Abstract
Obesity and chronic, treated HIV infection are both associated with persistent systemic inflammation and a similar constellation of metabolic and cardiovascular diseases, but the combined effects of excess adiposity and HIV on circulating proinflammatory cytokines and other biomarkers previously shown to predict disease risk is not well described. We measured inflammation biomarker levels in 158 predominantly virologically suppressed adults on long-term antiretroviral therapy (ART) with a range of body mass index (BMI) values from normal to morbidly obese. We assessed the relationship between BMI and each biomarker using multivariable linear regression adjusted for age, sex, race, CD4+ count, tobacco use, data source, protease inhibitor use, and routine nonsteroidal antiinflammatory drug (NSAID) or aspirin use. Among normal-weight (n=48) and overweight participants (n=41; BMI <30 kg/m2), incremental BMI increases were associated with significantly higher serum highly sensitive C-reactive protein (hsCRP; β=2.47, p=0.02) and tumor necrosis factor (TNF)-α receptor 1 levels (β=1.53, p=0.03), and significantly lower CD14 levels (β=0.84, p=0.01), but similar associations were not observed in the obese participants. Among the obese (n=69; BMI ≥30 kg/m2), however, higher serum levels of interleukin-6 (IL-6; β=1.30, p=0.02) and macrophage inflammatory protein-1α (β=1.77, p<0.01) were associated with higher BMI, a finding not observed among the nonobese. Among all participants, IL-6 and TNF-α receptor 1 levels were most closely associated with hsCRP (p<0.01). Further studies are needed to determine whether higher serum inflammation biomarker levels found in obese HIV-infected individuals on ART reflect an increased likelihood of adverse health outcomes, or if novel markers to estimate mortality and disease risk are needed in this population.
Introduction
The proportion of overweight and obese HIV-infected adults in the United States is approaching that of the general population, and recent studies have reported many patients gain weight after starting antiretroviral therapy (ART).1–3 Obesity and chronic, treated HIV infection are associated with a higher risk of developing a similar constellation of metabolic and cardiovascular diseases, and obese HIV-infected adults are more likely to have multiple concomitant comorbidities compared to normal-weight HIV-infected persons.4–6 While the etiology of the increased morbidity in HIV-infected obese individuals is likely multifactorial, persistent systemic inflammation is a hallmark of both excess adiposity and HIV infection, and a predictor of cardiovascular events, diabetes mellitus, and all cause mortality among persons on ART.7–9
HIV-infected men on ART with a normal body mass index (BMI; <25 kg/m2) have equivalent serum levels of highly sensitive C-reactive protein (hsCRP; a well-studied predictor of cardiovascular disease in the general population) as compared to obese (BMI >30 kg/m2) uninfected men, in addition to equivalent serum levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α; two biomarkers emerging as important predictors of mortality and adverse health outcomes in HIV infection). At present, however, there are few data on circulating inflammation biomarkers other than hsCRP in obese, HIV-infected individuals, though studies from HIV-uninfected populations have demonstrated that adipocytes from obese individuals express disproportionately high levels of IL-6, TNF-α, and other cytokines characteristic of a systemic inflammatory response.10–15
We hypothesized that circulating biomarkers of inflammation in treated HIV continue to rise with incremental increases in BMI among obese individuals on ART. In this study, we measured serum levels of hsCRP, IL-6, TNF-α receptor 1 and 2, and soluble CD14 (biomarkers previously reported as predictors of mortality and/or the development of cardiovascular and metabolic disease in treated HIV), in addition to macrophage chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α; two immune cell recruitment cytokines produced by adipose tissue which may also have immunomodulatory properties relevant to HIV infection) in a cohort of HIV-infected adults on long-term ART with a wide range of BMI values from normal to morbidly obese.7–9,16,17
Materials and Methods
We reviewed clinical records and analyzed contemporaneously collected plasma specimens from 158 predominantly virologically suppressed adults receiving HIV treatment at the Vanderbilt Comprehensive Care Clinic in Nashville, Tennessee. Our retrospective cohort included 105 participants enrolled in the previously described Vanderbilt Lipoatrophy and Neuropathy Cohort (LiNC) and 53 individuals enrolled in the Vanderbilt-Meharry Center for AIDS Research (CFAR) cohort with validated medical records and specimen repository samples.18 The inclusion criteria for all participants included age >18 years, ≥24 weeks of continuous ART with a regimen containing ≥2 nucleoside transcriptase inhibitors, a plasma HIV-1 RNA ≤10,000 within 180 days prior to sample collection, and no history of diabetes mellitus or myocardial infarction.
