Abstract
Antiretroviral therapy in resource-limited settings is monitored clinically and immunologically according to WHO guidelines. Frequent misclassification of virologic failure is reported, mostly in adults, leading to early therapy switch or late failure diagnosis. Pediatric treatment monitoring and resistance data upon first-line failure are limited, particularly when the 2010-WHO pediatric guidelines are used without routine viral load monitoring. We previously reported high treatment failure misclassification rates by pediatric 2010 guidelines in Cambodian children on first-line therapy. Here we determine the extent and patterns of resistance, with yearly viral load and 6-monthly CD4. Drug resistance mutations were determined using the IAS-USA 2011 list. Predicted resistance interpretation was determined with Stanford Database tools. Fifty-one children with available genotypes met inclusion criteria. All but one (subtype B) were CRF01_AE. The most common regimen was stavudine, lamivudine, and nevirapine (96%), taken for a median of 2.2 years. Resistance was seen in 98%; 96% to nucleoside and nonnucleoside reverse transcriptase inhibitors (NRTIs and NNRTIs); 51% with ≥4 mutations. The most common NRTI mutations were 184V/I and 67N and the most common NNRTI mutations were 181C/Y/I/V and 190A/S. A total of 22% had multiresistant mutations and 18% had predicted high-level resistance to subsequent therapy options didanosine, abacavir, etravirine, and tenofovir. In 98% of Cambodian children misclassified as nonfailing first-line therapy by 2010 guidelines, 51% had extensive drug resistance to current and 18% to subsequent antiretroviral therapy. Affordable routine viral load monitoring allowing for early and more accurate treatment failure diagnosis is desperately needed in resource-limited settings.
Introduction
Antiretroviral therapy (ART) in resource-limited settings (RLS) is typically monitored clinically and/or immunologically, according to World Health Organization (WHO) adult1 and pediatric2 guidelines. Frequent misclassification of virological failure in adult patients has been reported, leading to early ART switch or late failure diagnosis.3–5 In the latter circumstances, by the time treatment failure is recognized, patients may have already accumulated drug resistance mutations and high-level cross-resistance to subsequent antiretroviral regimens.6–8 Minimizing resistance is particularly important in RLS with limited ART options, usually restricted to first-line nonnucleoside reverse transcriptase inhibitor (NNRTI)-based and second-line protease inhibitor (PI)-based regimens.
In children failing ART, there are limited data on treatment monitoring and its association with drug resistance, particularly when recently published 2010-WHO pediatric guidelines are used without routine viral load (VL) monitoring.7,9,10 Children are twice as likely to experience virological failure to ART compared with adults.11 Furthermore, a large proportion of HIV-infected children (13–53%) are expected to experience virological failure within the first year of treatment and are therefore at a higher risk of developing drug resistance if failure is diagnosed late.7,10,12–14 Such resistance may have important consequences for second-line treatment strategies, particularly in children with reduced treatment options and formularies.
With increased global access to ART in countries where HIV-1 non-B subtypes predominate, it is important to investigate drug resistance patterns, their association with subtype genotypic variation, and their impact on optimum ART strategies. Mounting evidence suggests that naturally occurring polymorphisms among non-B subtypes can influence susceptibility to antiretroviral drugs,15 such as the higher incidence of nevirapine-resistant mutations K103N and Y181C in subtype C from South Africa16,17 or the thymidine analogue mutations (TAM) pathway variation18 observed in subtype C from Botswana.19 Most of these studies focus on adults, and whether specific pediatric variations exist is less clear.7,20
CRF01_AE is the most common HIV variant in Southeast Asia, accounting for 5% of all global HIV-1 infections.21 Data are limited on the patterns and extent of drug resistance mutations in adults and children from this region. Most first-line failure data in this HIV subtype are reported from Thailand, with NNRTI and NRTI resistance of 89–95% and 42–58% in adults and 97% and 98% in children, respectively.13,22–27
Data on drug resistance in Cambodian children failing ART are limited. In a recent systematic review of first-line ART failure among children,28 within 30 reviewed studies on 3,241 children, only 265 (8%) were from Cambodia and only 74 (2%) of those had genotypes available, some available only in poster form and after second-line ART failure. In the three available pediatric studies from Cambodia, reporting data from children on first-line ART with detectable VL and available genotypic resistance testing, 94% (34/36),29 100% (21/21),30 and 100% (2/2)31 had drug-resistant mutations to NRTI and NNRTI without specific reports on resistance patterns or extent.
We previously evaluated the WHO-2010 pediatric ART monitoring guidelines in 457 Cambodian children on first-line ART and reported misclassification rates of 33–40% among children with suppressed VL and of 12–24% among children with virological failure, depending on WHO stage.9 The majority of these children (98%) for whom genotypes were available had high levels of ART drug resistance and were not identified as having virological failure by the WHO guidelines. Here we further characterize the extent and patterns of drug resistance and their correlates in these children, mostly infected with CRF01_AE, and investigate resistance accumulation and the potential impact of such missed or late detection of drug resistance on second-line ART.
