Abstract
Purpose
The purpose of this study was to show that this two-stage procedure for ACL (anterior cruciate ligament) revision surgery could be straight-forward and provide satisfactory clinical and functional outcomes.
Materials
This is a five-year prospective analysis of clinical and functional data on 30 patients (19 men and 11 women; average age 29.1 ± 5.4) who underwent a two-stage ACL revision procedure after traumatic re-rupture of the ACL. Diagnosis was on Lachman and pivot-shift tests, arthrometer 30-lb KT-1000 side-to-side findings, and on MRI and arthroscopic assessments.
Results
Postoperative IKDC and Lysholm scores were significantly improved compared to baseline values (P < 0.001). At the last follow up, 20 of 30 patients (66.7 %) had returned to preoperative sport activity level (nine elite athletes, 11 county level), seven had changed to lower sport levels, and three had given up any sport activity. At the same appointment, 11 patients had degenerative changes. All these patients reported significantly lower Lysholm scores compared to patients without any degenerative change (p < 0.001).
Conclusions
In ACL revision surgery, when the first femoral tunnel has been correctly placed, this procedure allows safe filling of large bony defects, with no donor site comorbidities. It provides comfortable clinical, functional and imaging outcomes.
Introduction
Primary reconstruction of the anterior cruciate ligament (ACL) is successful in 75–93 % of patients [1–3]. Tunnel placement, graft type, loss of motion, arthrofibrosis, dysfunction of the extensor mechanism, arthritis, infection, neurovascular injury, and cyclops lesion are the main causes of failure of ACL reconstructions [4–6]. Many procedures have been used in ACL revision surgery, but the best management is still unknown. One-stage procedures without removing femoral fixation may be made when tunnels are inadequately located. On the other hand, when the position is correct, the graft and the fixation hardware should be removed and residual bone defects should be filled before performing another ACL reconstruction. We propose a two-stage ACL revision procedure. Specifically, we first perform an operation to remove femoral fixation and fill the defect using autologus bone grafts (OATS tube harvester grafting; Arthrex, Naples, FL) and then, once the bone implant has been healed, we reconstruct the ACL.
We report clinical, functional and imaging outcomes of 30 consecutive patients undergoing this two-stage procedure at five years from the index surgery. The hypothesis is that this approach could restore stability of the knee, and encourage return to pre-injury activity.
Materials and methods
Patient population
This is a retrospective analysis of prospectively collected clinical and functional data of 30 patients (19 men and 11 women) undergoing ACL revision surgery, operated on from January 2002 to April 2006.
The study was approved by the local Institutional Review Board (IRB).
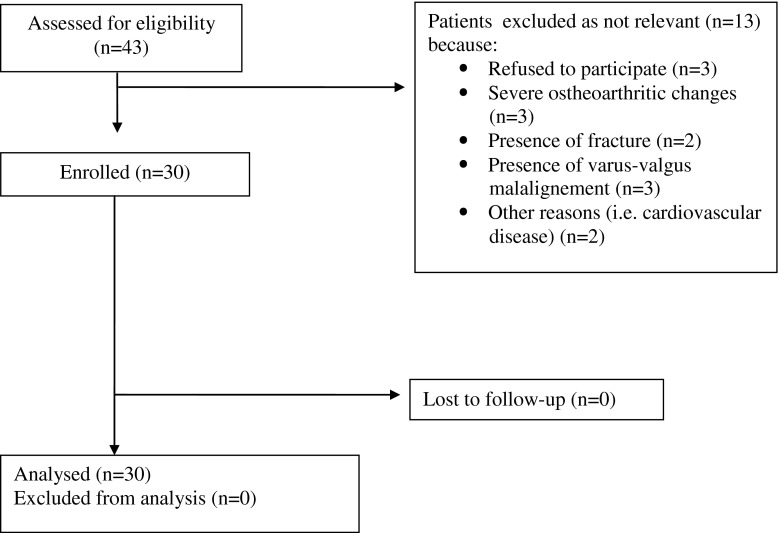
Inclusion criteria were post-traumatic knee instability and functional impairment in patients undergoing previous bone-patellar-tendon-bone ACL reconstruction. Patients with severe radiographic degenerative changes (joint space narrowing greater than 50 %), multi-ligament injury (posterior cruciate ligament, lateral and medial collateral ligaments), lower extremity fractures, cardiovascular disease, workers’ compensation claims or psychiatric illness were excluded (Fig. 1).
