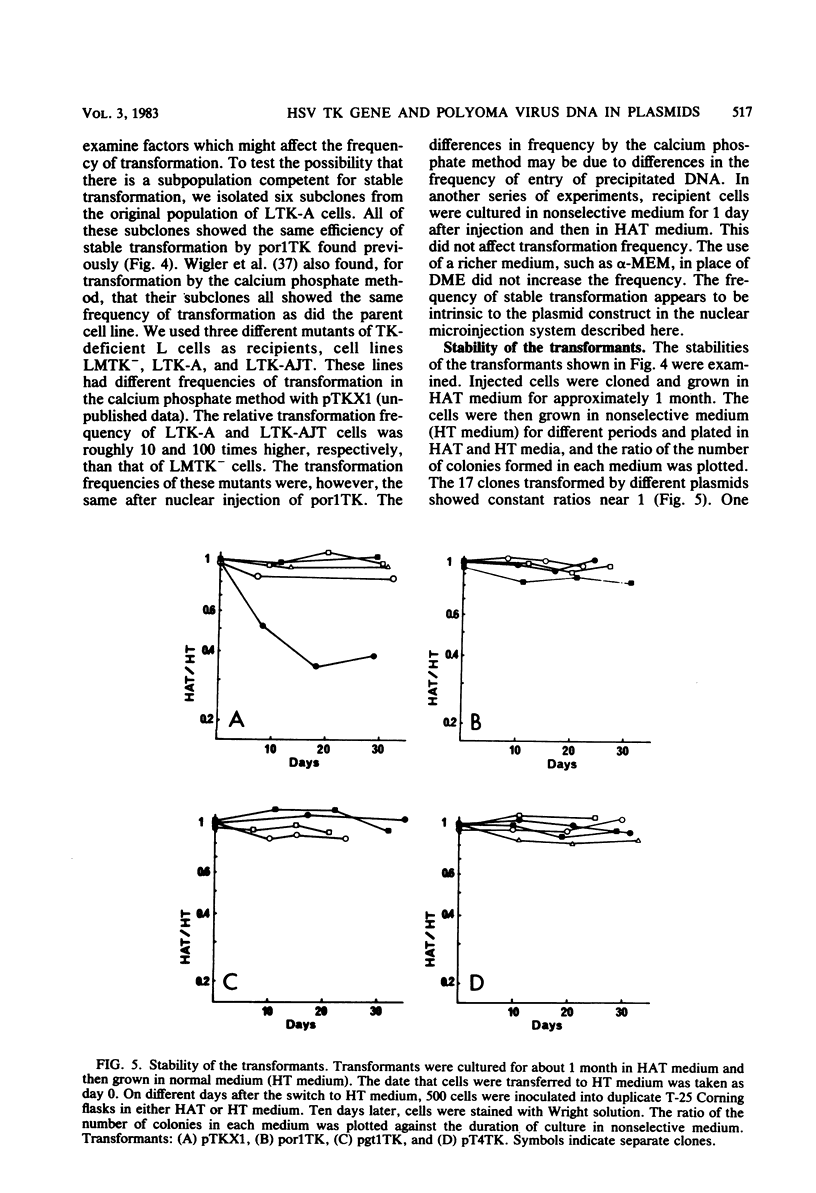
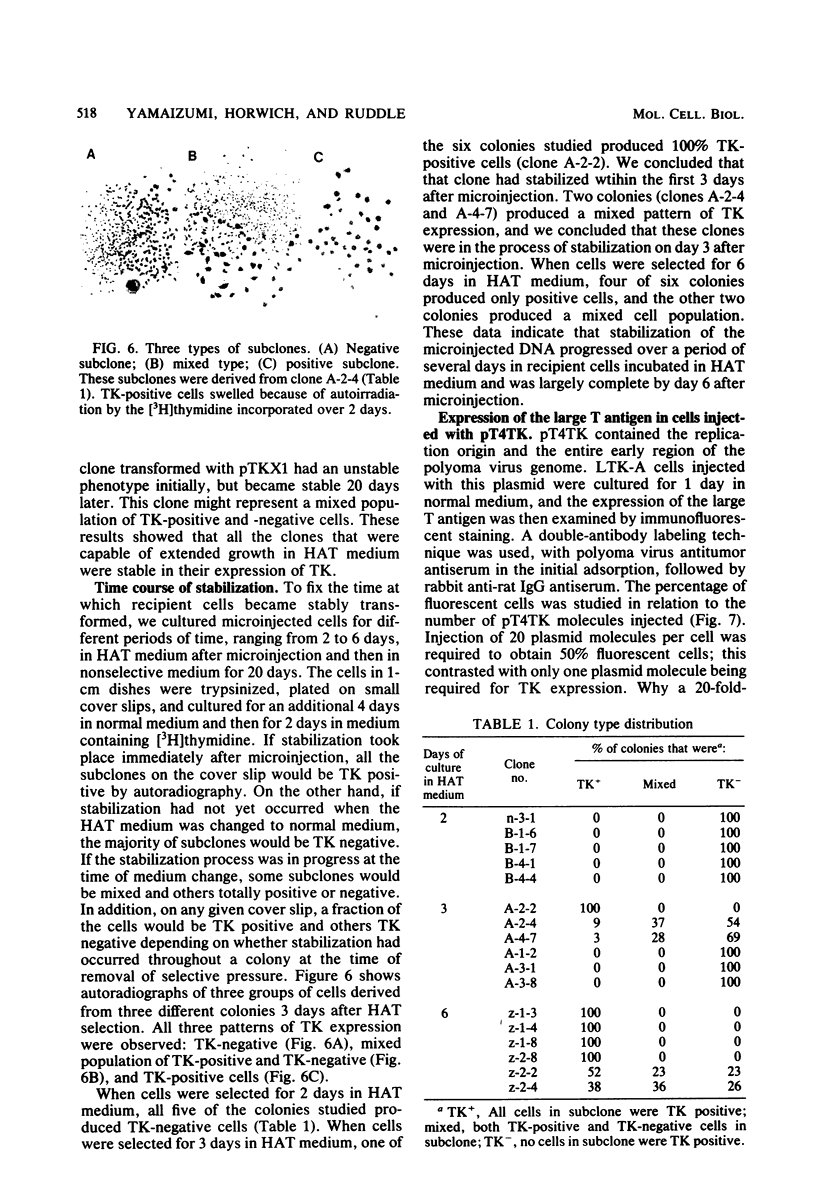
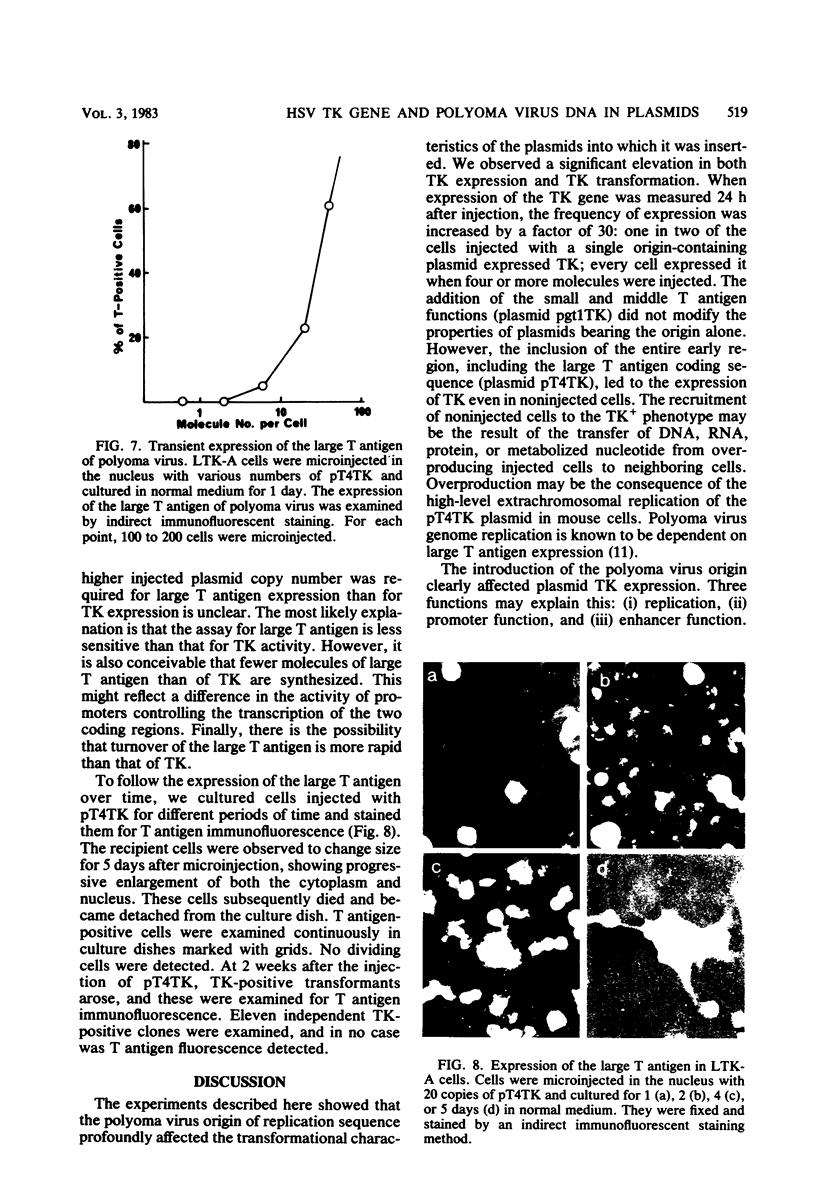
Abstract
To observe the effects of polyoma virus DNA on the expression of the herpes simplex virus (HSV) thymidine kinase (TK) gene early after transfer into TK-deficient mouse cells and the subsequent development of stable TK-positive transformants, we constructed a series of recombinant plasmids containing the herpes simplex virus TK gene joined with various segments of the polyoma virus genome and microinjected them into the nuclei or cytoplasm of LTK-A cells (TK−, APRT−). The frequency of nucleus-injected cells expressing TK after 1 day, measured by autoradiography of cells incubated with [3H]thymidine, increased approximately 30-fold when the plasmids contained the polyoma virus origin of replication. The origin includes sequences with homology to the simian virus 40 origin of replication and adjoining sequences, including a recently defined transcription-enhancing sequence. After microinjection of a single origin-containing plasmid molecule per cell, TK expression was detected in approximately 50% of the injected cells. When a larger number of origin-containing plasmid molecules were injected per cell, all cells showed early TK activity. When the entire polyoma virus early region was present, neighboring uninjected cells became TK positive. When plasmids were injected into the cell cytoplasm, approximately 400 times as many molecules per cell were needed to cause early TK activity. The frequency of stable transformation observed 2 weeks after nuclear injection of 10 to 20 polyoma virus origin-containing plasmid molecules per cell was at least 2 orders of magnitude greater than with plasmids containing the TK gene alone. The greatest enhancement of stable TK transformation was obtained with plasmids containing the origin alone, when the maximum frequency of stable transformation was 5%. The addition of the coding regions for the small and medium T antigens or the entire early region significantly decreased TK transformation frequency in a copy-dependent fashion. The timing of stabilization of TK-positive