Abstract
Objective
To test the hypothesis that the first stage of labor will be longer in nulliparous and multiparous women with diabetes compared to non-diabetic counterparts.
Methods
A retrospective analysis was performed from 228,668 deliveries between 2002–2008 from the Consortium of Safe Labor (NICHD, NIH). Patients with spontaneous onset of labor from 37 0/7 – 41 6/7 weeks gestation were included (71,282) and classified as nulliparous or multiparous. Pregnancies were further subdivided regarding presence of preexisting diabetes (preDM) or gestational diabetes (GDM), and normal controls. Labor curves were created matching for body mass index (BMI) and neonatal birth weight. Statistical analysis was performed on descriptive variables using χ2 with significance designated as p<0.05.
Results
Among nulliparous patients, there were 118 women with preDM and 475 women with GDM; 25,771 patients served as normal controls. Among multiparous women, there were 311 with preDM and 1,079 with GDM and 43,528 in the control group. Although differences in dilatation rates were observed in nulliparous and multiparous women with and without diabetes, labor curves were similar between the subgroups when matched for maternal BMI and birth weight.
Conclusions
Labor curves of women with preDM and GDM approximate those of non-diabetics, regardless of BMI, birth weight, or parity.
Keywords: obesity, gestational diabetes, type 2 diabetes mellitus, first stage, second stage
Introduction
The increased risks of adverse maternal and neonatal outcomes (e.g., macrosomia, shoulder dystocia, neonatal hypoglycemia, respiratory distress syndrome [RDS], cesarean delivery, and preeclampsia) are well described in pregnancies complicated by diabetes. [1–5] Sheiner et al. [6] noted that gestational diabetes mellitus (GDM) was an independent risk factor for failure of labor to progress during the first stage, even when adjusted for birth weight. They also found that providers may decide to proceed to cesarean delivery more readily in women with diabetes than in non-diabetic pregnancies having the knowledge of underlying disease. [6] Better understanding of normal labor progression in women with risk factors for cesarean delivery (e.g. diabetes, obesity) may allow clinicians to make better choices about labor management and help control the rate of cesarean birth in this high risk population. Given that obesity predisposes women to both preexisting diabetes (preDM) and GDM and that obese and/or diabetic women have increased rates of cesarean delivery, we sought to determine whether labor curves for pregnant women with diabetes differ from those of healthy women when matched by parity, BMI on admission, and neonatal birth weight.
Methods
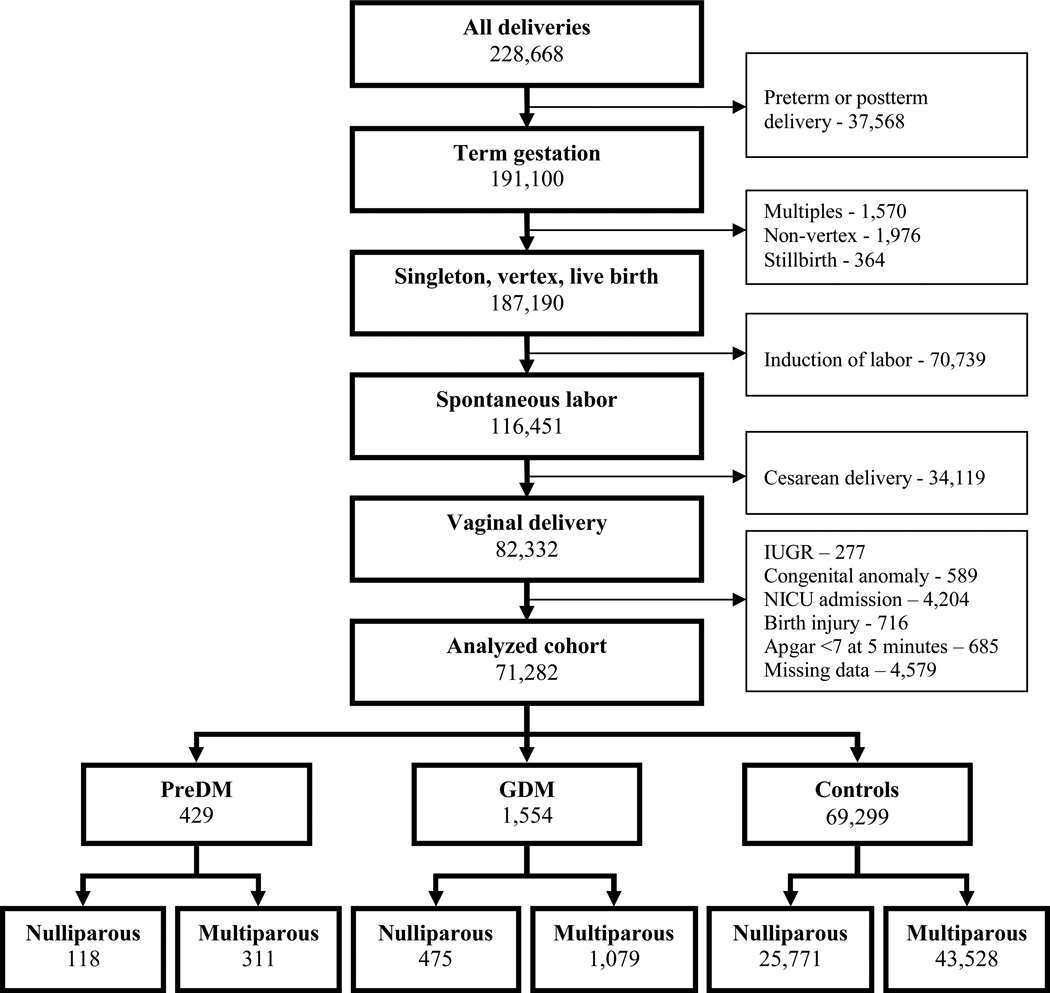
This was a retrospective cohort using the Consortium on Safe Labor database, Eunice Kennedy Shriver National Institute of Child Health and Human Development, which includes information on 228,668 deliveries of 233,844 neonates (including multiple gestations) from 2002–2008, encompassing 19 hospitals from 12 institutions. The study was approved by the MedStar Health Research Institute IRB. Detailed medical and demographic information, labor and delivery data, as well as information on newborn outcomes were abstracted from the electronic medical records (EMRs) from participating institutions. Data were validated using four key variables: 1) shoulder dystocia, 2) cesarean delivery for non-reassuring fetal heart rate tracing, 3) asphyxia, and 4) NICU admission for respiratory conditions, and found to be highly accurate, indicating that the Consortium data from EMRs were a reliable representation of medical charts. [7] Inclusion criteria were: term gestation (defined as gestational age between 37 0/7 – 41 6/7 weeks), singleton pregnancy in vertex presentation, and spontaneous labor resulting in vaginal birth of a live born neonate. We excluded data on infants with Apgar scores <7 at 5 minutes, birth injury, known fetal growth restriction, congenital anomalies or NICU admission (Figure 1).