Fig. 1.
Process of inclusion of patients
Preoperative assessment
The senior author (F.F.) assessed all the patients. Lachman and pivot-shift tests were positive in all instances, and KT-1000 side-to-side difference (30 lb) was also evident. MRI confirmed the ACL rupture in all the patients. Weight bearing anteroposterior (AP) and 30° flexion lateral radiographs were made to exclude fractures, for assessment of joint degeneration and varus–valgus misalignment. Before surgery, each patient underwent rehabilitation to gain a full range of motion of the knee, and keep the volume of the quadriceps adequate.
Surgical technique
All surgical procedures were performed under regional anaesthesia, with the patient supine, using a standard set-up. After exsanguination, a thigh tourniquet was inflated to 280 mmHg, and a 50-mmHg arthroscopic pump was used for irrigation. The mean tourniquet time was 69 min (range, 40–114). The mean time from failure to revision was 6.8 months (range, 4–9 months). A two-stage revision procedure was performed in all patients within three months from the diagnosis of ACL failure.
First stage procedure
The first arthroscopy allowed the surgeon to ascertain the femoral tunnel position and bony defect size. With the arthroscope in the antero-lateral portal, both the tibial and femoral tunnels were identified. After the head of each interference screw had been exposed, a screwdriver was introduced to remove the screw. If the screws were stripped, osteotomes or hollow drills were used. After the soft tissues were debrided and the tunnel entrance exposed, a gauge tool device was used to measure the diameter of the defect and measure the size of the bone plug. Tunnel dilators were then introduced to provide impaction of the bone and ensure a round hole. To harvest the bone graft, the patellar tendon was split in line with its fibres, one centimetre from its medial edge, allowing access to the safe zone on the anterior tibial metaphysis [7]. This site is located between the anterior roots of both the menisci, anterior to the insertion of the ACL to the tibia. The zone is not weight-bearing and is free from meniscal or cruciate ligament attachments. A bone plug was harvested from the safe zone of the tibia using an OATS tube harvester (Arthrex, Naples, FL), this plug was inserted arthroscopically into the defect using the same OATS harvester (press-fit technique). Cartilage lesions were encountered in 12 patients, all with grade 1–2 lesions according to the Outerbridge classification [8]. The site of chondral injury in six of 12 patients was located in the medial femoral condyle, in four of 12 patients in the lateral femoral condyle and in two patients at the patella surface.
All these patients underwent shaving and debridement of the cartilage lesions. Medial and lateral partial arthroscopic meniscectomies were performed in ten and eight patients, respectively.
Second stage procedure
After a minimum interval of three months from the first procedure, a routine ACL reconstruction with autologous hamstring tendons was performed [9]. A traditional trans-tibial technique was undertaken [10, 11]. The new ligament was fixed to the tibia by a metallic screw (Arthrex BioComposite Interference Screw) and to the femur by a bio-absorbable screw.
We did not refer to the diameter size and dilatation threshold of the tunnel as criteria for revision surgery, but we assessed only the arthroscopic position of the original femoral tunnel.
Rehabilitation program
After the first operation, all patients were allowed to weight bear on the operated leg as tolerated using elbow crutches. After ACL reconstruction, postoperative rehabilitation was in line with that previously described [12, 13]. Briefly, the knee was immobilised in a 0° locked brace. Patients were allowed home the day after surgery, and allowed to weight bear as much as they were able on the operated leg using elbow crutches. At four weeks, the brace was removed. Running was allowed at three months, and return to sports at six months. They were then followed up at three-month intervals and discharged at 24 months after the operation.
Follow up
For the purpose of this study, patients were assessed at three months from the first stage procedure, and at three, 12 months, and five years from the second stage operation. All examinations were performed by a single trained examiner (A.D.B.) not involved in the surgery. Symptoms, signs and functional evaluation were assessed according to the IKDC form, Lysholm score and mean arthrometer 30-lb KT-1000 side-to-side difference. Donor-site discomfort was also evaluated using the knee-walking test [9]. For each patient, the outcomes relative to the latest available follow up were reported and compared to preoperative status.