Serum hsCRP was measured on all samples using the Cobas Integra 800 analyzer particle-enhanced turbidimetric assay (Roche Diagnostics, Germany), but due to logistic constraints 44% of samples were processed by the Vanderbilt Clinical Chemistry Laboratory (Nashville, TN) and 66% of samples were sent to Laboratory Corporation of America (Birmingham, AL). Serum levels of IL-6, TNF-α receptor 1 and 2, MCP-1, and MIP-1α were measured using cytometric bead array (BD Biosciences, San Jose, CA), and soluble CD14 (sCD14) was measured using ELISA (R&D Systems, Minneapolis, MN). The inclusion of a protease inhibitor in the ART regimen at the time of sample collection and the CD4+ lymphocyte count and HIV-1 viral load values closest to the collection date (within 90 days) were obtained from the same electronic medical record for all participants. Cigarette smoking status and routine use of nonsteroidal antiinflammatory drugs (NSAIDs) or aspirin at the time of sample collection, factors which could affect systemic inflammation, were obtained by questioning or abstracted from the medical record.
We stratified the cohort according to National Heart, Lung, and Blood Institute (NHLBI) BMI guidelines to compare demographic and clinical characteristics: normal weight (BMI <25.0 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2); we did not include a separate category for underweight (BMI <18.5 kg/m2) due to the small number of subjects (n=1).6 In the first analysis, we calculated medians and interquartile ranges for continuous variables and percentages for categorical variables; the relationship between BMI and each demographic characteristic was assessed using the Spearman correlation test for continuous variables and the Wilcoxon rank sum test for categorical variables.
In the second analysis, we assessed the relationship between BMI and each of the inflammation biomarkers using multivariable linear regression adjusted for age, sex, race, CD4+ lymphocyte count, smoking status, data source (i.e., LiNC or CFAR cohort), routine NSAID or aspirin usage, and the inclusion of a protease inhibitor (PI) in the ART regimen. We initially analyzed the entire cohort and then analyzed nonobese and obese participants separately. The relationship between BMI and serum biomarkers was fit using restricted cubic splines with three knots to avoid assuming a linear relationship. To achieve normality of residuals, each of the serum biomarkers was natural logarithm-transformed. To improve interpretability, the regression coefficients were back transformed and expressed as a fold change in the dependent variable (e.g., serum hsCRP) for an interquartile range (IQR) increase in the independent variable (BMI). To compare the BMI biomarker relationships among nonobese versus obese participants, we calculated the fold change in each biomarker corresponding to a 10 unit increase in BMI from 20 kg/m2 to 30 kg/m2 to represent the nonobese BMI range and from 30 kg/m2 to 40 kg/m2 to represent the obese range.
In the third analysis, we assessed the relationship between circulating CRP, a late product of the inflammatory cascade, and cytokines (or circulating cytokine receptors) shown to promote hepatic CRP production (i.e., IL-6, TNF-α receptor 1 and TNF-α receptor 2) after adjustment for BMI and the other covariates listed above.
The analysis was performed using R 2.15.1 (www.r-project.org) and SPSS (IBM Corp., Armonk, NY). The study was approved by the Vanderbilt University Institutional Review Board. All participants provided written informed consent and the investigators adhered to the human experimentation guidelines of the United States Department of Health and Human Services.