Materials and Methods
Study setting
This retrospective cohort study was performed at Angkor Hospital for Children (AHC) in Siem Reap, Cambodia, one of 32 Pediatric AIDS Care sites recognized by the National Center for HIV/AIDS, Dermatology, and Sexually Transmitted Diseases (NCHADS). A nongovernmental organization-funded pediatric referral center, AHC had provided free care to a total of 913 HIV-infected children through September 2010. ART became available at AHC in 2003, and as of January 2011, 502 children had received ART. Children are seen every 8 weeks; WHO stage and medications are recorded in an electronic database at each visit. CD4 count and/or percent are determined every 6 months and routine VL is performed annually, as well as when immunological and/or clinical failure is suspected based on WHO and national guidelines as previously described.9 In viremic children (>1,000 copies/ml) whose clinicians were considering second-line ART, viral load was confirmed followed by drug resistance testing, with confirmed ART adherence by self-report of children and/or caregivers.
Patient cohort and laboratory testing
The inclusion criteria for this study were (1) HIV-infected children cared for at AHC between January 2005 and September 2010; (2) less than 15 years old; (3) on at least 6 months of first-line ART (zidovudine or stavudine + lamivudine + nevirapine or efavirenz); and (4) had HIV genotyping as described above. Second-line regimens in this setting include lopinavir/ritonavir with at least two NRTIs. CD4 measurements, VL, and genotyping were obtained as previously described.9 Briefly, CD4 measurements were performed at various laboratories over the study period, including the Cambodian Ministry of Health Reference Laboratory (Kampong Cham), the National Institute of Public Health (Phnom Penh), and the Pasteur Institute of Cambodia (IPC, Phnom Penh), by Becton Dickinson FACSCount, BD Biosciences, San Jose, CA. VL and genotyping were performed at IPC (Generic VL, Biocentric, Bandol, France).
Data analysis
Sequence alignment and quality assurance were performed by SQUAT.32 Sequence and phylogenetic analyses were done with BioEdit version 733 and Mega5.34 Subtyping was determined with the REGA tool.35 Resistance interpretation and susceptibility prediction were done according to the IAS-USA 2011 mutation list36 and with Stanford HIV Sequence Database tools (hivdb.stanford.edu). Extensive drug resistance was defined as ≥4 mutations from any drug class. Multidrug resistance was defined as the presence of K65R, Q151M, or at least three TAMs.36
Statistical analysis
Children were placed into ordinal groupings according to the number of NRTI and NNRTI mutations present. Proportional odds ordinal logistic regression was used to examine the association between key clinical covariates (VL, CD4, age, time on ART, and WHO stage) and the number of mutations. p values below 0.05 were considered statistically significant. All analysis was done using R version 2.14.2.37 FASTA sequences were submitted to GenBank with the following accession numbers (KC533594–KC533651).
Results
Patient characteristics
Fifty-one children met inclusion criteria and were included in analyses (Table 1). In this cohort 65% were male with a median age of 8 years (range 1.9–14.8), with the majority older than 5. According to WHO classification, 90% had disease stages I or II, with median CD4% 21 (range 5–36) and median VL 24,792 copies/ml (range 400–1,210,000). The most common ART regimen was stavudine + lamivudine + nevirapine (96%), taken for a median of 2.2 years (range 1.0–5.4). Based on the immunological criteria in the 2010-WHO pediatric guidelines, 50/51 (98%) of these children were not predicted to be failing ART, having CD4 counts above 100 cells/ml if older than 5 years, or CD4 counts above 200 cells/ml and CD4% above 10% if younger than 5 years, as we previously reported.9 The immunological and resistance characteristics of the one child that was identified as failing by the WHO guidelines were previously reported.9 All but one (subtype B) were CRF01_AE. Seven patients had a follow-up genotype available while on the same regimen.
Table 1.