Imaging assessment
Pre-operatively, ACL failure and position of previous tunnel were assessed on MRI scans in all the patients. Three months after the first procedure, tunnel state, bony plug placement, and bony defect filling were assessed by computer tomography (CT). Preoperatively and at a minimum follow up of five years (average 6.7, range five to nine years) from the revision, standard antero-posterior and lateral radiographs were undertaken to assess joint degeneration according to the Fairbank classification [10]. The insertion of the ACL to the femoral side was assessed according to the quadrant technique described by Bernard and Hertel [11]. This method defines the centre of the insertion site to the femur on conventional lateral radiographs. Four distances were measured: the total sagittal diameter of the lateral condyle measured along Blumensaat’s line (distance t), maximum intercondylar notch height (distance h), distance from the centre of the ACL to the most dorsal subchondral contour of the lateral femoral condyle (distance a), and the distance from the centre of the graft to the Blumensaat’s line (distance b). Since the length of distance a was considered as a partial distance of t and the length of distance b as a partial distance of h, they were expressed as percentages of t and h.
Statistical analysis
Unpaired t-tests and chi-square tests were used for comparison of pre- and postoperative continuous and categorical data, respectively. Univariate analysis of variance (ANOVA) was used to assess for significant changes in the arthrometer 30-lb KT-1000 side-to-side difference measures. The Pearson test was used to assess the correlation between osteoarthritis and the outcomes reported at the last follow up (Lysholm scores and KT laxity). Odds ratios were calculated to investigate whether the meniscectomy and cartilage lesions may predispose to develop degenerative changes. SPSS version 17.0 (SPSS, Chicago, IL, USA) was used to analyse the data. In all analyses, P < 0.05 indicated a statistically significant difference.
Results
The left knee was involved in 14 patients, the right in 16. Patients features are reported in Table 1, and mechanism of injury is detailed in Table 2.
Table 1.
Patient features
| Feature | Value |
|---|---|
| Number of patients | 30 |
| Males/females | 19/11 |
| Age at revision surgery, mean (range) | 29.1 years (19–42) |
| Time from ACL reconstruction to failure, mean (range) | 27 months (5–53) |
| Time from diagnosis of ACL failure to surgery, mean (range) | 6.8 months (4–9) |
| Follow up , mean (range) | 6.7 years (5–9) |
Table 2.
Mechanism of trauma
| Type of trauma | Number of patients |
|---|---|
| Direct impact | 8 |
| Indirect impact | 7 |
| Pivoting activity | 9 |
| Vehicle accident | 3 |
| Others | 3 |
At first surgery, the diameter of the bony defect averaged 10.4 mm (range, 8–11 mm), the average length measured 26.4 mm (range, 20–30 mm). We harvested bone plugs 1 mm larger and 1.5 mm longer than the tunnel size.
Clinical evaluation
At the last follow up IKDC scores were significantly improved compared to the baseline (Table 3). On clinical assessment, 27 of 30 patients (90 %) had less than 5° difference (normal IKDC rating) of flexion, and three (10 %) had a difference from 5° to 15° (nearly normal). Twenty-six of 30 (86.7 %) patients had full extension, and four (13.3 %) patients had lost more than 5° of extension (nearly normal). Twenty-four of 30 (80 %) patients were able to hop 90–100 % (grade A) compared to the uninjured side, and six of 30 (20 %) patients hopped from 50 to 90 % compared to the contra-lateral limb.
Table 3.
Clinical and functional outcomes
| Score | Pre-operative value | Postoperative value | P values |
|---|---|---|---|
| Mean KT-1000 arthrometer side-to-side difference IKDC | 7.4 mm (3–10) | 3.1 mm (1.3–3.8) | P < 0.001 |
| Lachman test | Grade II: 19 patients | 24 (80 %) patients had normal Lachman test results and 6 (20 %) patients had grade I laxity with a firm end point | P 0.001 |
| Grade III: 11 patients | |||
| Pivot-shift test | Grade I : 5 | Negative : 25 | P <0.0001 |
| Grade II : 13 | Grade I : 5 | ||
| Grade III : 12 | |||
| IKDC | 18 C; 12 D | 27 patients A and B; 3 C | P < 0.0001 |
| Mean Lysholm score | 65.4 (48–82, SD: 7.9) | 90.2 (72–100, SD: 7.9) | P < 0.001 |
At the last follow up, the KT-1000 side-to-side difference, Lachman and pivot-shift measures were also significantly improved compared to the results observed preoperatively (P < 0.001) (Table 3). The overall Lysholm score was also significantly higher than preoperatively (p < 0.001) (Table 3). The subgroup of patients who had received additional meniscectomy had significantly lower Lysholm scores than the others (p = 0.002). Comparable results were observed in patients with and without cartilage lesions (p = 0.97).
