Abstract
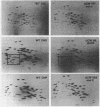
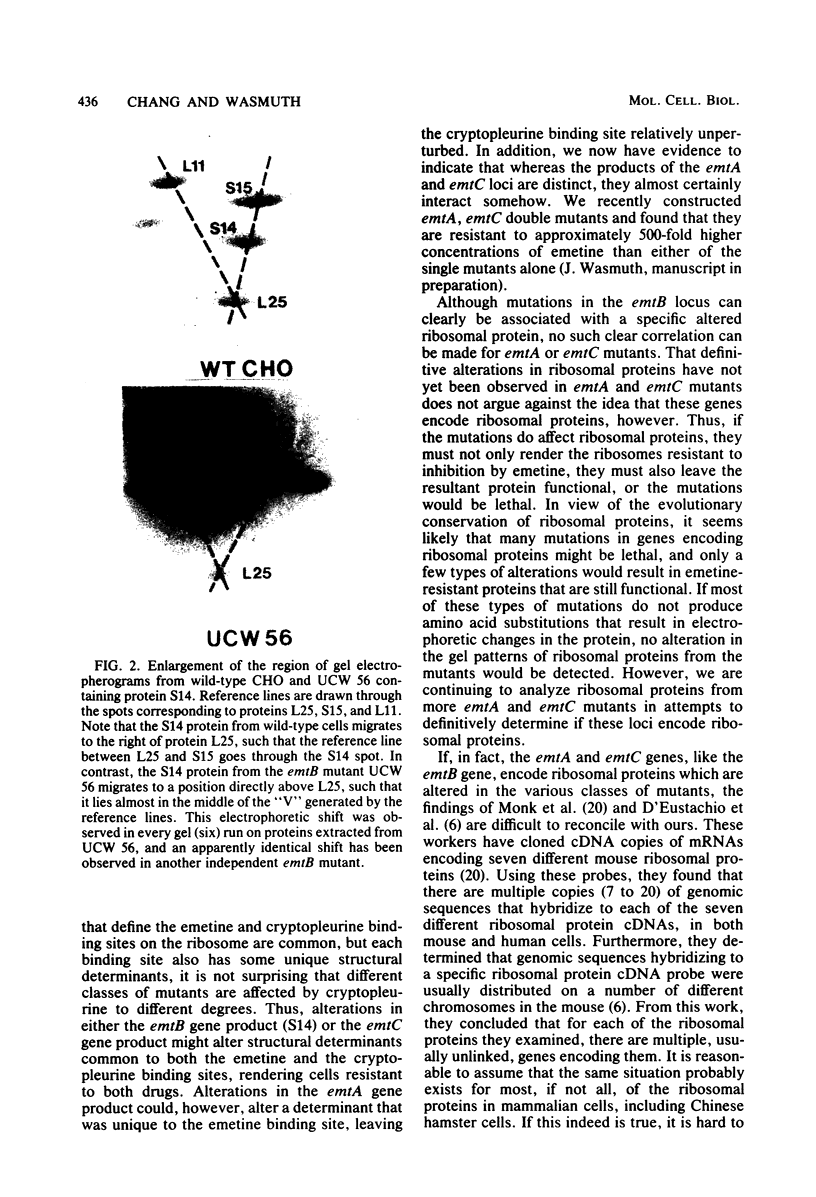
Genetic and biochemical experiments have enabled us to more clearly distinguish three genetic loci, emtA, emtB, and emtC, all of which can be altered to give rise to resistance to the protein synthesis inhibitor, emetine, in cultured Chinese hamster cells. Genetic experiments have demonstrated that, unlike the emtB locus, neither the emtA locus nor the emtC locus is linked to chromosome 2 in Chinese hamster cells, clearly distinguishing the latter two genes from emtB. emtA mutants can also be distinguished, biochemically, from emtB and emtC mutants based upon different degrees of cross-resistance to another inhibitor of protein synthesis, cryptopleurine. Two-dimensional gel electrophoretic analysis of ribosomal proteins failed to detect any electrophoretic alterations in ribosomal proteins from emtA or emtC mutants that could be correlated with emetine resistance. However, a distinct electrophoretic alteration in ribosomal protein S14 was observed in an emtB mutant. In addition, the parental Chinese hamster peritoneal cell line of an emtC mutant, and the emtC mutant itself, are apparently heterozygous for an electrophoretic alteration in ribosomal protein L9.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boersma D., McGill S. M., Mollenkamp J. W., Roufa D. J. Emetine resistance in Chinese hamster cells is linked genetically with an altered 40S ribosomal subunit protein, S20. Proc Natl Acad Sci U S A. 1979 Jan;76(1):415–419. doi: 10.1073/pnas.76.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma D., McGill S., Mollenkamp J., Roufa D. J. Emetine resistance in Chinese hamster cells. Analysis of ribosomal proteins prepared from mutant cells. J Biol Chem. 1979 Jan 25;254(2):559–567. [PubMed] [Google Scholar]
- D'Eustachio P., Meyuhas O., Ruddle F., Perry R. P. Chromosomal distribution of ribosomal protein genes in the mouse. Cell. 1981 May;24(2):307–312. doi: 10.1016/0092-8674(81)90320-2. [DOI] [PubMed] [Google Scholar]
- Dana S., Wasmuth J. J. Linkage of the leuS, emtB, and chr genes on chromosome 5 in humans and expression of human genes encoding protein synthetic components in human--Chinese hamster hybrids. Somatic Cell Genet. 1982 Mar;8(2):245–264. doi: 10.1007/BF01538680. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Grant P., Sánchez L., Jiménez A. Cryptopleurine resistance: genetic locus for a 40S ribosomal component in Saccharomyces cerevisiae. J Bacteriol. 1974 Dec;120(3):1308–1314. doi: 10.1128/jb.120.3.1308-1314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Diphtheria-toxin-resistant mutants of CHO cells affected in protein synthesis: a novel phenotype. Somatic Cell Genet. 1978 Sep;4(5):553–571. doi: 10.1007/BF01542926. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic and biochemical characterization of mutants of CHO cells resistant to the protein synthesis inhibitor trichodermin. Somatic Cell Genet. 1978 May;4(3):355–374. doi: 10.1007/BF01542848. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Mutants of CHO cells resistant to the protein synthesis inhibitor emetine: genetic and biochemical characterization of second-step mutants. Somatic Cell Genet. 1978 Jan;4(1):77–94. doi: 10.1007/BF01546494. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Mutants of CHO cells resistant to the protein synthesis inhibitors, cryptopleurine and tylocrebrine: genetic and biochemical evidence for common site of action of emetine, cryptopleurine, tylocrebine, and tubulosine. Biochemistry. 1977 Jul 12;16(14):3209–3214. doi: 10.1021/bi00633a026. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-emetine. Cell. 1976 Oct;9(2):213–219. doi: 10.1016/0092-8674(76)90112-4. [DOI] [PubMed] [Google Scholar]