Results
Among 158 participants included in the study, 48 (30%) had a BMI <25 kg/m2, 41 (26%) were overweight (BMI 25.0–29.9 kg/m2), and 69 (44%) were obese (BMI >30.0 kg/m2); within the obese group 13 (19%) were morbidly obese (BMI >40 kg/m2) (see Table 1). One-hundred and three (65%) had a plasma HIV-1 RNA level <50 copies/ml at the time of sample collection and among the remainder the median viral load was 177 copies/ml [interquartile range (IQR) 92, 350]. Overweight and obese participants were younger, more likely to be female and nonwhite, and were less likely to report routine use of aspirin or NSAIDs compared to the normal weight participants. There were no significant differences in CD4+ lymphocyte count, HIV-1 RNA level, smoking status, duration of ART treatment, or PI usage across BMI strata. Median leptin levels, a marker of adipose tissue mass, were significantly greater in the higher BMI categories (p<0.01).
Table 1.
Demographic and Clinical Characteristics of the Study Subjects
| |
Body mass index (kg/m2) |
|
||
|---|---|---|---|---|
| Parameter | <25.0 (normal) n=48 | 25.0–29.9 (overweight) n=41 | BMI ≥30.0 (obese) n=69a | p-value |
| Age, years | 47 (43, 51) | 46 (41, 53) | 43 (39, 48) | <0.01 |
| Female, n (%) | 9 (19%) | 8 (20%) | 30 (44%) | <0.01 |
| Nonwhite race, n (%) | 18 (38%) | 15 (37%) | 35 (51%) | 0.03 |
| Active tobacco use, n (%) | 26 (54%) | 21 (51%) | 29 (42%) | 0.27 |
| Routine NSAID or aspirin use, n (%) | 31 (65%) | 23 (56%) | 18 (26%) | <0.01 |
| CD4+ lymphocyte count, cells/μl | 515 (320, 755) | 502 (334, 708) | 500 (375, 786) | 0.47 |
| Plasma HIV-1 viral load, copies/ml | 50 (50, 101) | 50 (50, 106) | 50 (50, 87) | 0.65 |
| Duration of combination antiretroviral therapy, years | 2.9 (1.4, 4.6) | 2.0 (1.1, 4.5) | 2.9 (1.4, 6.2) | 0.21 |
| Protease inhibitor in treatment regimen, n (%) | 28 (58%) | 23 (56%) | 45 (65%) | 0.19 |
| Serum leptin, ng/ml | 195 (83, 470) | 310 (176, 672) | 815 (373, 1721) | <0.01 |
Within the obese group 13 (19%) were morbidly obese (BMI >40 kg/m2).
Data are presented as N (%) or median (interquartile range).
p-values were calculated using the Wilcoxon rank sum test for categorical variables and Spearman correlation for continuous variables.
NSAID, nonsteroidal antiinflammatory drug.
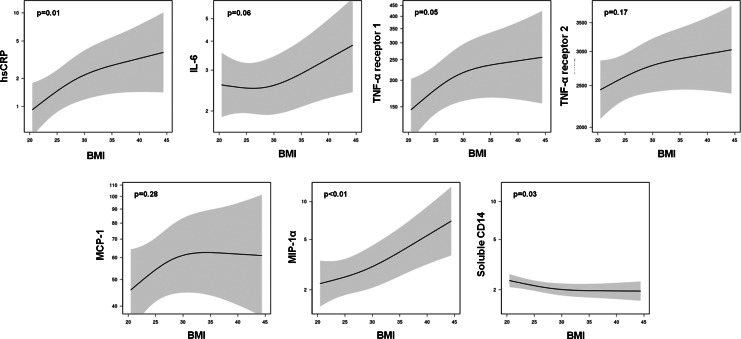
When the relationships between BMI and each serum marker were analyzed in the full cohort, a higher BMI was associated with higher circulating levels of hsCRP (β=1.90; p=0.01), and similar relationships were observed for IL-6 (β=1.08; p=0.06) and TNF-α receptor 1 (β=1.33; p=0.05) that did not reach statistical significance. There was also a statistically significant, positive relationship between BMI and MIP-1α (1.43; p=0.01), but we did not detect an association between BMI and circulating levels of MCP-1 (1.20; p=0.28) or TNF-α receptor 2 (β=1.10; p=0.17). Soluble CD14 was the only measured biomarker that decreased as BMI increased (β=0.90; p=0.03). The relationship of BMI and several of the biomarkers appeared to be nonlinear when displayed graphically (Fig. 1), though the term included to assess nonlinearity was not significant in any of the models (p>0.05).