Figure 1.
Patient selection diagram. GDM – gestational diabetes mellitus. PreDM – preexisting diabetes mellitus. IUGR – intrauterine growth restriction.
Patients were stratified based on parity (nulliparous vs. multiparous), and further subdivided into those with preDM, GDM, and normal non-diabetic controls. Maternal demographic information was analyzed with χ2 test for categorical variables and ANOVA for continuous variables with α=0.05.
In order to compare the labor progression between the groups, three approaches were utilized. First, the Kruskal-Wallis ANOVA was performed to compare the duration of the first and second stage of labor, stratified by parity status.
The second model used a repeated measure analysis with an eight-degree polynomial regression (previously described by Zhang et al. [8]) to determine average first and second stage labor curves stratified by parity and diagnosis of diabetes. The overall time period of first and second stage labor curve for each group was determined by the median of each subgroup population. Separate labor curves were created and matched for maternal BMI on admission and neonatal birth weight at a ratio of 1:1:3 for preDM, GDM, and control groups. As the goal of labor curve construction was to graphically estimate the pattern of labor for these patient populations, no statistical tests analyzing the shape of the curves were performed.
Finally, the rate of labor progression at each centimeter was evaluated with interval-regression model described by Zhang et al. [8], which assumes the log of duration is linear relative to the subgroup of diabetes, with normal parturients as the reference.
A separate log-normal regression analysis was performed to assess the impact of the following variables on duration of first and second stage of labor: parity; maternal age; BMI on admission; gestational age at delivery; dilatation, effacement and station on admission, oxytocin for labor augmentation, epidural use; neonatal birth weight, and type of diabetes.
Results
We excluded 4,579 (6.0%) of patients because of missing history of diabetes (4,557 patients) or those misclassified as having both GDM and preDM (22 patients). The final analyzed cohort included 71,282 women. Baseline demographic characteristics are presented in Table I. Among nulliparous patients, 118 had preDM, 475 had GDM, and 25,771 had neither condition. There were 311 multiparous patients with preDM and 1,079 with GDM, and 43,528 women with no diabetes. Patients with pregnancies complicated by either preDM or GDM were older and tended to have higher BMIs than the control population. Oxytocin augmentation of spontaneous labor did not differ statistically between the groups. Nulliparous patients were more likely to undergo an operative vaginal delivery as compared to multiparous women.
Table I.
Maternal and Labor Characteristics
| Nulliparous | Multiparous | |||||||
|---|---|---|---|---|---|---|---|---|
| PreDM | GDM | Normal | P-value | PreDM | GDM | Normal | P-value | |
| Age (years+/−SD) | 27.1 +/− 6.3 | 27.3 +/− 5.5 | 23.7 +/− 5.2 | <0.0001 | 30.4 +/− 5.6 | 31.4 +/− 5.2 | 28.2 +/− 5.4 | <0.0001 |