transformation was analyzed by releasing hypoxanthine-aminopterin-thymidine selection pressure at various times after microinjection, culturing the cells in nonselective medium, and assaying for TK activity. Stabilization was found to occur between 3 and 6 days after nuclear injection. Cells injected with a plasmid containing the origin and the early region were examined for expression of the large T antigen with polyoma virus antitumor serum and immunofluorescent staining. The expression of the large T antigen was clearly associated with a cytopathic effect. TK-positive clones observed 2 weeks after injection of the plasmid were uniformly T antigen negative. Cytotoxicity may be the result of plasmid replication and toxic levels of T antigen or TK. In addition, expression of the large T antigen may block stabilization by preventing the integration of origin-containing plasmid molecules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Killos L., Sanders-Haigh L., Kretschmer P. J., Diacumakos E. G. Replication and expression of thymidine kinase and human globin genes microinjected into mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5399–5403. doi: 10.1073/pnas.77.9.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Characterization of early simian virus 40 transcriptional complexes: late transcription in the absence of detectable DNA replication. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5443–5447. doi: 10.1073/pnas.74.12.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Sharp P. A. Expression of early and late simian virus 40 transcripts in the absence of protein synthesis. J Virol. 1980 Jun;34(3):592–597. doi: 10.1128/jvi.34.3.592-597.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. A., Topp W. C., Rifkin D. B., Moreau P. E. Transformation of rat embryo fibroblasts by cloned polyoma virus DNA fragments containing only part of the early region. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3978–3982. doi: 10.1073/pnas.77.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A., Koop A. H., Eckhart W. Expression of the gene for the polyoma small T antigen in Escherichia coli. J Virol. 1980 Oct;36(1):125–132. doi: 10.1128/jvi.36.1.125-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Ruddle F. H. Phenotype stabilisation and integration of transferred material in chromosome-mediated gene transfer. Nature. 1979 Aug 23;280(5724):657–660. doi: 10.1038/280657a0. [DOI] [PubMed] [Google Scholar]