At the last follow up, 20 of 30 patients (66.7 %) had returned to the preoperative sport activity level (nine elite athletes, 11 county level), seven had changed to lower non-impact sports, and three had given up any sport activity. All patients were able to undertake the knee walking test without any pain.
Imaging results
Three months after the first procedure, CT scans showed complete integration of the bone plug into the tunnel. Preoperatively, 25 of 30 (83.3 %) patients showed no degenerative (grade 0) Fairbanks changes, and five of 30 (16.7 %) had grade I Fairbanks changes. Postoperatively, 19 of 30 (63.3 %) patients had any degenerative changes, seven (23.3 %) had grade I changes, and four (13.3 %) had grade II changes. At five years, patients with degenerative changes had significantly lower Lysholm scores compared to patients without any change (p < 0.001). The incidence of degenerative changes was negatively associated with postoperative Lysholm scores (P < 0.001).
Eighteen (60 %; 18 of 30) patients who had undergone medial or lateral meniscectomy had a 5.8 OR of development of degenerative changes (95 % CI, 1.2–27.8, p = 0.03) compared with patients without meniscal tear. Six of 12 (50 %) patients with cartilage lesions developed Fairbank changes (2.0 OR, 95 % CI, 0.5–8.6, p = 0.45); the presence of cartilage lesions at surgery was not significantly predisposing to further degeneration. The centre of the ACL was located at an average of 23 % (range 17–25 %) of the height of the lateral femoral condyle and of 20 % (range 15–23 %) along the Blumensaat’s line.
Complications
Five patients reported hypoaesthesia and numbness over the anterior medial aspect of the tibia, but only one patient still had an area of hypoesthesia at the final follow up, with no inconvenience. Eight patients reported joint stiffness, but all of them recovered after physiotherapy. None of patients reported fracture, re-ruptures, vascular complication, or infection.
Discussion
The main finding of this study was that this two-stage ACL revision procedure provides satisfying clinical and functional outcomes after a minimum of five years from the index surgery. The first stage procedure was undertaken to remove the ACL graft and fixation devices and to fill the femoral tunnel with a bone auto-graft harvested from a safe zone of the knee. Later, when the graft was healed, the ACL was reconstructed using ipsilateral quadrupled hamstrings. We acknowledge that single stage ACL revision procedures are also effective [13], but the reason for our approach was that all patients reported a post-traumatic ACL re-rupture, and we attempted to restore the integrity of the femoral condyle before performing a revision procedure. After removal of hardware we found a large hole (defect) in the femur that we supposed to be large enough to compromise any ACL revision procedure. Bone auto and allograft may be used. Iliac crest auto-grafts are at lower risk of infection and provide better osteointegration than allografts [14], but some pain may persist at the donor site. We harvested a large bone plug from the proximal tibial metaphysis in a load-bearing free zone commonly used as entry point when performing intramedullary tibial nailing fixation [3, 7]. The harvest is relatively safe because of the low risk of damage to the knee [15, 16]. The purpose of implanting the graft is to restore the anatomy and structural integrity of the bone. We now point out that some failures and re-ruptures may occur after ACL surgery because of weakening and micro-fracturing of the bone, and we propose that a previous tunnel would compromise the integrity of a new reconstruction, and predispose to bone disruption. This justifies the first stage of the procedure, which aims to fill a large defect in the lateral femoral condyle [3]. We acknowledge that a three-month interval period may not be long enough to ensure bone healing, but all patients presented satisfying graft integration to the bone on CT imaging assessment.
We used autologous hamstring tendon graft. These tendons provide good stability and strength [13], with evidence on MRI scans of tendon regeneration and almost normal insertion point on the pes anserinum six years after surgery. When comparing the operated and contralateral sides, even though cross sectional areas are similar, some strength deficits may persist, but the actual clinical impact of this is unknown [17]. On the other hand, the contralateral patellar tendon was not used to avoid the risk of patellar bone fracture and patellar tendon rupture [18].
