Abstract
Elevated serum levels of inflammatory biomarkers have been associated with increased mortality and morbidity among HIV-infected individuals receiving combination antiretroviral therapy (cART) in European and U.S. cohorts. Few similar data are available from sub-Saharan Africa, where most cART-treated adults reside and the prevalence of advanced immunosuppression and opportunistic infections (OIs) at cART initiation is higher. This was a retrospective nested case-control analysis of clinical trial data from the completed Adult Antiretroviral Treatment and Drug Resistance (“Tshepo”) study, 2002–2007, Gaborone, Botswana. We measured pretreatment serum levels of interleukin-6 (IL-6), high sensitivity C-reactive protein, and D-dimer in stored plasma samples from 32 deceased participants (cases) and 64 survivors (controls), matched for age, sex, baseline CD4+ cell count, and plasma HIV-1 RNA. Multivariate conditional logistic regression analyses were used to compare inflammatory biomarker levels, adjusting for pretreatment body mass index (BMI) and the presence of OIs. A total of 37 (5.7%) of 650 patients died on study, for a crude mortality rate of 20.6/1,000 person-years. Of 37 (86%) study participants who died on study 32 were included in this analysis. Causes of death (n=32) included non-AIDS-defining events (31.3%), HIV-related OIs (28.1%), cART/toxicity-related (21.9%), other infectious etiologies (15.6%), and unknown (3.1%). Median time to death was 31 weeks [interquartile range (IQR) 14–64]. Median baseline levels of all three biomarkers were higher in cases compared to matched controls. After adjusting for BMI and the presence of OIs, only baseline and most recent (near time of event) levels of IL-6 remained as significant predictors of all-cause mortality [adjusted OR (aOR)=1.25, 95% CI (1.05–1.48); p=0.012; and aOR=1.48 (1.05–2.09); p=0.027, respectively]. Serum IL-6 levels are important predictors of all-cause mortality in this adult urban sub-Saharan African cART-treated population. Future translational studies are warranted to better elucidate pathophysiology and inform the design of novel interventions to ameliorate the risk of death among these “at-risk” individuals.
Introduction
Over the past two decades, the widespread use of combination antiretroviral therapy (cART) has significantly reduced AIDS-related mortality and morbidity in resource-replete settings.1 The past decade has seen the rapid scale-up and provision of cART to eligible adults in sub-Saharan Africa, leading to dramatic increases in the quality and quantity of life among HIV-infected persons residing in the African subcontinent, where presently more than 4.0 million persons are receiving potentially life-saving cART. However, this has come at some cost as regional studies have documented higher than expected mortality rates among cART-treated adults, especially within the first 3–6 months following cART initiation.2–5 This risk has been shown to be particularly high among those having advanced immunosuppression (i.e., CD4+ cell counts less than 50 cells/μl), anemia, advanced WHO clinical stage, and malnutrition [i.e., body mass index (BMI) <18.5] at the time of cART initiation.3,6–10 Causes of mortality in these patients have not been well characterized and may be related to immune reconstitution, cART-related toxicities, gender differences, and suboptimal adherence (and resultant immunologic and/or virologic failure).
Although overall death rates among persons on cART continue to decline, evidence from cohorts in resource-repleted and resource-limited settings suggests the proportion of deaths attributable to non-AIDS diseases is increasing with longer cART treatment.8,10–12 Traditional risk factors for cardiovascular, hepatic, and pulmonary disease may contribute to higher mortality13–17; however, recent studies demonstrate that HIV infection itself and a resultant state of chronic inflammation may also contribute to this increased morbidity and mortality. The Strategies for Management of Antiretroviral Therapy (SMART) trial found major cardiovascular, renal, and hepatic disease and all-cause mortality were higher in individuals with unsuppressed viral replication.18 Further analyses of the SMART study data and also the Fat Redistribution and Metabolic Change in HIV infection (FRAM) study indicate an association between increased levels of inflammatory and coagulation markers, specifically interleukin-6 (IL-6), D-dimer, and high-sensitivity C-reactive protein (hsCRP), with increased all-cause mortality. The SMART study showed that higher baseline levels of hsCRP, IL-6, and D-dimer were associated with 2.0, 8.3, and 12.4 increased odds of all-cause mortality, respectively.19,20 These markers of inflammation and coagulation remain important predictors of death even at high CD4+ cell counts.20
While evidence suggests an important role for persistent immune activation and inflammation in the pathogenesis of many long-term complications of HIV infection and resultant mortality in high-income settings, there are limited similar data from sub-Saharan Africa,3,15 where the majority of cART-treated adults reside and the prevalence of advanced immunosuppression and opportunistic infections (OIs) at cART initiation is greater. In particular, evidence evaluating the predictors of mortality beyond the first 6 months following cART commencement is scarce in this setting. Recent data from our cohort in Botswana highlight the important contribution of non-AIDS diseases to mortality (29.7% non-AIDS-defining event-related mortality).6 Consequently, the potential role of inflammatory processes in the deaths in this cohort warrants further investigation.