Demographic, Clinical, and Laboratory Patient Characteristics
| Variable | Value |
|---|---|
| Number of children | 51 |
| Gender: male (%) | 33/51 (65%) |
| Age (years) at sequence; median (range) | 8 (2, 14) |
| <2 | 1/51 (2%) |
| ≥2 and <5 | 7/51 (14%) |
| ≥5 | 43/51 (84%) |
| Age (years) at HIV diagnosis; median (range) | 5 (0.6, 10) |
| Current WHO stage | |
| 1 | 40/51 (78%) |
| 2 | 6/51 (12%) |
| 3 | 4/51 (8%) |
| 4 | 1/51 (2%) |
| Current CD4 count: median (range) | 680 (94, 3341) |
| Current CD4%: median (range) | 21 (5, 36) |
| Current VL copies/ml: median (range) | 24,792 (400 to 1,210,000) |
| 400 < VL ≤ 1,000 | 5/51 (10%) |
| 1,000 < VL ≤ 5,000 | 4/51 (8%) |
| VL>5,000 | 42/51 (82%) |
| Regimen | |
| d4T, 3TC, NVP | 49/51 (96%) |
| d4T, 3TC, EFV | 1/51 (2%) |
| 3TC, NVP, AZT | 0/51 (0%) |
| 3TC, EFV, AZT | 1/51 (2%) |
| Years on HAART: median (range) | 2.2 (1.0, 5.4) |
VL, viral load; d4T, stavudine; 3TC, lamivudine; NVP, nevirapine; EFV, efavirenz; AZT, zidovudine; HAART, highly active antiretroviral treatment.
Genotypic drug resistance
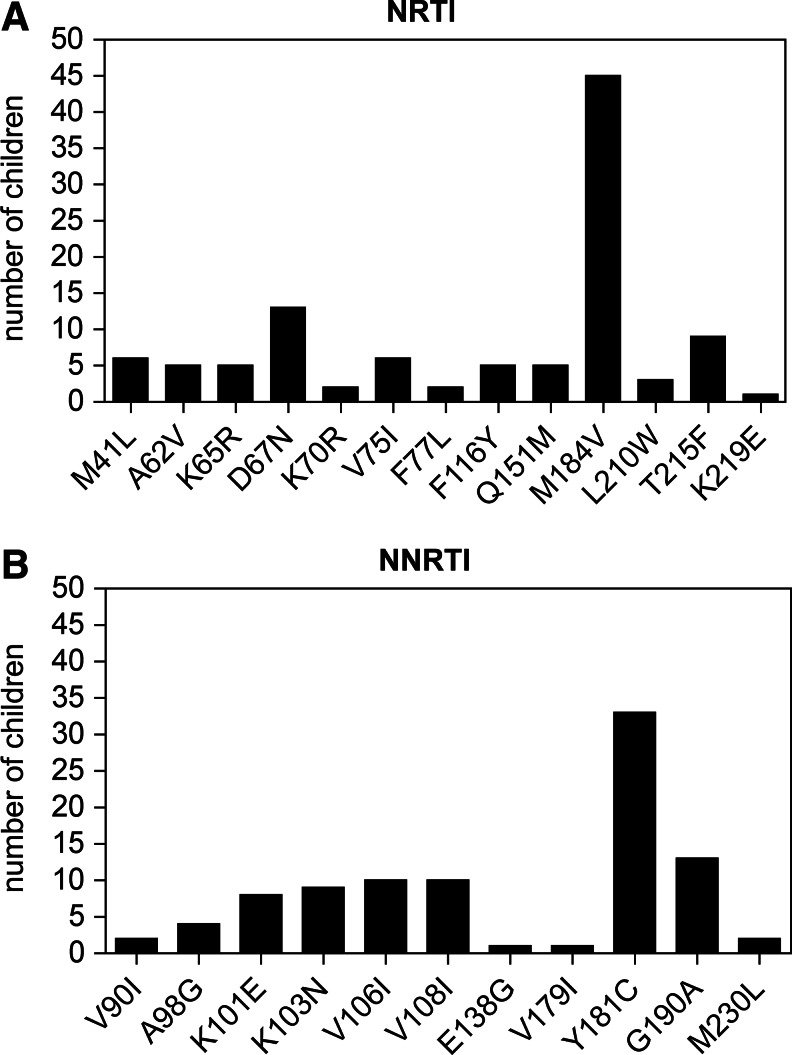
The majority of children (50/51; 98%) had drug resistance in a wide range of patterns and severity (Table 2 and Fig. 1). All but two children had at least one and up to seven NRTI-associated mutations. The most common NRTI mutations were 184V/I (45/51, 88%) and 67N (13/51, 25%). One to four NNRTI mutations were observed in all but one child with the most common NNRTI mutations being 181C/Y/I/V (33/51, 65%) and 190A/S (13/51, 25%). Twenty-six children (51%) had ≥4 resistance mutations to both NRTI and NNRTIs, with up to nine drug resistance mutations identified within some patients. Multidrug resistance mutations (K65R, Q151M, or at least three TAMs) were present in 11 (21%) children. TAMs associated with only pathway-2 (67N, 70R, 215F, and 219E) were observed in 14 patients (27%) and six patients (12%) had only pathway-1 mutations (41L, 67N, 210W, and 215Y).18 Two samples had a “combined” pathway, including mutations 215F (TAM-2) and 210W (TAM-1). Only two patients (4%) had either one or no resistance mutations despite failing ART, suggesting that most children in this cohort were to some extent compliant rather than completely noncompliant with their ART. Each child was placed into one of three ordinal groups defined as (1) 0 to 2 mutations, (2) 3 to 4 mutations, and (3) >4 mutations. Higher VL [OR=4.0 (95% CI 1.7, 9.2) per log10 higher VL, p<0.01], lower CD4 count [OR=1.3 (95% CI 1.1, 1.5) per 100 cell lower CD4, p<0.01], and lower CD4% [OR=1.1 (95% CI 1.0, 1.2) per 1% lower CD4%, p=0.02] were associated with more resistance mutations. Age, time on ART, and WHO stage were not found to be associated with the number of resistance mutations.