FIG. 1.
Relationship between body mass index and circulating biomarkers. Regression lines are adjusted age, sex, race, CD4+ lymphocyte count, smoking status, data source, and aspirin, NSAID, and protease inhibitor usage; the shaded areas represent 95% confidence intervals.
We next calculated the fold-change in each biomarker observed when comparing a hypothetical BMI 30 kg/m2 subject to one with a BMI of 20 kg/m2 (i.e., to represent participants within the normal-weight and overweight range) and the change observed when comparing a hypothetical BMI 40 kg/m2 subject to one with a BMI of 30 kg/m2 (i.e., to represent participants within the obese range). Among the normal-weight and overweight participants, the 10-unit incremental increase in BMI was associated with significantly higher serum hsCRP (β=2.47, p=0.02) and TNF-α receptor 1 levels (β=1.53, p=0.03) and significantly lower CD14 levels (β=0.84, p=0.01; see Table 2), but no statistically significant relationships were observed for IL-6, TNF-α receptor 2, MIP-1α, or MCP-1 levels. Conversely, among the obese participants, serum levels of IL-6 (β=1.30, p=0.02) and MIP-1α (β=1.77, p<0.01) were significantly higher with the incremental increase in BMI, but no statistically significant relationships were observed for hsCRP, TNF-α receptors 1 or 2, CD14, or MCP-1 levels in this group.
Table 2.
Fold-Change in Inflammation Biomarkers Accompanying a 10 kg/m2 Body Mass Index Increase Among Nonobese Subjects Compared to Obese Subjects
| Serum biomarker | Regression coefficientaBMI 30 vs. 20 kg/m2 | p-value | Regression coefficientbBMI 40 vs. 30 kg/m2 | p-value |
|---|---|---|---|---|
| hsCRP | 2.47 (1.18, 5.15) | 0.02 | 1.50 (0.95, 2.37) | 0.08 |
| IL-6 | 0.99 (0.70, 1.41) | 0.97 | 1.30 (1.05, 1.62) | 0.02 |
| TNF-α receptor 1 | 1.53 (1.05, 2.23) | 0.03 | 1.14 (0.90, 1.44) | 0.28 |
| TNF-α receptor 2 | 1.15 (0.96, 1.37) | 0.12 | 1.06 (0.95, 1.19) | 0.27 |
| Soluble CD14 | 0.84 (0.74, 0.96) | 0.01 | 0.97 (0.90, 1.06) | 0.52 |
| MCP-1 | 1.36 (0.93, 1.99) | 0.11 | 1.01 (0.80, 1.28) | 0.93 |
| MIP-1α | 1.38 (0.86, 2.21) | 0.18 | 1.77 (1.32, 2.37) | <0.01 |
Represents the fold change in corresponding biomarkers comparing a hypothetical subject with a BMI of 30 kg/m2 and a hypothetical subject with a BMI of 20 kg/m2.
Represents the fold change in corresponding biomarkers comparing a hypothetical subject with a BMI of 40 kg/m2 and a hypothetical subject with a BMI of 30 kg/m2.
All models adjusted for age, sex, race, CD4+ lymphocyte count, smoking status, data source, and ASA, NSAID, or protease inhibitor usage.
hsCRP, highly sensitive C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor alpha; MCP-1, macrophage chemotactic protein 1; MIP-1α, macrophage inflammatory protein-1α.