- Loyter A., Scangos G. A., Ruddle F. H. Mechanisms of DNA uptake by mammalian cells: fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):422–426. doi: 10.1073/pnas.79.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Ruddle F. H. Co-transfer of human X-linked markers into murine somatic cells via isolated metaphase chromosomes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3346–3350. doi: 10.1073/pnas.75.7.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G. A., Huttner K. M., Juricek D. K., Ruddle F. H. Deoxyribonucleic acid-mediated gene transfer in mammalian cells: molecular analysis of unstable transformants and their progression to stability. Mol Cell Biol. 1981 Feb;1(2):111–120. doi: 10.1128/mcb.1.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser C. A., Steglich C., deWet J. R., Scheffler I. E. Cell cycle-dependent regulation of thymidine kinase activity introduced into mouse LMTK- cells by DNA and chromatin-mediated gene transfer. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1119–1123. doi: 10.1073/pnas.78.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Schaffhausen B., Benjamin T. Tumor antigens induced by nontransforming mutants of polyoma virus. Cell. 1978 Oct;15(2):485–496. doi: 10.1016/0092-8674(78)90018-1. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. Autoradiography. Methods Enzymol. 1979;58:279–292. doi: 10.1016/s0076-6879(79)58143-9. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Mekada E., Uchida T., Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978 Sep;15(1):245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W. A small segment of polyoma virus DNA enhances the expression of a cloned beta-globin gene over a distance of 1400 base pairs. Nucleic Acids Res. 1981 Dec 11;9(23):6251–6264. doi: 10.1093/nar/9.23.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]