| Pre-pregnancy BMI (kg/m2) | 27.2 | 25.8 | 23.5 | <0.0001 | 28.6 | 27.6 | 24.9 | <0.0001 |
| Admission BMI (kg/m2) | 31.3 | 30.7 | 29.2 | <0.0001 | 33.1 | 31.7 | 30.0 | <0.0001 |
| Tobacco use | 6.8 | 5.9 | 5.2 | NS | 3.2 | 6.5 | 6.8 | 0.0438 |
| Gestational age at delivery | 38.7 | 38.7 | 39.1 | <0.0001 | 38.6 | 38.6 | 39.1 | <0.0001 |
| Race | 0.0001 | <0.0001 | ||||||
| Caucasian | 36 | 52 | 53 | 25 | 48 | 51 | ||
| African American | 20 | 9 | 18 | 31 | 10 | 20 | ||
| Hispanic | 26 | 14 | 15 | 31 | 25 | 19 | ||
| Other/unknown | 18 | 25 | 13 | 13 | 18 | 10 | ||
| Cervical dilatation on admission (cm) | 3.7 | 3.8 | 4.0 | NS | 4.5 | 4.7 | 5.0 | <0.0001 |
| Effacement on admission (%) | 77 | 86 | 85 | <0.0001 | 74 | 82 | 83 | <0.0001 |
| Station on admission | −2.0 | −2.0 | −1.8 | 0.0206 | −2.1 | −2.1 | −1.8 | 0.0003 |
| Oxytocin augmentation | 71 | 63 | 67 | NS | 62 | 53 | 55 | NS |
| Epidural | 33 | 83 | 75 | 0.0001 | 30 | 78 | 71 | <0.0001 |
| Operative vaginal delivery | 6 | 14 | 12 | NS | 4 | 3 | 3 | NS |
| Length of first stage of labor (hr) | 5.1 (12.3) | 4.6 (11.9) | 4.7 (11.3) | 0.7095 | 3.4 (8.9) | 2.7 (8.4) | 2.6 (7.8) | 0.0001 |
| Length of second stage of labor (hr) | 0.9 (2.7) | 1.2 (2.7) | 1.0 (2.7) | 0.0007 | 0.2 (0.8) | 0.3 (1.1) | 0.3 (1.0) | 0.0171 |
| Birth weight (grams+/−SD) | 3211+/−391 | 3292+/−399 | 3275+/−397 | NS | 3437+/−496 | 3418+/−442 | 3369+/−420 | <0.0001 |
| Macrosomia | NS | <0.0001 | ||||||
| Birth weight 4000 g+ | 0.85 | 4.7 | 3.74 | 9.9 | 7.8 | 6.6 | ||
| Birth weight 4500 g+ | 0 | 0.21 | 0.23 | 2.3 | 1.2 | 0.6 | ||
| Birth weight 5000 g+ | 0 | 0 | 0.02 | 0 | 0.3 | 0.04 | ||
Data presented in %, mean +/−SD, or medians (90th percentile) unless otherwise noted. NS – not statistically significant at α=0.05.
PreDM – preexisting diabetes mellitus. GDM – gestational diabetes mellitus.
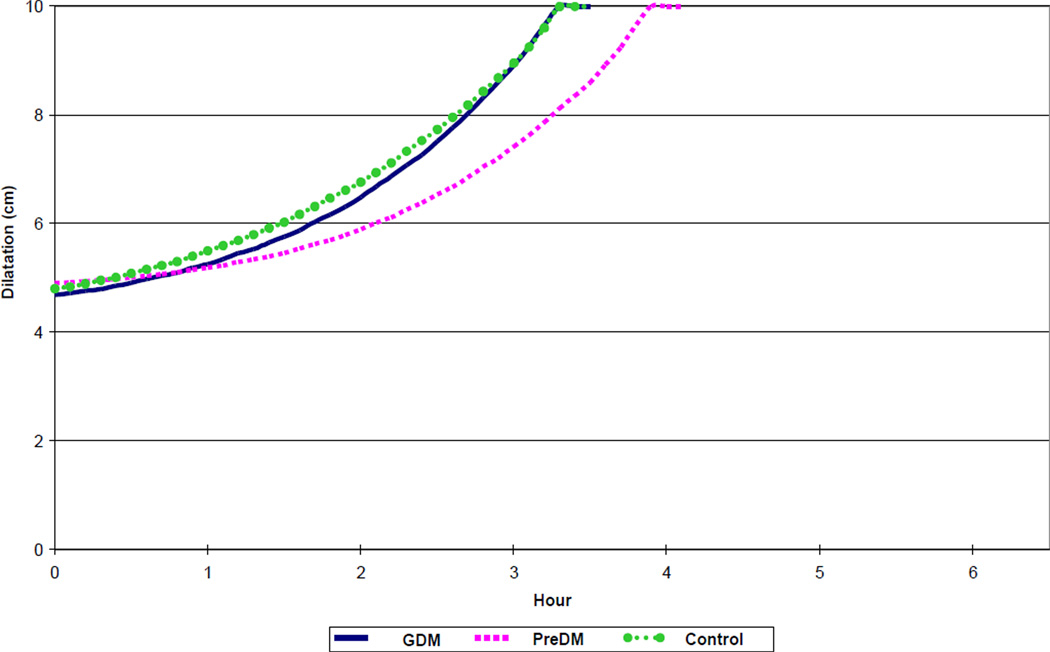
Labor curves are presented in Figures 2, 3, 4 and 5 for nulliparous and multiparous patients, unmatched and matched for BMI at admission and neonatal birth weight. As expected, durations of the first and second stages of labor were longer for nulliparous patients as compared to multiparous patients (Tables II and III). The duration of the first stage reported as median hours (90th percentile) for women matched by BMI and neonatal birth weight was 5.1 hours (10.1 hours), 4.7 hours (13.4 hours), and 5.3 hours (10.8 hours) for nulliparous preDM, GDM and control groups, respectively; and 3.8 hours (9.1 hours), 3.2 hours (8.9 hours), and 3.2 hours (8.8 hours) for multiparous preDM, GDM and controls, respectively. The duration of second stage of labor reported as median hours (90th percentile) was 0.9 hours (2.1 hours), 1.0 hour (3.1 hours), and 0.9 hours (2.8 hours) for nulliparous preDM, GDM and control groups, respectively; and 0.2 hours (0.8 hours), 0.3 hours (0.9 hours), and 0.3 hours (1.0 hour) for multiparous preDM, GDM and controls, respectively. Tables II and III summarize details of labor progression in nulliparous and multiparous patients with and without diabetes.