Table 2.
Nonnucleoside Reverse Transcriptase Inhibitor and Nucleoside Reverse Transcriptase Inhibitor Drug-Resistant Mutations and Individual Patient Demographics
| Patient ID | NRTI | NNRTI | CD4 | Viral load |
|---|---|---|---|---|
| <2 years | CD4% | |||
| 1 | M184V | A98G, K101E, Y181C | na | 336,845 |
| 2 to <5 years | CD4% | |||
| 2 | M184V, T215F | V108I, Y181C | na | 2,710,000 |
| 3 | D67DN, M184V | A98G, Y181C | 19 | 21,393 |
| 4 | D67N, M184V | K103N | na | 33,244 |
| 5 | M184V | K101E, G190A | 32 | 35,037 |
| 6 | M184V | K101E, Y181C | na | 37 |
| 7 | M184V | K103N, V108I | 32 | 51,860 |
| 8 | M184V | Y181C | 20 | 591,096 |
| ≥5 years | CD4 count | |||
| 9 | A62V, D67N, V75I, F77L, F116Y, Q151M, M184V | K101E, G190A | 549 | 58,287 |
| 10 | D67N, K70R, M184V, T215F, K219E | A98G, V108I, Y181C | na | 32,990 |
| 11 | M41L, D67N, M184V, L210W, T215Y | V106I, G190A | na | 786,933 |
| 12 | A62V, V75I, F116FY, Q151M, M184V | E138G, G190A | 701 | 267,409 |
| 13 | D67N, M184V, L210W, T215F | K101E, G190S | 380 | 69,149 |
| 14 | D67N, F77FL, F116Y, Q151M, M184V | Y181C | 878 | 14,940 |
| 15 | A62V, M184I, L210W, T215F | V90I, Y181C | 856 | 87,104 |
| 16 | D67N, M184V, T215Y | K101E, G190A | 423 | 57,093 |
| 17 | M41L, K65R, M184V | V106I, Y181C | 1,018 | 227,554 |
| 18 | V75IV, F116Y, Q151M, M184V | K103N | 422 | 27,274 |
| 19 | K65R, F116Y, Q151M | K103N, Y181C | 130 | 1,070,000 |
| 20 | M41LM, M184V | V106I, Y181CY, G190AG | 758 | 11,085 |
| 21 | M184V | K103N, V106I, V108I, V179IT | 1,042 | 0 |
| 22 | A62V, V75I, M184V | Y181C | na | 16,347 |
| 23 | K70KR, M184V | K103N, Y181C | 727 | 7,448 |
| 24 | K65R, D67N | Y181C, G190A | 321 | 101,467 |
| 25 | V75I, M184I | V108I, Y181C | 1,383 | 400 |
| 26 | M184V, T215F | V108I, Y181C | 332 | 42,378 |
| 27 | M41LM, V75I, M184V | G190A | 2,236 | 9,533 |
| 28 | D67DN, M184V | V90I, Y181C | 820 | 400 |
| 29 | M184V, T215Y | V106IV, Y181C | 225 | 112,223 |
| 30 | D67N, M184V | V106I, Y181V | 644 | 7,605 |
| 31* | M184V | K103N, V106I, M230L | 818 | 3,627 |
| 32 | M184V | V108I, Y181C | 1,274 | 250 |
| 33 | D67DN, M184V | Y181C | 971 | 95,874 |
| 34 | D67DN, M184V | G190A | na | 0 |
| 35 | M184V | V108I, Y181C | 256 | 2,111 |
| 36 | M184V | Y181C, G190A | na | 26,041 |
| 37 | K65R | V106I, Y181C | 523 | 400 |
| 38 | K65KR | K103KN, Y181CY | 409 | na |
| 39 | M184V, T215FS | Y181C | na | 250 |
| 40 | M184V | K101E, G190A | 394 | 24,792 |
| 41 | M184V | A98G, Y181C | 620 | 13,992 |
| 42 | M184V | V108I, Y181C | 567 | 3,985 |
| 43 | A62V M184V | G190A | 1,545 | 32,362 |
| 44 | M184V | V108I, Y181C | 464 | 16,353 |
| 45 | M184V | K101EK, Y181C | 275 | 12,161 |
| 46 | M184V | V106I, Y181C | 721 | 11,199 |
| 47 | M184V | Y181C | na | 38,697 |
| 48 | M184V | Y181C | 3,341 | 10,098 |
| 49* | M184MV | K103KN | 849 | 3,783 |
| 50# | none | V106I | na | 2,582 |
| 51 | none | none | 962 | 400 |
Samples are grouped according to the WHO age classification and are listed based on the total number of reported resistance mutations. All patients were on a stavudine+amivudine+nevirapine regimen, except otherwise indicated (*), and classified as HIV-1 CRF_AE subtype, except otherwise indicated (#). Data not available are indicated (na).
NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitors.
FIG. 1.
Number of nucleoside reverse transcriptase inhibitor (NRTI) (A) and nonnucleoside reverse transcriptase inhibitor (NNRTI) (B) resistance mutations in children failing first-line antiretroviral therapy (ART).
Predicted drug resistance
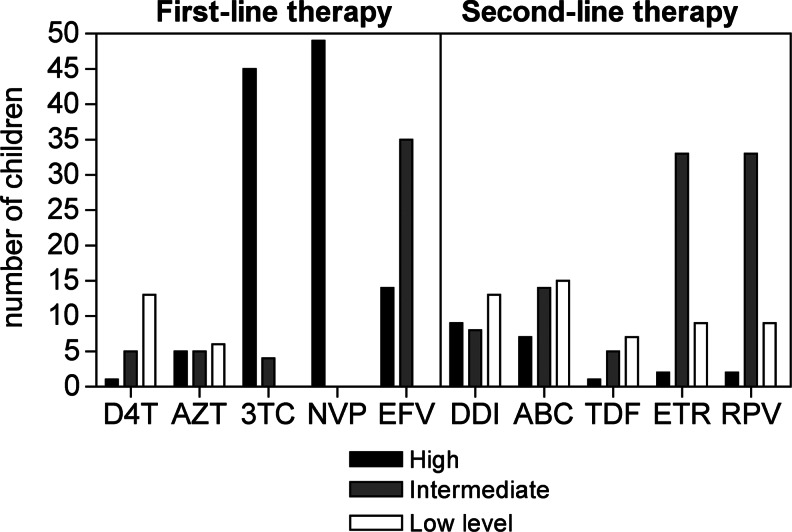
As expected, most children failing first-line ART regimens had high-level predicted drug resistance to lamivudine (45/51, 88%, 95% CI 76–96%) and nevirapine (49/51, 96%, 95% CI 87–100%) (Fig. 2). Forty children (78%, 95% CI 65–89%) had predicted intermediate or high-level drug resistance to second-line ART, with 22 (43%, 95% CI 29–58%) resistant to any second-line NRTIs including didanosine, abacavir, and tenofovir; 35 (69%, 95% CI 54–81%) to any second-line NNRTIs including etravirine and rilpivirine; and 19 (37%, 95% CI 24–52%) to both second-line NNRTIs and NRTIs.
FIG. 2.
Predicted drug susceptibility for first-line and second-line ART.
HIV drug resistance accumulation
In seven Cambodian children for whom follow-up sequences were available while on the same ART (Table 3) the median time between sequences was 4.2 months (range 2.1–20.5). Four (57%) of the patients accumulated new drug resistance mutations during that time, six NRTI associated and two NNRTI associated. The acquired mutations in two patients (patient 24 and 26 in Table 3) suggest a TAM-2 pathway and in one (patient 30) a TAM-1 pathway. These mutations increased predicted drug resistance to potential second-line drugs such as etravirine and rilpivirine (patient 11), abacavir (patient 24), and didanosine and tenofovir (patient 26).
Table 3.
Accumulation of Drug Resistance Mutations Over Time on a Failing First-Line Regimen
| Patient | Sample | Months between sample | NRTI | NNRTI |
|---|---|---|---|---|
| 11 | 1 | M41L, D67N, M184V, L210W, T215Y | V106I, G190A | |
| 2 | 20.4 | M41L, D67N, M184V, L210W, T215Y | V106I, Y181C,* G190A | |
| 22 | 1 | A62V, V75I, M184V | Y181C | |
| 2 | 4.2 | A62V, V75I, M184V | Y181C | |
| 24 | 1 | K65R, D67N | Y181C, G190A | |
| 2 | 4 | K70R,* M184V,* K219E* | Y181C, G190A | |
| 26 | 1 | M184V, T215F | V108I, Y181C | |
| 2 | 2.1 | M41L,* M184V, T215F | V108I, Y181C | |
| 30 | 1 | D67N, M184V | V106I, Y181V | |
| 2 | 7.2 | M41L,* D67N, M184V, T215Y* | V106I, V108IV,* Y181V | |
| 35 | 1 | M184V | V108I, Y181C | |
| 2 | 5.5 | M184V | V108I, Y181C | |
| 45 | 1 | M184V | K101EK, Y181C | |
| 2 | 2.3 | M184V | K101E, Y181C |
New mutations observed in follow-up sample are indicated with an asterisk (*).