As countries in sub-Saharan Africa move to provide simpler (one pill per day), more potent, better tolerated, and earlier cART (based on evolving “when to start” guidelines), it is anticipated that the incidence of long-term complications of cART and therefore attributable mortality will increase. It is therefore of paramount importance to identify simple and reliable predictors of mortality for those receiving cART in this region. Identifying those at highest risk, i.e., persons having higher levels of a particular serum inflammatory biomarker or biomarkers (e.g., highest tertile), could allow for more intensive clinical monitoring and/or additional therapeutic interventions [e.g., statin or angiotensin-converting enzyme (ACE) inhibitor therapy] to detect and treat risk factors for future non-AIDS complications. To evaluate the relationship between inflammatory biomarkers and mortality further, we conducted a nested case-control study and measured levels of hsCRP, D-dimer, and IL-6 at cART initiation and at the time closest to death. We hypothesized that higher levels of baseline inflammatory biomarkers, and the persistence of elevated serum levels of these markers despite cART treatment, would be a significant predictor of all-cause mortality in this at-risk population.
Materials and Methods
Study population
We retrospectively analyzed blood plasma samples from 32 HIV-1-infected adults who died within 3 years of initiating cART in Gaborone, Botswana and 64 HIV-infected matched controls who survived >3 years. The cohort comprised patients enrolled in the completed Adult Antiretroviral Treatment and Drug Resistance (“Tshepo”) study between December 1, 2002 and December 31, 2007.21 Tshepo was an open-label, randomized 3×2×2 factorial design study conducted at Princess Marina Hospital in Gaborone, Botswana, evaluating the efficacy, tolerability, and incidence of drug resistance mutations among six different first-line cART regimens: zidovudine, lamuvidine, plus nevirapine (ZDV/3TC/NVP); zidovudine, lamuvidine, plus efavirenz (ZDV/3TC/EFV); zidovudine, didanosine, plus nevirapine (ZDV/ddI/NVP); zidovudine, didanosine, plus efavirenz (ZDV/ddI/EFV); stavudine, lamuvidine, plus nevirapine (d4T/3TC/NVP); and stavudine, lamuvidine, plus efavirenz (d4T/3TC/EFV). An examination of secondary endpoints, including time to death for any reason, was included in the initial study aims.
Study participants were followed for 3 years with monthly scheduled study visits (up to 5 years of follow-up time as study enrollment occurred over a 2-year period). At study entry, participants were asked to consent to have plasma stored at baseline and at subsequent follow-up visits. Study participants qualified for cART based on existing Botswana national antiretroviral (ARV) treatment guidelines,22,23 namely, an AIDS-defining illness and/or CD4+ cell count <200 cells/mm3 or by having a CD4+ cell count between 201 and 350 cells/mm3 and a corresponding plasma HIV-1 RNA level greater than 55,000 copies/ml, which was consistent with consensus U.S. adult treatment guidelines at the time the study was designed. Further inclusion and exclusion criteria have been described elsewhere.24 OIs were documented within the parent study as described elsewhere.21 Briefly, all study participants were screened prior to enrollment, at the time of enrollment, as well as on study (longitudinally) for the presence of OIs, including pulmonary tuberculosis (TB), extrapulmonary TB, Pneumocystis jiroveci (formerly carinii) pneumonia, Kaposi's sarcoma, Candida esophagitis, Cytomegalovirus retinitis, wasting syndrome, and Cryptococcus neoformans (Cryptococcal) meningitis, as per existing standard of care.