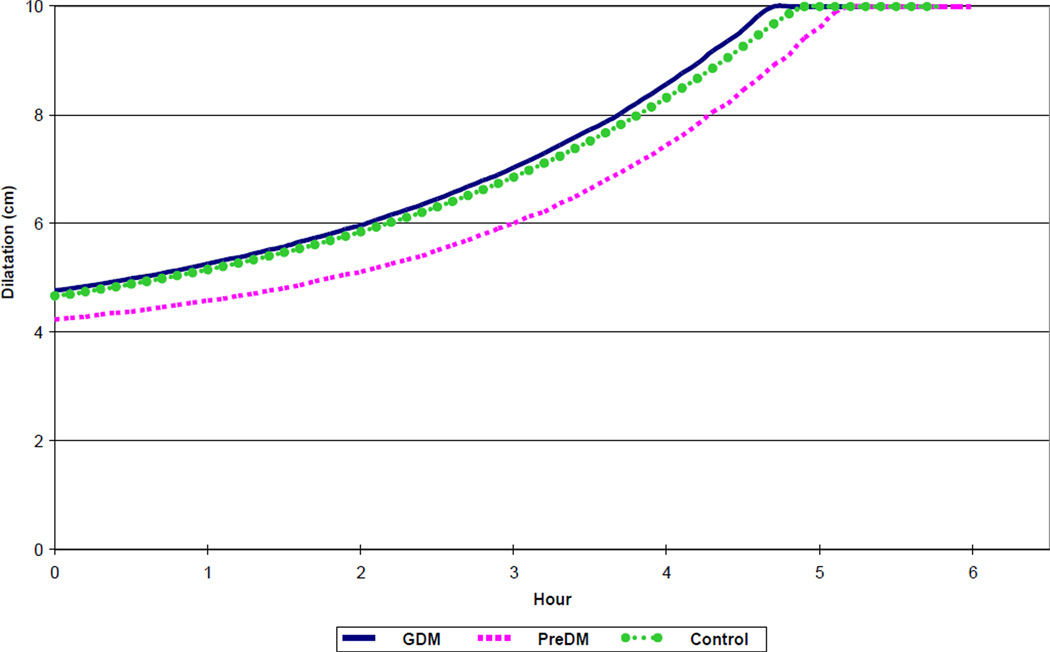
Figure 2.
Spontaneous labor progression in nulliparous women with diabetes and normal controls, resulting in vaginal birth of live born newborns. GDM – gestational diabetes mellitus. PreDM – preexisting diabetes mellitus.
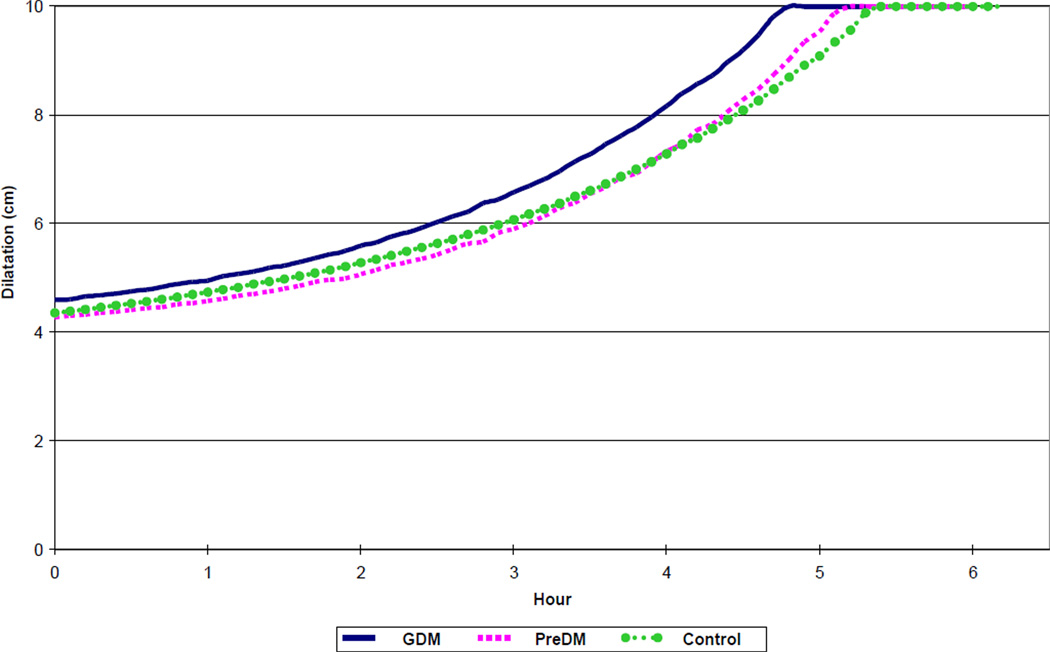
Figure 3.
Spontaneous labor progression in nulliparous women with diabetes and normal controls, resulting in vaginal birth of live born newborns, matched for neonatal birth weight and maternal BMI on admission. GDM – gestational diabetes mellitus. PreDM – preexisting diabetes mellitus. BMI – body mass index (kg/m2).