Discussion
We report extensive drug resistances in 51 Cambodian children infected mostly with HIV-1 CRF01_AE and failing first-line ART, despite being classified as nonfailing based on WHO-2010 guidelines. All but two children (98%) had developed up to nine drug resistance mutations to NRTIs and/or NNRTIs and 51% to both. In 21% those mutations were multidrug resistant conferring cross-resistance to other NRTIs and 80% had intermediate or high-level predicted resistance to second-line medications. A higher number of resistance mutations was associated with higher VL and lower CD4 values. Continuation of failing ART regimens resulted in resistance accumulation in 57% of children, further increasing predicted resistance to subsequent regimens.
Although the use of NNRTI-based first-line regimens in adults and children has been established as effective and safe, it can be limited by rapid development of high-level resistance in circumstances such as low adherence or treatment interruptions. This is an important consequence to consider, particularly in children who will require ART for long periods of time. According to reviewed pediatric data from RLS28 and specifically from Cambodia29–31 and Thailand,13,22,23 57–100% of children failing first-line NNRTI-based ART had extensive NRTI and NNRTI resistance, with most common NRTI mutations M184V/I and D67N, and NNRTI mutations Y181C and G190A. Our results confirm and extend these observations, contribute to the limited genotypic data available for children failing first-line therapy in Cambodia, and emphasize the risk of the development of extensive drug resistance when guidelines that are not based on routine VL monitoring are used.
Multidrug resistance mutations after first-line ART failure have been reported in 23–38% of CRF01_AE-infected Thai children.22,23 Similar information from Cambodia is limited. In prior reports from Cambodia before 2007, extensive resistance was reported at 14% (5/36) after 12 months of first-line ART29 and at 27% (6/22) after 24 months.30 Despite the observed effectiveness of ART in these studies, with such little data, the extent of resistance and its effect on subsequent regimens in Cambodian CRF01_AE-infected children are not known.
In this study we extend these findings and report that 21% of children carry multidrug resistance that included K65R, Q151M, or at least three TAMs. Collectively these findings highlight the development of extensive and complex drug resistance mutations when children on ART are monitored clinically, immunologically, or even with annual VL testing. NRTI and NNRTI mutation cooccurrence and the extent of per patient resistance and cross-resistance described here further highlight the need for increased awareness of the potential consequences of such resistance in children in RLS.
Data presented here extend the evidence for diverse resistance patterns in HIV-1 subtypes and recombinant forms. We observed a more prevalent TAM-2 compared to the TAM-1 pathway, similar to other reports in CFR01_AE-infected adults and children.26,38 These are in contrast to subtype B, where the TAM-1 pathway is twice as frequent as TAM-2.18 TAMs are clinically important because they influence the virological response to some NRTIs and a specific rather than random mutation accumulation. The factors that drive the development toward a specific TAM pathway are still unclear and are probably influenced by host and virus, but even less is known about the development of TAM pathways in non-B subtype-infected patients, particularly children. To our knowledge this is the first study to observe “combined” TAM-2 pathway (215F) and TAM-1 pathway (210W) associated mutations in this setting. Such variation has been reported in an adult subtype C cohort failing zidovudine/didanosine-containing ART in Botswana,19 but additional data are needed to determine if such variation is common in CRF01_AE-infected children and its effect.
The suggested second-line regimen in the Cambodian setting, similar to other RLS, includes lopinavir/ritonavir with at least two NRTIs. This second-line regimen is associated with a high rate of virological suppression and immune reconstitution after 24 months of follow-up in adults from Cambodia,39 but similar data are lacking for children in Cambodia. Predicted drug resistance from data presented here indicates intermediate or high-level resistance in 80% of children to second-line NNRTIs and/or NRTIs. Despite good virological response to second-line ART in children,40 this is a major concern, particularly due to the need for life-long treatment and the limited available subsequent drugs, restricting treatment management in these RLS.
In addition to the limitations previously discussed for this cohort,9 which include the paucity of children younger than 2, no specific adherence information, and the absence of CD4 and VL data for some patients, our analysis used population-based sequencing, and potentially existing minority resistance variants were not detected. Moreover, we had only genotypic, without phenotypic, resistance estimation. This study is also a cross-sectional analysis with very few follow-up samples that might assist in understanding the systematic development of drug resistance in children. Given these limitations, this study highlights the need for larger investigations focusing on pediatric cohorts in these settings monitored using WHO guidelines.