Lastly, we analyzed the relationships between hsCRP, a terminal product of the inflammation cascade, and serum cytokines known to promote hepatic CRP production. Higher circulating levels of IL-6 (β=1.83, p<0.01) and TNF-α receptor 1 (β=1.48, p<0.01) were associated with higher hsCRP (see Table 3). We did not detect an association between hsCRP and TNF-α receptor 2.
Table 3.
Relationship Between Inflammation-Related Serum Biomarkers and Highly Reactive C-Reactive Protein Level (n=158)
| Serum biomarker | Regression coefficient | p-value |
|---|---|---|
| IL-6 | 1.83 (1.40, 2.40) | <0.01 |
| TNF-α receptor 1 | 1.48 (1.22, 1.80) | <0.01 |
| TNF-α receptor 2 | 1.34 (1.00, 1.79) | 0.10 |
All models adjusted for BMI, age, sex, race, CD4+ lymphocyte count, smoking status, data source, and ASA, NSAID, or protease inhibitor usage. Serum biomarkers were included in the models as nonlinear terms. The regression coefficient represents the fold change in hsCRP for a one interquartile range increase in biomarkers.
Each model included a term to assess for a nonlinear relationship of each biomarker to hsCRP, which was not significant (p>0.05) for any model.
IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha.
Discussion
In a cohort of HIV-infected adults on long-term, effective ART, progressive increases in BMI were associated with higher circulating levels of several inflammation biomarkers after adjustment for clinical characteristics, tobacco use, and common antiinflammatory medications. The rise in hsCRP and TNF-α receptor 1 levels observed with interval increases in BMI leveled off above a value of approximately 30 kg/m2. In contrast, the incremental rise in circulating levels of IL-6 and MIP-1α was more pronounced, and statistically significant, among obese participants compared to nonobese participants. Serum levels of CD14, a soluble receptor produced during monocyte activation and a previously described marker of microbial translocation, were inversely related to BMI among the nonobese, but this biomarker remained relatively constant in the higher BMI range. These findings suggest the association between BMI and hsCRP previously reported in studies of predominantly nonobese HIV-infected persons is attenuated among the obese, and the inflammatory state in heavier patients may be better characterized by IL-6 rather than biomarkers further along the biochemical pathway.12,18
The higher circulating IL-6 levels observed among obese individuals in our study may have important implications for estimating mortality and disease risk in this population, but a major uncertainty is whether the rise in this cytokine reflects the exacerbation of an immune-mediated pathophysiologic process promoting end-organ damage or derives from a different, potentially more innocuous, source. Recent evidence suggests that higher serum IL-6 levels are associated with an increased risk of death in HIV-infected individuals, and IL-6 may actually be a stronger predictor of cardiovascular disease morbidity and mortality compared to the more commonly studied hsCRP.9,16 In an analysis of SMART subjects, pretreatment serum IL-6 levels in the highest quartile were associated with increased risk of fatal and nonfatal cardiovascular events compared to the lowest quartile (aOR 4.6, 95% CI: 2.6–8.3), which represented a higher odds of disease events compared to pretreatment hsCRP levels (aOR 2.1, 95% CI: 1.4–3.2). While these results suggest IL-6 may be an important biomarker of inflammation-related disease processes, the median BMI of the SMART participants studied was 25.7 kg/m2 for those with a cardiovascular event and 25.0 kg/m2 for those without an event, and additional studies are needed to determine if the high serum IL-6 levels observed in our obese participants represent further attributable risk.
