an inhibitor of phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:910–9. doi: 10.1074/jbc.M209733200. [DOI] [PubMed] [Google Scholar]
- 4.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–72. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Addio F, Yuan X, Habicht A, Williams J, Ruzek M, Iacomini J, Turka LA, Sayegh MH, Najafian N, Ansari MJ. A novel clinically relevant approach to tip the balance toward regulation in stringent transplant model. Transplantation. 2010;90:260–9. doi: 10.1097/tp.0b013e3181e64217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 7.Swanson SJ, Hale DA, Mannon RB, Kleiner DE, Cendales LC, Chamberlain CE, Polly SM, Harlan DM, Kirk AD. Kidney transplantation with rabbit antithymocyte globulin induction and sirolimus monotherapy. Lancet. 2002;360:1662–4. doi: 10.1016/S0140-6736(02)11606-0. [DOI] [PubMed] [Google Scholar]
- 8.Ozaki KS, Camara NO, Galante NZ, Camargo LF, Pacheco-Silva A. Decreased Cytomegalovirus infection after antilymphocyte therapy in sirolimus-treated renal transplant patients. Int Immunopharmacol. 2005;5:103–6. doi: 10.1016/j.intimp.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, Ford ML, Ahmed R, Kirk AD, Larsen CP. Sirolimus Enhances the Magnitude and Quality of Viral-Specific CD8+ T cell Responses to Vaccinia Virus Vaccination in Rhesus Macaques. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2010.03407.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown NF, Stefanovic-Racic M, Sipula IJ, Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism. 2007;56:1500–7. doi: 10.1016/j.metabol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Sipula IJ, Brown NF, Perdomo G. Rapamycin-mediated inhibition of mammalian target of rapamycin in skeletal muscle cells reduces glucose utilization and increases fatty acid oxidation. Metabolism. 2006;55:1637–44. doi: 10.1016/j.metabol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185:2004–8. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev. 2010;235:234–43. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–95. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–75. [PubMed] [Google Scholar]
- 19.Macdonald WA, Chen Z, Gras S, Archbold JK, Tynan FE, Clements CS, Bharadwaj M, Kjer-Nielsen L, Saunders PM, Wilce MC, Crawford F, Stadinsky B, Jackson D, Brooks AG, Purcell AW, Kappler JW, Burrows SR, Rossjohn J, McCluskey J. T cell allorecognition via molecular mimicry. Immunity. 2009;31:897–908. doi: 10.1016/j.immuni.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Amir AL, D’Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, van der Hoorn MA, Kester MG, Doxiadis, Falkenburg JH, Claas FH, Heemskerk MH. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–57. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 21.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 23.Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, Joosten I. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39:537–45. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 24.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–63. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 27.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–64. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–61. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 29.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–76. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 30.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]