Clinical outcomes
Mortality data were obtained from serious adverse event logs and verbal autopsy forms. Monthly visits allowed regular contact between clinical research staff and study participants and family members (when applicable). Family members were contacted within 1 week after the death had been confirmed to obtain additional details surrounding the death. In addition, clinical in-patient hospital records were reviewed for participants hospitalized at Princes Marina Hospital. Two patients underwent autopsies. The current investigation focuses on “all-cause mortality” due to the lack of comprehensive physical autopsy data.
Case-control study
Of the 37 enrolled participants who died, five cases were excluded from this analysis as death was due to violent injury (three cases; road traffic accident and burns), one participant died after study completion, and an additional case was excluded as it was not possible to find matching controls. Plasma samples obtained at study entry and the time-point closest to death were retrieved for the remaining 32 participants (termed “cases”). Two matched controls were chosen for each case (64 controls). Controls were matched to cases by age (±5 years), sex, date of randomization (±3 months), baseline CD4+ cell count (<200 or 200–350), and baseline plasma HIV-1 level (closest match). These characteristics were chosen as matching variables based on a previously demonstrated association with mortality. Date of randomization was chosen to ensure that the latest biomarker levels for cases and controls were measured at approximately the same time after enrollment.
HIV-negative reference group
To determine “background” immune activation and inflammation, as has been reported in non-HIV-infected African adults compared to similar groups in the United States and Europe,25 we measured IL-6, D-dimer, and hsCRP levels in stored plasma from HIV-negative participants recruited for immunology research at the Botswana-Harvard AIDS Institute Partnership. Forty-seven participants (25 women and 22 men) from a similar age range as cases and controls were selected for the study.
Biomarkers
IL-6, hsCRP, and the coagulation marker D-dimer were measured from frozen plasma samples. These biomarkers were selected based on prior studies suggesting an association with all-cause mortality in both HIV-uninfected and HIV-infected populations,19,26–29 and for their high reproducibility in the laboratory.29 IL-6 levels were measured using the R&D Systems Human IL-6 Quantikine HS ELISA (R&D Systems, Minneapolis, MN) as per the manufacturer's instructions. hsCRP and D-dimer levels were measured simultaneously using the Roche COBAS Integra 400 plus (Roche Diagnostics GmbH, Mannheim, Germany) in routine use at the Botswana-Harvard Partnership HIV Reference Laboratory, Gaborone, Botswana.
Statistical methods
Summary statistics appropriate for matched case-control studies19,30 were used to summarize the principal findings. Conditional logistic regression analyses for matched case-control studies were conducted to investigate the associations of baseline biomarker levels and the value closest to death or study completion with all-cause mortality. Separate analyses by quartile were performed, reporting odds ratios with 95% confidence intervals using the lowest quartile as reference. Odds ratios associated with one IQR higher biomarker level after log10 transformation were calculated in unadjusted and adjusted models. All analyses were adjusted for baseline BMI and the presence of OIs at treatment initiation; the analysis of biomarker levels immediately preceding death or study completion was adjusted for baseline biomarker values. Because cases and controls were matched for age, baseline CD4+ cell count, baseline plasma HIV-1 RNA level, and sex, these terms were not included in the model. All statistical analysis were performed using Stata 11.1 (College Station, TX). All reported p-values were two-sided and considered significant if less than 0.05.
Ethical approvals
Ethical review board approval was obtained from the Ministry of Health, Botswana (Health Research Development Committee) and the Harvard School of Public Health (Office of Human Research Administration).
Results
Thirty seven (5.7%) of 650 patients died on study, 12 (32%) male and 25 (68%) females, for a crude mortality rate of 20.6 per 1,000 person years. Of study participants who died on study 32 of 37 (86%) were included in this analysis. Causes of death (n=32) by category were as follows: non-AIDS-defining event (NADE) related (31.3%), HIV-related/opportunistic infections (28.1%), cART/toxicity related (21.9%), other infectious etiologies (15.6%), and unknown (3.1%). Median time to death was 31 weeks [interquartile range (IQR)=14–64]. The median CD4+ cell count among cases was 170.5 cells/μl (IQR 93.5–230.5) compared to 163.5 cells/μl (IQR 92–236.5) for controls.