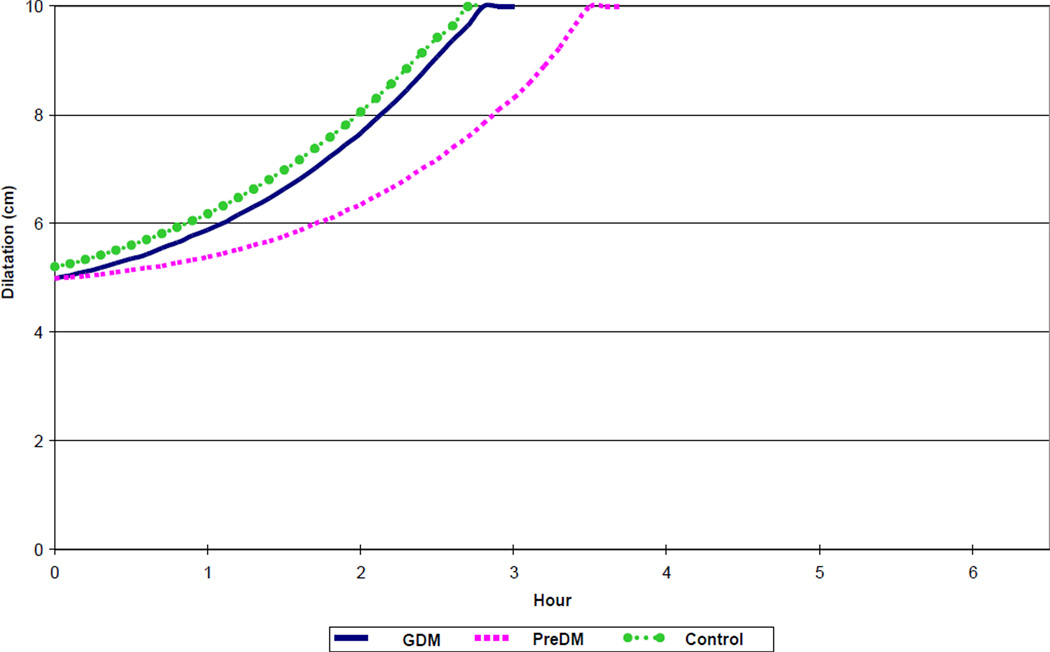
Figure 4.
Spontaneous labor progression in multiparous women with diabetes and normal controls, resulting in vaginal birth of live born newborns. GDM – gestational diabetes mellitus. PreDM – preexisting diabetes mellitus.
Figure 5.
Spontaneous labor progression in multiparous women with diabetes and normal controls, resulting in vaginal birth of live born newborns, matched for neonatal birth weight and maternal BMI on admission. GDM – gestational diabetes mellitus. PreDM – preexisting diabetes mellitus. BMI – body mass index (kg/m2).
Table II.
Length of first stage of spontaneous labor (in hours) by cervical dilatation on admission, parity, and the diagnosis of diabetes
| Nulliparous | Multiparous | |||||
|---|---|---|---|---|---|---|
| PreDM | GDM | Normal | PreDM | GDM | Normal | |
|
Cervical Dilatation (cm) |
||||||
| 3–4 | 1.80 (0.69, 4.69) | 1.48 (0.40, 5.54) | 1.52 (0.55, 4.26) | 1.72 (0.62, 4.74) | 1.33 (0.37, 4.74) | 1.45 (0.47, 4.40) |
| 4–5 | 0.92 (0.29, 2.92) | 0.95 (0.26, 3.55) | 1.11 (0.35, 3.56) | 1.08 (0.26, 4.50) | 0.93 (0.25, 3.47) | 0.99 (0.28, 3.54) |
| 5–6 | 0.50 (0.09, 2.87) | 0.43 (0.08, 2.35) | 0.63 (0.17, 2.35) | 0.64 (0.15, 2.75)* | 0.55 (0.15, 2.02) | 0.50 (0.11, 2.18) |
| 6–7 | 0.21 (0.05, 0.85)* | 0.42 (0.12, 1.51) | 0.39 (0.09, 1.67) | 0.32 (0.07, 1.51) | 0.26 (0.05, 1.34) | 0.27 (0.05, 1.40) |
| 7–8 | 0.34 (0.11, 1.02) | 0.33 (0.08, 1.36) | 0.27 (0.06, 1.23) | 0.14 (0.02, 0.98) | 0.14 (0.02, 0.81) | 0.14 (0.02, 0.88) |
| 8–9 | 0.23 (0.05, 0.98) | 0.26 (0.06, 1.13) | 0.24 (0.05, 1.11) | 0.09 (0.01, 0.68) | 0.06 (0.01, 0.51)* | 0.09 (0.01, 0.63) |
| 9–10 | 0.51 (0.14, 1.78) | 0.61 (0.21, 1.75) | 0.57 (0.18, 1.87) | 0.20 (0.04, 0.96) | 0.19 (0.04, 0.89) | 0.20 (0.04, 0.86) |
All data presented as medians (10th, 90th percentile).
Denotes statistical significance at α=0.05 compared to the control group.
PreDM – preexisting diabetes mellitus. GDM – gestational diabetes mellitus.
Table III.
Length of second stage of spontaneous labor (in hours) by parity and diagnosis of diabetes
| Nulliparous | Multiparous | |||||
|---|---|---|---|---|---|---|
| PreDM | GDM | Normal | PreDM | GDM | Normal | |
|
Second Stage of Labor (hrs) |
||||||
| With epidural | 1.02 (0.33, 3.13) |
1.13 (0.40, 3.18) |
1.03 (0.37, 2.87) |
0.34 (0.13, 0.87) |
0.33 (0.10, 1.12) |
0.31 (0.10, 1.01) |
| Without epidural | 0.66 (0.18, 2.34) |
0.74 (0.21, 2.63) |
0.68 (0.21, 2.21) |
0.21* (0.06, 0.74) |
0.16 (0.04, 0.63) |
0.17 (0.05, 0.62) |
All data presented as medians (10th, 90th percentile).