We attribute the greater rise in serum IL-6 levels per unit increase in BMI observed among obese individuals to the potent expression of this cytokine by hypertrophied adipocytes.13,15 The expansion of fat depots results in increased expression of IL-6 and TNF-α per unit of tissue, both of which are major promoters of hepatic CRP production.13,15 Adipocyte hypertrophy increases MCP-1 and MIP-1α expression, which promote monocyte and other immune cell migration into adipose tissue and increased macrophage production of TNF-α and interleukin-1β.14,19–21 Additionally, in vitro studies have shown that exposure to HIV viral particles and ART have profound effects on adipocyte biology, including increased TNF-α, IL-6, and other cytokine expression, and increased macrophage infiltration.22–24 While the combined effect of these changes is higher circulating IL-6 and other proinflammatory cytokine levels, the origin of these biomarkers in adipose tissue rather than from activated immune cells at other sites in the body may have implications for interpreting the relationship between biomarker levels and clinical outcomes.
Higher sCD14 levels in HIV-infected individuals have been attributed to increased microbial translocation across the bowel wall, and in the SMART cohort higher sCD14 levels were associated with increased hsCRP levels and risk of mortality, a finding that could be related to the direct effects of the sCD14 and endotoxin complex on endothelial cells or the stimulation of proinflammatory cytokines from immune cells.17,25 CD14 is a glycoprotein that mediates the interaction of lipopolysaccharide (LPS), a major constituent of bacterial endotoxin, with monocycles, macrophages, and other immune cells, and circulating levels of sCD14 approximate the membrane bound form (mCD14) upregulated in the presence of toxin.25–28 In the context of prior reports, our findings were unusual as we observed that sCD14 decreased while hsCRP increased among heavier subjects, while in the SMART cohort hsCRP increased in direct proportion to sCD14. We attribute this difference in the sCD14–hsCRP relationship to our observational cohort study design versus the nested case-control design of the SMART analysis, which matched deceased subjects (who appeared to have distinct serum biomarker profiles) with controls. Of note, our results are consistent with a recent study of immune cell subsets in healthy lean, overweight, and obese HIV uninfected adults, which found CD14+ monocyte counts were lowest in obese subjects and negatively associated with BMI.29 The inverse relationship between adiposity and sCD14 levels observed in our cohort may be due to fewer circulating monocytes (i.e., fewer cells available for activation) or differences in microbial translocation across the bowel, the sCD14 to mCD14 ratio, or the rate of endotoxin clearance, among other factors, between obese and nonobese participants.
Circulating MIP-1α levels were significantly higher among obese participants in our cohort, but the clinical significance of this finding to HIV disease progression and treatment outcomes is unclear. Higher serum MIP-1α may reflect increased gene expression of this cytokine from adipose tissue depots, as previously reported in studies of both obese HIV-uninfected individuals and nonobese HIV-infected individuals with ART-associated lipodystrophy, or other cellular sources including T cells and macrophages.21,30–32 Increased expression of MIP-1α in adipose tissue promotes immune cell infiltration and in situ inflammation, and there is some evidence that MIP-1α, along with MIP-1β and RANTES, can inhibit CCR5-tropic HIV-1 infection of CD4+ T cells, macrophages, and dendritic cells.33–35 This raises the possibility that the higher serum levels of MIP-1α in obese individuals could affect HIV-1 entry or replication in peripheral CD4+ T cells, but current published data on the relationship between serum MIP-1α and HIV replication or the response to ART are conflicting.36–38 We hypothesize that the increased circulating MIP-1α derives from adipose deposits, but further studies are needed to determine whether higher serum levels affect the HIV disease state or are simply a reflection of excess adiposity.
The primary limitation of our study was the cross-sectional design and lack of clinical endpoints necessary to assess the relationship between serum biomarker levels and health outcomes among obese participants. Our cohort had a higher proportion of females and nonwhite individuals in the upper BMI range, a disparity also reported in other studies, and while our analyses adjusted for age, sex, and race, additional confounders may have been present.39 Additionally, the self-reported use of tobacco, a proinflammatory stimulus, and aspirin or NSAIDS, two antiinflammatory agents, may have been subject to recall bias. Our study combined stored samples from two separate research cohorts and while the same inclusion criteria were used for all subjects, samples were collected contemporaneously, laboratory assays were performed in a single batch (excluding CRP, which was processed at two sites using the same analyzer platform), and all regression models were adjusted for the data source, there may have been additional unrecognized confounders. A future study might address these issues by using a prospective design, additional clinical evaluations (e.g., for other infectious, allergic, or rheumatologic conditions that promote inflammation), exclusion of patients using any antiinflammatory medications, and larger sample size with adequate numbers of participants in each BMI category to permit detailed subgroup analyses.