We performed a single bundle ACL revision in which the femoral tunnel was drilled through a transtibial approach. The radiographic assessment by Bernard and Hertel of the ACL position showed that the ligament was relatively high and vertical. We now drill the femoral tunnel through an anteromedial portal to achieve a lower, more horizontal and, hence, more anatomical position of the ACL [19, 20]. However, the clinical relevance of this is still the subject of debate; at present, none of these procedures fully restores both kinematics and biomechanics of the knee [21]. Almost all the patients had gained good stability and good to excellent clinical and functional outcomes, better than those reported in previous studies. When asked about sport activity, almost all of them had returned to the same sport activity level they practised before the injury.
On the other hand, 11 of 30 patients had evidence of degenerative changes to the knee. In agreement with published data [22–24], all these patients had undergone secondary operations to cartilage and menisci, and reported significantly lower results.
The strength of the study is that a fully trained orthopaedic surgeon operated on all patients, and an independent investigator performed all clinical and functional examinations. Postoperative management was uniform throughout the study, and all patients were enrolled according to strict selection criteria (traumatic re-rupture) and were followed up for a minimum of five years from the index revision procedure.
The indications for two-stage revision procedures have narrowed in recent years. Shortcomings are additional costs, morbidity related to two separate operations, and the prolonged rehabilitation period, but we now stress that, in the presence of anatomical or near anatomical tunnels, this two stage procedure could be a suitable alternative. We are aware that the evidence given for assessing postsurgical outcomes is not as strong as that produced by a randomised controlled trial. The study design, namely, a prospective case series, may present selection bias. The relatively small sample size of the study does not allow us to draw definitive conclusions. Without comparing this approach with an alternative, we cannot conclude that our outcomes arise from staging, bone graft choice, ACL graft choice, technique (anatomic single bundle ACL reconstruction), or other factors.
Conclusions
This two-stage procedure is safe and effective in this relatively small sample.
Acknowledgments
Conflict of interest
No financial or material support was received.
Contributor Information
Francesco Franceschi, Email: f.franceschi@unicampus.it.
Rocco Papalia, Email: r.papalia@unicampus.it.
Angelo Del Buono, Email: a.delbuono@unicampus.it.
Biagio Zampogna, Email: b.zampogna@unicampus.it.
Lorenzo Diaz Balzani, Email: lorenzo.diaz87@gmail.com.
Nicola Maffulli, Email: n.maffulli@qmul.ac.uk.
Vincenzo Denaro, Email: denaro@unicampus.it.
References
- 1.Ramjug S, Ghosh S, Walley G, Maffulli N. Isolated anterior cruciate ligament deficiency, knee scores and function. Acta Orthop Belg. 2008;74:643–651. [PubMed] [Google Scholar]
- 2.Lovric V, Kanazawa T, Nakamura Y, Oliver R, Yu Y, Walsh WR (2011) Effects of gaps induced into the ACL tendon graft on tendon-bone healing in a rodent ACL reconstruction model muscles. Ligaments Tendons J I:91–99 [PMC free article] [PubMed]
- 3.Franceschi F, Papalia R, Di Martino A, Rizzello G, Allaire R, Denaro V. A new harvest site for bone graft in anterior cruciate ligament revision surgery. Arthroscopy. 2007;23(558):e551–e554. doi: 10.1016/j.arthro.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DL, Fu FH. Anterior cruciate ligament reconstruction: why do failures occur? Instr Course Lect. 1995;44:391–406. [PubMed] [Google Scholar]
- 5.Felmet G. Anatomic double bundle single tunnel foreign material free ACL-reconstruction—a technical note. Muscle Ligaments Tendons J. 2011;1:147–151. [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillon MS, Ball K, Prabhakar S. Differences among mechanoreceptors in healthy and injured anterior cruciate ligaments and their clinical importance. Muscle Ligaments Tendons J. 2012;2:38–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Tornetta P, Riina J, Geller J, Purban W. Intraarticular anatomic risks of tibial nailing. J Orthop Trauma. 1999;13:247–251. doi: 10.1097/00005131-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med. 2003;31:83–86. doi: 10.1177/03635465030310012601. [DOI] [PubMed] [Google Scholar]