In conclusion, this study demonstrates high prevalence, extensive patterns, and accumulation of drug resistance in Cambodian children infected with HIV-1 CRF01_AE who are failing first-line ART, particularly with higher VL and lower CD4 values. The majority (98%) of these children were not identified as failing by current pediatric monitoring guidelines, and thus they were not suspected of having drug resistance, and would have remained unidentified without routine virological monitoring. These data emphasize that misclassification of treatment failure using WHO-2010 pediatric guidelines leads to an inability for early detection of treatment failure with resulting extensive drug resistance and predicted high-level cross resistance. The resulting limited second-line therapy options available to children reinforces the belief that affordable routine VL monitoring is desperately needed in RLS. Further research is warranted to better define resistance pathways in diverse HIV-1 subtypes, particularly in children.
Acknowledgments
This study was supported by the Brown University Framework in Global Health Program (National Institutes of Health Grant R25-TW008102). Drs. Coetzer and Kantor and Ms. DeLong and Ms. Schreier are supported by National Institutes of Health Grants RO1-AI66922 and P30AI042853.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. 2010. [PubMed]
- 2.WHO. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access; Recommendations for a Public Health Approach. 2010. [PubMed]
- 3.Hosseinipour MC. van Oosterhout JJ. Weigel R, et al. The public health approach to identify antiretroviral therapy failure: High-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore DM. Awor A. Downing R, et al. CD4+ T-cell count monitoring does not accurately identify HIV-infected adults with virologic failure receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;49(5):477–484. doi: 10.1097/QAI.0b013e318186eb18. [DOI] [PubMed] [Google Scholar]
- 5.Kantor R. Diero L. Delong A, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Infect Dis. 2009;49(3):454–462. doi: 10.1086/600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DM. Schooley RT. Running with scissors: Using antiretroviral therapy without monitoring viral load. Clin Infect Dis. 2008;46(10):1598–1600. doi: 10.1086/587110. [DOI] [PubMed] [Google Scholar]
- 7.Ruel TD. Kamya MR. Li P, et al. Early virologic failure and the development of antiretroviral drug resistance mutations in HIV-infected Ugandan children. J Acquir Immune Defic Syndr. 2011;56(1):44–50. doi: 10.1097/QAI.0b013e3181fbcbf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantor R. Shafer RW. Follansbee S, et al. Evolution of resistance to drugs in HIV-1-infected patients failing antiretroviral therapy. AIDS. 2004;18(11):1503–1511. doi: 10.1097/01.aids.0000131358.29586.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westley BP. Delong AK. Tray CS, et al. Prediction of treatment failure using 2010 WHO guidelines is associated with high misclassification rates and drug resistance among HIV-infected Cambodian children. Clin Infect Dis. 2012;55:432–440. doi: 10.1093/cid/cis433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kebe K. Thiam M. Diagne Gueye NR, et al. High rate of antiretroviral drug resistance mutations in HIV type 1-infected Senegalese children in virological failure on first-line treatment according to the World Health Organization guidelines. AIDS Res Hum Retroviruses. 2013;29(2):242–249. doi: 10.1089/aid.2011.0300. [DOI] [PubMed] [Google Scholar]
- 11.Kamya MR. Mayanja-Kizza H. Kambugu A, et al. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 12.Reddi A. Leeper SC. Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jittamala P. Puthanakit T. Chaiinseeard S. Sirisanthana V. Predictors of virologic failure and genotypic resistance mutation patterns in Thai children receiving non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009;28(9):826–830. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 14.Wamalwa DC. Farquhar C. Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45(3):311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wainberg MA. Brenner BG. The impact of HIV genetic polymorphisms and subtype differences on the occurrence of resistance to antiretroviral drugs. Mol Biol Int. 2012;2012:256982. doi: 10.1155/2012/256982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JA. Li JF. Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192(1):16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 17.Flys T. Nissley DV. Claasen CW, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192(1):24–29. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- 18.Marcelin AG. Delaugerre C. Wirden M, et al. Thymidine analogue reverse transcriptase inhibitors resistance mutations profiles and association to other nucleoside reverse transcriptase inhibitors resistance mutations observed in the context of virological failure. J Med Virol. 2004;72(1):162–165. doi: 10.1002/jmv.10550. [DOI] [PubMed] [Google Scholar]
- 19.Novitsky V. Wester CW. DeGruttola V, et al. The reverse transcriptase 67N 70R 215Y genotype is the predominant TAM pathway associated with virologic failure among HIV type 1C-infected adults treated with ZDV/ddI-containing HAART in southern Africa. AIDS Res Hum Retroviruses. 2007;23(7):868–878. doi: 10.1089/aid.2006.0298. [DOI] [PubMed] [Google Scholar]