Denotes statistical significance at α=0.05 compared to the control group.
PreDM – preexisting diabetes mellitus. GDM – gestational diabetes mellitus.
Although differences in rates of dilatation were observed in nulliparous and multiparous women with and without diabetes, labor progression was similar between the subgroups when matched for maternal BMI and birth weight. Statistically significant differences in labor progression were observed only when assessing the duration of the second stage of labor in the multiparous preDM group. It is noteworthy that these patients had the highest prepregnancy and admission BMIs compared to the other subgroups (28.6 and 33.1 kg/m2, respectively). Tables IV and V present the rate of progression of cervical dilatation for different subgroups.
Table IV.
Cervical dilatation rate in the first stage of labor in nulliparous and multiparous women with and without diabetes
| Nulliparous | Multiparous | |||||
|---|---|---|---|---|---|---|
| PreDM | GDM | Normal | PreDM | GDM | Normal | |
| Cervical Dilatation (cm/hr) | 1.2 (0.6, 2.9) | 1.0 (0.5, 2.7) | 1.1 (0.6, 2.6) | 1.3 (0.6, 4.7) | 1.6 (0.7, 5.5) | 1.6 (0.7, 5.5) |
All data presented as medians (10th, 90th percentile).
Denotes statistical significance at α=0.05.
PreDM – preexisting diabetes mellitus. GDM – gestational diabetes mellitus.
Table V.
Rate of progression of spontaneous first stage of labor in nulliparous and multiparous women with and without diabetes
| Cervical Dilation (cm) | Admitted at 3 cm | Admitted at 4 cm | Admitted at 5 cm | ||||
|---|---|---|---|---|---|---|---|
| N | M | N | M | N | M | ||
|
PreDM (n=429) |
From 3 to 4 | 2.05 (4.23) | 1.99 (5.02) | - | - | - | - |
| 4 to 5 | 0.65 (2.01) | 1.04 (3.72) | 1.71 (4.05) | 1.32 (5.44) | - | - | |
| 5 to 6 | 0.56 (2.78) | 0.65 (2.06) | 0.54 (3.07) | 0.48 (2.55) | 0.87 (6.29) | 0.94 (3.85) | |
| 6 to 7 | 0.83 (0.84) | 0.12 (0.77) | 0.30 (0.30) | 0.28 (1.16) | 0.42 (0.97) | 0.24 (1.42) | |
| 7 to 8 | 0.67 (0.78) | 0.09 (0.46) | 0.18 (0.78) | 0.20 (1.13) | 0.28 (0.81) | 0.12 (1.10) | |
| 8 to 9 | 0.34 (0.34) | 0.10 (1.08) | 0.23 (1.05) | 0.07 (0.42) | 0.37 (1.34) | 0.02 (0.34) | |
| 9 to 10 | 0.53 (1.04) | 0.16 (1.18) | 0.41 (1.52) | 0.18 (0.64) | 0.70 (0.99) | 0.15 (0.75) | |
|
GDM (n=1,554) |
From 3 to 4 | 2.06 (6.55) | 1.63 (5.04) | - | - | - | - |
| 4 to 5 | 1.28 (3.82) | 0.83 (2.87) | 1.31 (4.11) | 1.21 (4.55) | - | - | |
| 5 to 6 | 0.49 (1.83) | 0.46 (1.42) | 0.56 (2.31) | 0.52 (2.00) | 0.80 (4.06) | 0.82 (2.90) | |
| 6 to 7 | 0.40 (1.43) | 0.10 (0.66) | 0.48 (1.45) | 0.20 (1.07) | 0.49 (1.84) | 0.35 (1.58) | |
| 7 to 8 | 0.42 (1.24) | 0.10 (0.59) | 0.31 (1.39) | 0.15 (0.63) | 0.53 (1.48) | 0.12 (0.86) | |
| 8 to 9 | 0.28 (1.02) | 0.05 (0.37) | 0.14 (1.01) | 0.05 (0.31) | 0.40 (1.43) | 0.05 (0.41) | |
| 9 to 10 | 0.63 (1.69) | 0.22 (0.74) | 0.55 (1.73) | 0.18 (1.00) | 0.68 (2.04) | 0.19 (0.92) | |
|
Normal (n=69,299) |
From 3 to 4 | 1.83 (4.89) | 1.69 (4.83) | - | - | - | - |
| 4 to 5 | 1.08 (3.41) | 0.81 (3.04) | 1.55 (4.51) | 1.30 (4.20) | - | - | |
| 5 to 6 | 0.52 (1.96) | 0.35 (1.58) | 0.72 (2.51) | 0.48 (2.05) | 1.07 (3.50) | 0.80 (3.07) | |
| 6 to 7 | 0.29 (1.31) | 0.18 (0.94) | 0.41 (1.59) | 0.24 (1.21) | 0.48 (1.98) | 0.28 (1.45) | |
| 7 to 8 | 0.22 (1.00) | 0.10 (0.59) | 0.27 (1.20) | 0.13 (0.73) | 0.30 (0.07) | 0.15 (0.92) | |
| 8 to 9 | 0.21 (0.98) | 0.07 (0.44) | 0.24 (1.07) | 0.08 (0.53) | 0.28 (1.12) | 0.09 (0.60) | |
| 9 to 10 | 0.56 (1.81) | 0.20 (0.80) | 0.57 (1.84) | 0.20 (0.82) | 0.55 (1.85) | 0.19 (0.80) | |
Data presented as medians (90th percentiles) in hours.