Patients in this southern African Botswana cohort were younger and more likely to be female, and had lower CD4+ cell counts at the time of cART initiation when compared to cohorts of HIV-infected adults from resource-repleted settings.24 Baseline characteristics of cases and controls are given in Table 1. Cases and controls are well matched for sex (both 72% female), age (p=0.92), CD4+ cell count (p=0.18), and plasma HIV-1 RNA level (p=0.71). Median BMI was also similar between cases and controls (p=0.48).
Table 1.
Characteristics of Cases (Deaths) and Matched Controls at Baseline
| Category | Cases (n=32) | Controls (n=64) | p-value |
|---|---|---|---|
| Age Median (25th, 75th %ile) | 36 (30.5, 40.5) | 35 (31, 41.5) | 0.92 |
| Sex (% female) | 71.9 | 71.9 | NA |
| CD4 (cells/mm3) median (25th, 75th) %ile) | 170.5 (93.5, 230.5) | 163.5 (92, 236.5) | 0.18 |
| Plasma HIV-1 RNA (copies/ml) median (25th, 75th %ile) | 323k (95k, 750k) | 231k (97k, 714k) | 0.71 |
| BMI (kg/m2) median (25th, 75th %ile) | 19.81 (17.7–30.96) | 21.30 (18.97–23.93) | 0.48 |
BMI, body mass index.
Viral load data at the latest time-point (time-point closest to death in cases and equivalent time-point for controls) were not available for all cases and controls. However, of the available viral load measurements, 85% of cases (17/20) and 94% of controls (34/36) were virologically suppressed (viral load <400 copies/ml).
Baseline biomarker levels as predictors of mortality
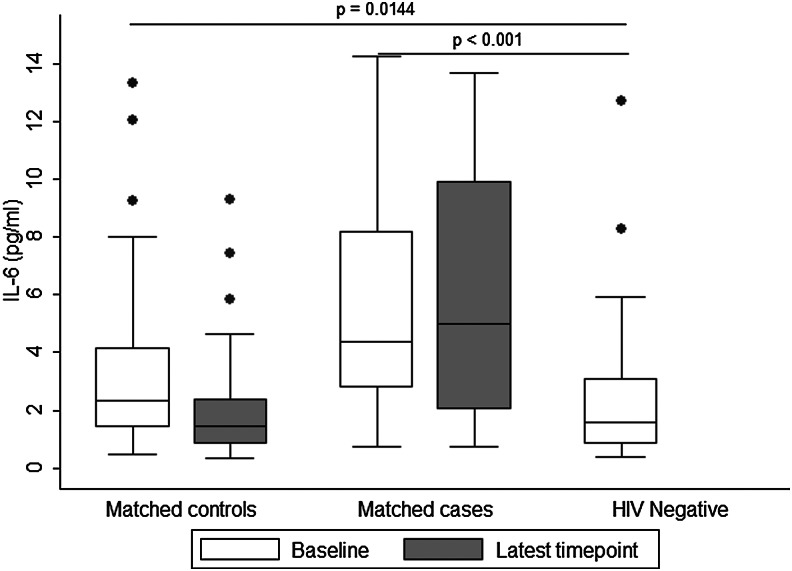
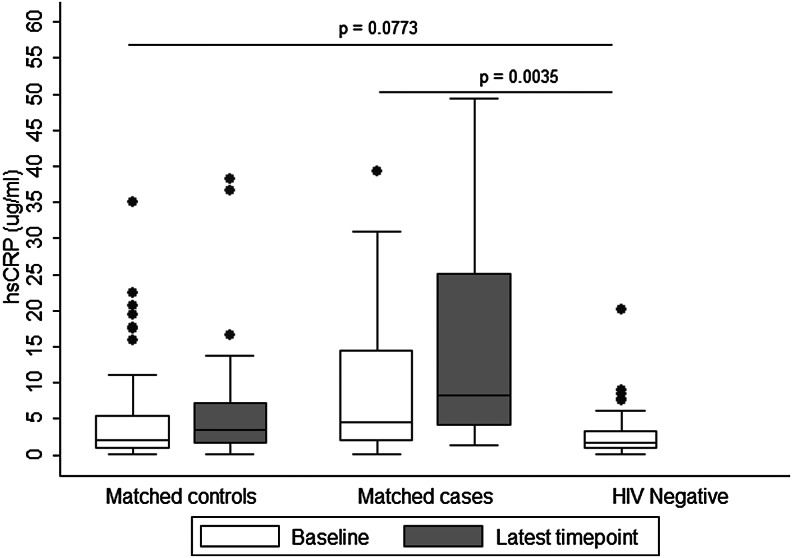
Table 2 describes the median and quartile levels of IL-6, hsCRP, and D-dimer in cases and controls at cART initiation (baseline) and at the time-point closest to death/same duration on study for matched control (latest level). Median baseline levels of all three biomarkers were higher in cases as compared to their matched controls. In the unadjusted analysis, cases had significantly higher levels of IL-6 and hsCRP when compared to controls. For each pg/ml increase in IL-6 there was a 1.3-fold increase in the odds of death in cases compared to controls [odds ratio (OR)=1.30 (95% CI 1.09–1.54), p=< 0.001], and for each μg/ml increase in hsCRP there was an approximately 1.2-fold increase in the odds of death in cases compared to controls [OR=1.17 (95% CI 1.01–1.35), p=0.04]. However, after adjusting for BMI and OIs, only IL-6 was a significant predictor of all-cause mortality [adjusted OR (aOR)=1.25; 95% CI 1.05–1.48, p=0.012].