- 20.Tolle M. Howard L. Kirk B. Gomila A. Schwarzwald H. Anabwani G. Reverse transcriptase genotypes in pediatric patients failing initial antiretroviral therapy in Gaborone, Botswana. J Int Assoc Physicians AIDS Care (Chic) 2012;11(4):260–268. doi: 10.1177/1545109711422273. [DOI] [PubMed] [Google Scholar]
- 21.Hemelaar J. Gouws E. Ghys PD. Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS. 2011;25(5):679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puthanakit T. Jourdain G. Hongsiriwon S, et al. HIV-1 drug resistance mutations in children after failure of first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. HIV Med. 2010;11(9):565–572. doi: 10.1111/j.1468-1293.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 23.Sungkanuparph S. Apiwattanakul N. Thitithanyanont A. Chantratita W. Sirinavin S. HIV-1 drug resistance mutations in children who failed non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Southeast Asian J Trop Med Public Health. 2009;40(1):83–88. [PubMed] [Google Scholar]
- 24.Chetchotisakd P. Anunnatsiri S. Kiertiburanakul S, et al. High rate multiple drug resistances in HIV-infected patients failing nonnucleoside reverse transcriptase inhibitor regimens in Thailand, where subtype A/E is predominant. J Int Assoc Physicians AIDS Care (Chic) 2006;5(4):152–156. doi: 10.1177/1545109706294288. [DOI] [PubMed] [Google Scholar]
- 25.Sungkanuparph S. Win MM. Kiertiburanakul S. Phonrat B. Maek-a-nantawat W. HIV-1 drug resistance at virological failure versus immunological failure among patients failing first-line antiretroviral therapy in a resource-limited setting. Int J STD AIDS. 2012;23(5):316–318. doi: 10.1258/ijsa.2011.011337. [DOI] [PubMed] [Google Scholar]
- 26.Ngo-Giang-Huong N. Jourdain G. Amzal B, et al. Resistance patterns selected by nevirapine vs. efavirenz in HIV-infected patients failing first-line antiretroviral treatment: A bayesian analysis. PLoS One. 2011;6(11):e27427. doi: 10.1371/journal.pone.0027427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manosuthi W. Butler DM. Chantratita W. Sukasem C. Richman DD. Smith DM. Patients infected with HIV type 1 subtype CRF01_AE and failing first-line nevirapine- and efavirenz-based regimens demonstrate considerable cross-resistance to etravirine. AIDS Res Hum Retroviruses. 2010;26(6):609–611. doi: 10.1089/aid.2009.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigaloff KC. Calis JC. Geelen SP. van Vugt M. de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: A systematic review. Lancet Infect Dis. 2011;11(10):769–779. doi: 10.1016/S1473-3099(11)70141-4. [DOI] [PubMed] [Google Scholar]
- 29.Janssens B. Raleigh B. Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: Evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120(5):e1134–1140. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 30.Isaakidis P. Raguenaud ME. Te V, et al. High survival and treatment success sustained after two and three years of first-line ART for children in Cambodia. J Int AIDS Soc. 2010;13:11. doi: 10.1186/1758-2652-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sophan S. Meng CY. Pean P, et al. Virologic and immunologic outcomes in HIV-infected Cambodian children after 18 months of highly active antiretroviral therapy (HAART) Southeast Asian J Trop Med Public Health. 2010;41(1):126–137. [PMC free article] [PubMed] [Google Scholar]
- 32.Delong AK. Wu M. Bennett D, et al. Sequence quality analysis tool for HIV type 1 protease and reverse transcriptase. AIDS Res Hum Retroviruses. 2012;28(8):894–901. doi: 10.1089/aid.2011.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 34.Tamura K. Peterson D. Peterson N. Stecher G. Nei M. Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Oliveira T. Deforche K. Cassol S, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21(19):3797–3800. doi: 10.1093/bioinformatics/bti607. [DOI] [PubMed] [Google Scholar]
- 36.Johnson VA. Calvez V. Gunthard HF, et al. 2011 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19(4):156–164. [PMC free article] [PubMed] [Google Scholar]
- 37.R Foundation for Statistical Computing, [computer program]. Version; Vienna, Austria: 2012. R: A language and environment for statistical computing. [Google Scholar]
- 38.Zolfo M. Schapiro JM. Phan V, et al. Genotypic impact of prolonged detectable HIV type 1 RNA viral load after HAART failure in a CRF01_AE-infected cohort. AIDS Res Hum Retroviruses. 2011;27(7):727–735. doi: 10.1089/aid.2010.0037. [DOI] [PubMed] [Google Scholar]
- 39.Ferradini L. Ouk V. Segeral O, et al. High efficacy of lopinavir/r-based second-line antiretroviral treatment after 24 months of follow up at ESTHER/Calmette Hospital in Phnom Penh, Cambodia. J Int AIDS Soc. 2011;14:14. doi: 10.1186/1758-2652-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puthanakit T. Jourdain G. Suntarattiwong P, et al. High virologic response rate after second-line boosted protease inhibitor-based antiretroviral therapy regimens in children from a resource limited setting. AIDS Res Ther. 2012;9(1):20. doi: 10.1186/1742-6405-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]