- 9.Kartus J, Ejerhed L, Sernert N, Brandsson S, Karlsson J. Comparison of traditional and subcutaneous patellar tendon harvest. A prospective study of donor site-related problems after anterior cruciate ligament reconstruction using different graft harvesting techniques. Am J Sports Med. 2000;28:328–335. doi: 10.1177/03635465000280030801. [DOI] [PubMed] [Google Scholar]
- 10.Fairbank TJ. Examination of the knee joint. Br Med J. 1969;3:220–222. doi: 10.1136/bmj.3.5664.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard M, Hertel P, Hornung H, Cierpinski T. Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg. 1997;10:14–21. [PubMed] [Google Scholar]
- 12.Osti L, Papalia R, Del Buono A, Merlo F, Denaro V, Maffulli N. Simultaneous surgical management of chronic grade-2 valgus instability of the knee and anterior cruciate ligament deficiency in athletes. Knee Surg Sports Traumatol Arthrosc. 2010;18:312–316. doi: 10.1007/s00167-009-0966-y. [DOI] [PubMed] [Google Scholar]
- 13.Weiler A, Schmeling A, Stohr I, Kaab MJ, Wagner M. Primary versus single-stage revision anterior cruciate ligament reconstruction using autologous hamstring tendon grafts: a prospective matched-group analysis. Am J Sports Med. 2007;35:1643–1652. doi: 10.1177/0363546507303114. [DOI] [PubMed] [Google Scholar]
- 14.Brown CH, Jr, Carson EW. Revision anterior cruciate ligament surgery. Clin Sports Med. 1999;18:109–171. doi: 10.1016/S0278-5919(05)70133-2. [DOI] [PubMed] [Google Scholar]
- 15.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51:871–879. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 16.Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2012;37:279–284. doi: 10.1007/s00264-012-1757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JY, Ha JK, Kim YW, Shim JC, Yang SJ, Kim JG. Relationships among tendon regeneration on MRI, flexor strength, and functional performance after anterior cruciate ligament reconstruction with hamstring autograft. Am J Sports Med. 2012;40:152–162. doi: 10.1177/0363546511424134. [DOI] [PubMed] [Google Scholar]
- 18.Kartus J, Stener S, Lindahl S, Eriksson BI, Karlsson J. Ipsi- or contralateral patellar tendon graft in anterior cruciate ligament revision surgery. A comparison of two methods. Am J Sports Med. 1998;26:499–504. doi: 10.1177/03635465980260040401. [DOI] [PubMed] [Google Scholar]
- 19.Dargel J, Schmidt-Wiethoff R, Fischer S, Mader K, Koebke J, Schneider T. Femoral bone tunnel placement using the transtibial tunnel or the anteromedial portal in ACL reconstruction: a radiographic evaluation. Knee Surg Sports Traumatol Arthrosc. 2009;17:220–227. doi: 10.1007/s00167-008-0639-2. [DOI] [PubMed] [Google Scholar]
- 20.Brown CH, Spalding T, Robb C. Medial portal technique for single-bundle anatomical anterior cruciate ligament (ACL) reconstruction. Int Orthop. 2013;37:253–269. doi: 10.1007/s00264-012-1772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ristanis S, Stergiou N, Siarava E, Ntoulia A, Mitsionis G, Georgoulis AD. Effect of femoral tunnel placement for reconstruction of the anterior cruciate ligament on tibial rotation. J Bone Joint Surg Am. 2009;91:2151–2158. doi: 10.2106/JBJS.H.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89–106. doi: 10.1093/bmb/ldq043. [DOI] [PubMed] [Google Scholar]
- 23.Garofalo R, Djahangiri A, Siegrist O. Revision anterior cruciate ligament reconstruction with quadriceps tendon-patellar bone autograft. Arthroscopy. 2006;22:205–214. doi: 10.1016/j.arthro.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 24.Struewer J, Ziring E, Frangen TM, Efe T, Meissner S, Buecking B, Bliemel C, Ishaque B. Clinical outcome and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using hamstring graft: follow-up after two and ten years. Int Orthop. 2013;37:271–277. doi: 10.1007/s00264-012-1653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]