Table 2.
Baseline Biomarker Levels and Risk of All-Cause Mortality for Cases and Matched Controls
| |
Cases |
Controls |
Unadjusteda |
Adjustedb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampling point/biomarker | Median (25th, 75th %ile) | Median (25th, 75th %ile) | OR | 95% CI | p-value | AOR | 95% CI | p-value | ||
| Baseline | ||||||||||
| IL-6 (pg/ml) | 4.37 (2.76, 8.16) | 2.34 (1.39, 4.14) | 1.30 | 1.09 | 1.54 | <0.001 | 1.25 | 1.05 | 1.48 | 0.012 |
| hsCRP (μg/ml) | 8.38 (3.02, 20.06) | 2.34 (0.88, 5.9) | 1.17 | 1.01 | 1.35 | 0.04 | 1.14 | 0.97 | 1.35 | 0.123 |
| D-dimer (μg/ml) | 0.87 (0.52, 1.68) | 0.59 (0.46, 0.93) | 1.48 | 0.94 | 2.34 | 0.09 | 1.35 | 0.77 | 2.36 | 0.293 |
| Latest level | ||||||||||
| IL-6 (pg/ml) | 5.01 (2.04, 9.93) | 1.46 (0.84, 2.38) | 1.58 | 1.16 | 2.14 | <0.001 | 1.48 | 1.05 | 2.09 | 0.027 |
| hsCRP (μg/ml) | 24.26 (4.44, 86.74) | 3.55 (1.66, 9.63) | 1.04 | 1.00 | 1.09 | 0.04 | 1.02 | 0.95 | 1.09 | 0.573 |
| D-dimer (μg/ml) | 0.67 (0.40, 1.77) | 0.45 (0.33, 0.73) | 1.09 | 0.89 | 1.33 | 0.41 | 1.42 | 0.66 | 3.04 | 0.366 |
p-values obtained from a univariate conditional logistic model.
p-values obtained from a conditional logistic model with adjustments for baseline BMI and OIs.
OR, odds ratio; CI, confidence interval; AOR, adjusted odds ratio; IL-6, interleukin-6; hsCRP, high-sensitivity C-reactive protein; OIs, opportunistic infections.
The latest levels of IL-6 and hsCRP were significant predictors of mortality after adjusting for the corresponding baseline values; however, only IL-6 remained significant after adjusting for baseline BMI and OIs (aOR 1.48 95% CI 1.05–2.09, p=0.027).
Due to their known potential to cause toxicities,31 the influence of didanosine- or stavudine (d4T)-containing regimens (ZDV/ddI or d4T/3TC) versus not (ZDV/3TC) was also investigated using the conditional logistic regression model. However, exposure to ddI- or d4T-containing regimens (compared to exposure to non-d-drug containing regimens; ZDV/3TC) was not a significant predictor of mortality (aOR=0.91; 95% CI 0.21–4.03, p=0.902).