In summary, we observed that the relationship between BMI and circulating levels of inflammation biomarkers differed between obese and nonobese HIV-infected individuals on long-term ART, a finding that may have important implications for the use of these biomarkers as predictors of mortality and morbidity risk. In particular, serum levels of IL-6, an emerging predictor of mortality and cardiovascular disease in treated HIV, rose markedly among the obese, but prior studies of adipocyte biology suggest that much of the circulating IL-6 may originate from adipose tissue deposits rather than, for example, reactive inflammatory processes accompanying end-organ damage. At present, the clinical relevance of higher inflammation biomarker levels observed in obese individuals should not be extrapolated from prior studies of predominantly nonobese individuals. As the prevalence of obesity continues to rise among HIV-infected persons, further studies are needed to characterize health outcomes in this population and identify serum biomarkers most useful for risk stratification.
Acknowledgments
This work was supported by an NIH/NIAID Career Development Award (Grant K23 100700) to J.K., an NIH/NCCAM Career Development Award (Grant K23 AT002508) to T.H., the Vanderbilt Meharry Center for AIDS Research (Grant P30 AI54999), a Vanderbilt Diabetes Research and Training Center Pilot and Feasibility Award (supported by NIH Grant P60 DK020593), a K24 award to T.S. (NIH Grant A1065298), and a CTSA award (Grant UL1TR000445) from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. Additional support for statistical analyses was provided by an award to the Vanderbilt Multidisciplinary Clinical Research Center (NIH Grant P60 AR056116).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Amorosa V. Synnestvedt M. Gross R, et al. A tale of 2 epidemics: The intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39(5):557–561. [PubMed] [Google Scholar]
- 2.Crum-Cianflone N. Tejidor R. Medina S. Barahona I. Ganesan A. Obesity among patients with HIV: The latest epidemic. AIDS Patient Care STDS. 2008;22(12):925–930. doi: 10.1089/apc.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakey WC. Yang LY. Yancy W. Chow SC. Hicks CB. From wasting to obesity: Initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 2013;29(3):435–440. doi: 10.1089/aid.2012.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DJ. Westfall AO. Chamot E, et al. Multimorbidity patterns in HIV-infected patients: The role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr. 2012;61(5):600–605. doi: 10.1097/QAI.0b013e31827303d5. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prospective Studies Collaboration. Whitlock G. Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Heart L, and Blood Institute (NHLBI) The Evidence Report: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services; Bethesda, MD: 1998. NIH Publication No. 98-4083. [Google Scholar]
- 7.Brown TT. Tassiopoulos K. Bosch RJ. Shikuma C. McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33(10):2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triant VA. Meigs JB. Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuller LH. Tracy R. Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samaras K. Gan SK. Peake PW. Carr A. Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 2009;17(1):53–59. doi: 10.1038/oby.2008.500. [DOI] [PubMed] [Google Scholar]
- 11.Koethe JR. Bian A. Shintani AK, et al. Serum leptin level mediates the association of body composition and serum C-reactive protein in HIV-infected persons on antiretroviral therapy. AIDS Res Hum Retroviruses. 2012;28(6):552–557. doi: 10.1089/aid.2011.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reingold J. Wanke C. Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: The fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48(2):142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skurk T. Alberti-Huber C. Herder C. Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 14.Bruun JM. Lihn AS. Pedersen SB. Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90(4):2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 15.Jernas M. Palming J. Sjoholm K, et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20(9):1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 16.Duprez DA. Neuhaus J. Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandler NG. Wand H. Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boger MS. Shintani A. Redhage LA, et al. Highly sensitive C-reactive protein, body mass index, and serum lipids in HIV-infected persons receiving antiretroviral therapy: A longitudinal study. J Acquir Immune Defic Syndr. 2009;52(4):480–487. doi: 10.1097/qai.0b013e3181b939e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg SP. McCann D. Desai M. Rosenbaum M. Leibel RL. Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancello R. Henegar C. Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH. Nair S. Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: Increased expression of inflammation-related genes. Diabetologia. 2005;48(9):1776–1783. doi: 10.1007/s00125-005-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lihn AS. Richelsen B. Pedersen SB, et al. Increased expression of TNF-alpha, IL-6, and IL-8 in HALS: Implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab. 2003;285(5):E1072–1080. doi: 10.1152/ajpendo.00206.2003. [DOI] [PubMed] [Google Scholar]
- 23.Bastard JP. Caron M. Vidal H, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359(9311):1026–1031. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 24.Jan V. Cervera P. Maachi M, et al. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther. 2004;9(4):555–564. [PubMed] [Google Scholar]
- 25.Brenchley JM. Price DA. Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 26.Wright SD. Ramos RA. Tobias PS. Ulevitch RJ. Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 27.Ulevitch RJ. Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 28.Landmann R. Knopf HP. Link S. Sansano S. Schumann R. Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64(5):1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viardot A. Heilbronn LK. Samocha-Bonet D. Mackay F. Campbell LV. Samaras K. Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev. 2012;28(5):447–454. doi: 10.1002/dmrr.2302. [DOI] [PubMed] [Google Scholar]
- 30.Sevastianova K. Sutinen J. Kannisto K. Hamsten A. Ristola M. Yki-Jarvinen H. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2008;295(1):E85–91. doi: 10.1152/ajpendo.90224.2008. [DOI] [PubMed] [Google Scholar]
- 31.Canque B. Rosenzwajg M. Gey A. Tartour E. Fridman WH. Gluckman JC. Macrophage inflammatory protein-1alpha is induced by human immunodeficiency virus infection of monocyte-derived macrophages. Blood. 1996;87(5):2011–2019. [PubMed] [Google Scholar]
- 32.Annunziato F. Galli G. Nappi F, et al. Limited expression of R5-tropic HIV-1 in CCR5-positive type 1-polarized T cells explained by their ability to produce RANTES, MIP-1alpha, and MIP-1beta. Blood. 2000;95(4):1167–1174. [PubMed] [Google Scholar]
- 33.Uguccioni M. D'Apuzzo M. Loetscher M. Dewald B. Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1 alpha and MIP-1 beta on human monocytes. Eur J Immunol. 1995;25(1):64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 34.Cocchi F. DeVico AL. Garzino-Demo A. Arya SK. Gallo RC. Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+T cells. Science. 1995;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 35.Scarlatti G. Tresoldi E. Bjorndal A, et al. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3(11):1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie SW. Dallalio G. North M. Frame P. Means RT:, Jr Serum chemokine levels in patients with non-progressing HIV infection. AIDS. 1996;10(9):F29–33. doi: 10.1097/00002030-199610090-00001. [DOI] [PubMed] [Google Scholar]
- 37.Krowka JF. Gesner ML. Ascher MS. Sheppard HW. Lack of associations of chemotactic cytokines with viral burden, disease progression, or lymphocyte subsets in HIV-infected individuals. Clin Immunol Immunopathol. 1997;85(1):21–27. doi: 10.1006/clin.1997.4411. [DOI] [PubMed] [Google Scholar]
- 38.Ye P. Kazanjian P. Kunkel SL. Kirschner DE. Lack of good correlation of serum CC-chemokine levels with human immunodeficiency virus-1 disease stage and response to treatment. J Lab Clin Med. 2004;143(5):310–319. doi: 10.1016/j.lab.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Buchacz K. Baker RK. Palella FJ, Jr, et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther. 2013;18(1):65–75. doi: 10.3851/IMP2450. [DOI] [PubMed] [Google Scholar]