- Haralson M. A., Roufa D. J. A temperature-sensitive mutation affecting the mammalian 60 S ribosome. J Biol Chem. 1975 Nov 25;250(22):8618–8623. [PubMed] [Google Scholar]
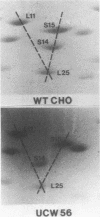
- Madjar J. J., Arpin M., Buisson M., Reboud J. P. Spot position of rat liver ribosomal proteins by four different two-dimensional electrophoreses in polyacrylamide gel. Mol Gen Genet. 1979 Mar 20;171(2):121–134. doi: 10.1007/BF00269998. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Michel S., Cozzone A. J., Reboud J. P. A method to identify individual proteins in four different two-dimensional gel electrophoresis systems: application to Escherichia coli ribosomal proteins. Anal Biochem. 1979 Jan 1;92(1):174–182. doi: 10.1016/0003-2697(79)90641-9. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Nielsen-Smith K., Frahm M., Roufa D. J. Emetine resistance in chinese hamster ovary cells is associated with an altered ribosomal protein S14 mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1003–1007. doi: 10.1073/pnas.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjar J. J., Traut R. R. Differences in electrophoretic behaviour of eight ribosomal proteins from rat and rabbit tissues and evidence for proteolytic action on liver proteins. Mol Gen Genet. 1980;179(1):89–101. doi: 10.1007/BF00268450. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Bielka H., Gordon J., Lastick S. M., Lin A., Ogata K., Reboud J. P., Traugh J. A., Traut R. R., Warner J. R. Proposed uniform nomenclature for mammalian ribosomal proteins. Mol Gen Genet. 1979 Jan 16;169(1):1–6. doi: 10.1007/BF00267538. [DOI] [PubMed] [Google Scholar]
- Moehring T. J., Danley D. E., Moehring J. M. Codominant translational mutants of Chinese hamster ovary cells selected with diphtheria toxin. Somatic Cell Genet. 1979 Jul;5(4):469–480. doi: 10.1007/BF01538881. [DOI] [PubMed] [Google Scholar]
- Monk R. J., Meyuhas O., Perry R. P. Mammals have multiple genes for individual ribosomal proteins. Cell. 1981 May;24(2):301–306. doi: 10.1016/0092-8674(81)90319-6. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Lofgren D. J., Adair G. M. CHO cell mutants for arginyl-, asparagyl-, glutaminyl-, histidyl- and methionyl-transfer RNA synthetases: identification and initial characterization. Cell. 1977 May;11(1):157–168. doi: 10.1016/0092-8674(77)90326-9. [DOI] [PubMed] [Google Scholar]
- WALLER J. P., HARRIS J. I. Studies on the composition of the protein from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1961 Jan 15;47:18–23. doi: 10.1073/pnas.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Chu L. Y. Linkage in cultured Chinese hamster cells of two genes, emtB and leuS, involved in protein synthesis and isolation of cell lines with mutations in three linked genes. J Cell Biol. 1980 Dec;87(3 Pt 1):697–702. doi: 10.1083/jcb.87.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Hill J. M., Vock L. S. Biochemical and genetic evidence for a new class of emetine-resistant Chinese hamster cells with alterations in the protein biosynthetic machinery. Somatic Cell Genet. 1980 Jul;6(4):495–516. doi: 10.1007/BF01539152. [DOI] [PubMed] [Google Scholar]
- Wasmuth J. J., Hill J. M., Vock L. S. Identification and characterization of a third complementation group of emetine-resistant Chinese hamster cell mutants. Mol Cell Biol. 1981 Jan;1(1):58–65. doi: 10.1128/mcb.1.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Duff C., Campbell C. E. Marker segregation without chromosome loss at the emt locus in Chinese hamster cell hybrids. Somatic Cell Genet. 1980 Mar;6(2):199–213. doi: 10.1007/BF01538796. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]