N – nulliparous. M – multiparous. PreDM – preexisting diabetes mellitus. GDM – gestational diabetes mellitus.
In the log-normal regression analysis, after controlling for other variables, nulliparity had the strongest effect with the average duration of first or second stage of labor lasting 30.5% longer than in multiparous group (p<0.0001) (data not shown).
Discussion
The original data reporting the normal progression of labor were first published more than 50 years ago by Friedman. [9,10] Based on those patterns, abnormal active labor progression was defined as cervical dilatation <1.2 cm/hour in nulliparous and <1.5 cm/hour in multiparous women. A recent publication by Zhang and colleagues examined spontaneous labor curves in nulliparous and multiparous women based on information available in the Consortium on Safe Labor database that reflects current obstetrical practice in the US. [8] The analysis demonstrated that labor progress as determined by cervical dilatation from 4 cm to 6 cm was slower than previously described; the report concluded that allowing for a longer time period to reach active labor may decrease the rate of cesarean deliveries for the indication of arrest of dilatation.
Explanations for differences in labor curves over the past 50 years include facts that current parturients are older and heavier and their babies are larger, all factors known to influence the rate of labor progression and confirmed in contemporary publications. Verdiales et al. evaluated the effect of maternal obesity on the course of labor, noting a statistically significantly higher rate of arrest of dilatation in the obese group (BMI >35 kg/m2) compared to normal weight parturients (BMI <26 kg/m2). [11] Vahratian and colleagues found that the active phase of labor was significantly longer in overweight and obese women compared to those of normal weight after adjusting for fetal weight, [12] In Naylor’s 1996 publication evaluating the relationship between birth weight and mode of delivery among pregnant women with untreated borderline GDM, treated GDM, and controls [13], cesarean rates remained high after adjustment for multiple variables, even though treatment of GDM normalized birth weights. It is possible that more cesarean deliveries are performed because GDM may affect labor progression in these individuals or due to concerns for shoulder dystocia in this high-risk population, suggesting that practice style is a contributor to the nationally increasing rates of cesarean delivery.
With cesarean delivery rates increased by almost 60% from 1996 to an all-time high of 32.9% in 2009 [14], definitions of “normal” labor progression have the potential of decreasing the number of cesareans performed for dystocia of labor in the first stage. Factors that may affect first stage of labor include premature rupture of membranes at term, increased BMI, fetal macrosomia, nulliparity, and gestational diabetes. [6,11,12,15–17] The mechanism of prolonged first stage of labor in parturients with increased BMI is not completely understood, however, as demonstrated by Zhang et al. [18], may involve poor uterine myometrium contractility as seen in obese patients. Several authors have proposed that a larger fetus and more adipose tissue deposits in the pelvis predispose to slower progression of labor, requiring stronger contractions and more time. Vahratian and colleagues noted that for overweight women (BMI 26.1–29.0 kg/m2), protracted dilatation occurred with cervical dilation 4 cm to 6 cm, while in obese women (BMI >29.0 kg/m2), labor was prolonged before the cervix was 7 cm dilated, consistent with the current findings. Zhang’s analysis of contemporary labor curves, not stratifying patients by BMI, noted that it may take more than 6.5 hours (at the 95th percentile) to progress from 4 – 5 cm cervical dilatation, and 4.5 hours from 5 – 6 cm cervical dilatation in nulliparous women. This finding calls into question the current definition of active labor, usually defined as cervical dilatation at 4 cm. Peisner et al. noted that among women with normal active phase progression, approximately 50%, 74% and 89% entered active phase by 4, 5, and 6 cm respectively. [19] These findings suggest that active phase of labor is not a specific threshold, and “normal” labor can have a later onset but still result in a vaginal delivery of healthy neonate.
In our analysis of spontaneous normal labor progression among women with diabetes, we demonstrated that the rates of cervical dilatation were similar among all subgroups during the active stage of labor. The duration of second stage of labor also was similar, except for multiparous women with preexisting diabetes who had a longer second stage (without an epidural), even after adjusting for neonatal birth weight and maternal BMI at the time of admission. Analysis of overall diabetes effect (present or absent) was not statistically significant, implying that factors other than the diabetes itself may have a more profound effect on labor progression (data not shown). The findings of active labor progression in our study are similar to that of Zhang and colleagues [8]: it may take up to 6 hours at the 90th percentile (Table II) to observe cervical change from 4 to 6 cm, regardless of parity. We also saw that multiparous women with preexisting diabetes had a statistically significant slower progression from 5 to 6 cm, even after accounting for maternal BMI and neonatal birth weight.
There are several strengths and limitations of this study. The former is that the Consortium database encompassed both academic and community hospitals covering the nine ACOG districts in the United States and is representative of the general population. The current study was meant only to describe spontaneous labor progression in diabetic women that results in a vaginal delivery with normal neonatal outcomes. As with different provider management styles, arrest of labor progression could have been diagnosed at cervical dilatation of 4 – 6 cm, before active labor was achieved, resulting in bias due to performance of cesarean delivery prematurely in labor that could have potentially resulted in normal vaginal delivery if more time was allowed for labor progression. Cervical examination is a subjective measure, and results could have been affected by the experience of the person performing the evaluation. As several academic centers contributed to the database, residents as well as other mid-level providers would be expected to perform cervical examinations. Constructed labor curves were based on median cervical dilatation, and represent the exam at the time of admission in active labor. Therefore, it was not possible to describe the length of the latent phase from the current analysis, and explains the lack of an obvious inflection point which would be occurring at the time of or prior to admission to Labor and Delivery.
We used neonatal birth weight when adjusting for labor progression, understanding that fetuses of women with diabetes have increased adipose tissue deposition. Using the ponderal index, which takes into account the length of the neonate, may be a more appropriate measure, however, was out of the scope of the variables available in the database. Another limitation is that the medications used for treatment of diabetes as well as the degree of diabetes control cannot be determined from the available data, but may have significant clinical implications for management of the delivery by the obstetric provider.
The current study demonstrates that active labor progression is similar in nulliparous and multiparous women with preDM and GDM as compared to normal controls matched by neonatal birth weight, parity, and maternal BMI, and that a vaginal delivery can be achieved even with slightly slower progression of spontaneous labor progression in women with diabetes. Our future studies will focus on labor progression in women with diabetes who undergo an induction of labor, further delineating risk factors for success and failure.
Acknowledgements
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH (Contract No. HHSN267200603425C). Statistical support for this project was provided through the MedStar Health Research Institute, a component of the Georgetown-Howard Universities Center for Clinical and Translational Science and supported by Grant 1UL1RR031975 from the NCRR, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Declaration of Interest Statement
The authors report no declarations of interest.
References
- 1.Langer O, Anyaegbunam A, Brustman L, Divon M. Management of women with one abnormal oral glucose tolerance test value reduces adverse outcome in pregnancy. Am J Obstet Gynecol. 1989;161(3):593–599. doi: 10.1016/0002-9378(89)90361-x. [DOI] [PubMed] [Google Scholar]
- 2.Sermer M, Naylor CD, Gare DJ, Kenshole AB, Ritchie JW, Farine D, Cohen HR, McArthur K, Holzapfel S, Biringer A. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. The Toronto tri-hospital gestational diabetes project. Am J Obstet Gynecol. 1995;173(1):146–156. doi: 10.1016/0002-9378(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 3.Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90(6):869–873. doi: 10.1016/s0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 60, March 2005. Pregentational diabetes mellitus. Obstet Gynecol. 2005;105(3):675–685. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001. Gestational diabetes. Obstet Gynecol. 2001;98(3):525–538. [PubMed] [Google Scholar]
- 6.Sheiner E, Levy A, Feinstein U, Hershkovitz R, Hallak M, Mazor M. Obstetric risk factors for failure to progress in the first versus the second stage of labor. J Matern Fetal Neonatal Med. 2002;11(6):409–413. doi: 10.1080/jmf.11.6.409.413. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, Landy HJ, Hibbard JU, Haberman S, Ramirez MM, Bailit JL, Hoffman MK, Gregory KD, Gonzalez-Quintero VH, Kominiarek M, Learman LA, Hatjis CG, van Veldhuisen P Consortium on Safe Labor. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD, Hatjis CG, Ramirez MM, Bailit JL, Gonzalez-Quintero VH, Hibbard JU, Hoffman MK, Kominiarek M, Learman LA, Van Veldhuisen P, Troendle J, Reddy UM Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FRIEDMAN E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–1575. doi: 10.1016/0002-9378(54)90311-7. [DOI] [PubMed] [Google Scholar]
- 10.FRIEDMAN EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6(6):567–589. [Google Scholar]
- 11.Verdiales M, Pacheco C, Cohen WR. The effect of maternal obesity on the course of labor. J Perinat Med. 2009;37(6):651–655. doi: 10.1515/JPM.2009.110. [DOI] [PubMed] [Google Scholar]
- 12.Vahratian A, Zhang J, Troendle JF, Savitz DA, Siega-Riz AM. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obstet Gynecol. 2004;104(5 Pt 1):943–951. doi: 10.1097/01.AOG.0000142713.53197.91. [DOI] [PubMed] [Google Scholar]
- 13.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: Pathophysiology or practice style? Toronto tri-hospital gestational diabetes investigators. JAMA. 1996;275(15):1165–1170. [PubMed] [Google Scholar]
- 14.Brady E, Hamilton PD, Joyce A, Martin MPH, Stephanie J, Ventura MA. Births: Preliminary data for 2009. National Vital Statistics Reports : From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;59(3) [PubMed] [Google Scholar]
- 15.Sheiner E, Levy A, Feinstein U, Hallak M, Mazor M. Risk factors and outcome of failure to progress during the first stage of labor: A population-based study. Acta Obstet Gynecol Scand. 2002;81(3):222–226. [PubMed] [Google Scholar]
- 16.Nuthalapaty FS, Rouse DJ, Owen J. The association of maternal weight with cesarean risk, labor duration, and cervical dilation rate during labor induction. Obstet Gynecol. 2004;103(3):452–456. doi: 10.1097/01.AOG.0000102706.84063.C7. [DOI] [PubMed] [Google Scholar]
- 17.Cheng YW, Shaffer BL, Bryant AS, Caughey AB. Length of the first stage of labor and associated perinatal outcomes in nulliparous women. Obstet Gynecol. 2010;116(5):1127–1135. doi: 10.1097/AOG.0b013e3181f5eaf0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG. 2007;114(3):343–348. doi: 10.1111/j.1471-0528.2006.01233.x. [DOI] [PubMed] [Google Scholar]
- 19.Peisner DB, Rosen MG. Transition from latent to active labor. Obstet Gynecol. 1986;68(4):448–451. [PubMed] [Google Scholar]