In aggregate analysis of all cases and controls, a baseline BMI of less than 18.0 was a significant predictor of mortality (OR=3.07; 95% CI 1.31–7.20, p=0.01), even after adjusting for baseline hsCRP and opportunistic infections (OR=2.42; 95% CI 1.10–6.56, p=0.03) (Table 3).
Table 3.
Baseline Predictors of Mortality
| |
Unadjusteda |
Adjustedb |
||||
|---|---|---|---|---|---|---|
| Baseline characteristics | OR | 95% CI | p-value | AOR | 95% CI | p-valueb |
| BMI<18kg/m2 | 3.07 | 1.31–7.20 | 0.010 | 2.42 | 1.10–6.56 | 0.030 |
| hsCRP baseline>10 | 3.16 | 1.24–8.05 | 0.016 | 2.69 | 0.88–6.66 | 0.087 |
| Baseline OIs | 1.58 | 0.66–3.75 | 0.305 | 1.20 | 0.45–3.21 | 0.712 |
p-values obtained from a univariate conditional logistic model.
p-values obtained from a conditional logistic model with variables BMI baseline hsCRP and baseline OIs.
Elevated baseline hsCRP of greater than 10 μg/ml was a significant predictor of mortality (p=0.016) but was not significant after adjusting for baseline opportunistic infections and BMI.
In further analysis we compared the risk of death per interquartile range (IQR) increase in each biomarker (Table 4). In both adjusted and unadjusted analyses, patients having one IQR higher baseline levels of IL-6 and hsCRP had an approximate 2-fold higher risk of mortality; IL-6 aOR 2.21 (95% CI 1.19–4.12, p=0.012), hsCRP aOR 1.92 (95% CI 1.11–3.29, p=0.019). Lower values were observed for D-dimer; however, these results were not statistically significant 1.27 (95% CI 0.79–2.04, p=0.329).
Table 4.
Risk of All-Cause Mortality Associated with Biomarker Levels at Baseline
|
OR associated with IQR higher biomarker level after log10 transformation | |||||
|---|---|---|---|---|---|
|
Univariate analysis |
Adjusted analysis |
||||
| Biomarker | OR (95% CI) | p-valuea | Biomarker | OR (95% CI) | p-valueb |
| IL-6 (pg/ml) | 2.41 (1.36–4.28) | 0.002 | IL-6 (pg/ml) | 2.21 (1.19–4.12) | 0.012 |
| hsCRP (μg/ml) | 2.14 (1.31–3.48) | 0.002 | hsCRP (μg/ml) | 1.92 (1.11–3.29) | 0.019 |
| D-dimer (μg/ml) | 1.45 (0.95–2.22) | 0.089 | D-dimer (μg/ml) | 1.27 (0.79–2.04) | 0.329 |
p-values obtained from a aunivariate and bmultivariate conditional logistic model corresponding to log10 transformed biomarker. Multivariate model included BMI, OIs, and corresponding biomarker.
The significant findings regarding IL-6 and hsCRP at baseline (prior to treatment) were analyzed against HIV-uninfected individuals for comparison. The HIV-uninfected reference group was similar to HIV-infected cases and controls in age 35 (27, 42) years vs. 35 (31, 41.5) years [median (25th, 75th %ile), p=0.86], and roughly half were female (53%). No further data for HIV-uninfected participants were collected. Baseline (pre-cART initiation) levels of IL-6 and hsCRP were significantly higher in cases: IL-6: 4.37 (2.76, 8.16) pg/ml (median (25th, 75th %ile), hsCRP: 8.38 (3.02, 20.06) μg/ml compared to the reference HIV-uninfected group: IL-6: 1.56 (0.82, 3.08) pg/ml, hsCRP: 1.79 (0.82, 3.34) μg/ml (p<0.001 and p=0.0035, respectively). IL-6 values were slightly higher in HIV-infected controls [2.34 (1.39, 4.14) pg/ml (p=0.0144)] when compared to values in the uninfected reference group. There was no difference in hsCRP levels between the uninfected reference group (above) and controls for hsCRP [2.34 (0.88, 5.90) μg/ml (p=0.077)].
A summary of results regarding baseline levels of IL-6 and hsCRP among cases, controls, and HIV-uninfected participants is presented in Figs. 1 and 2, respectively.
FIG. 1.
Baseline and latest levels of serum interleukin (IL)-6 (pg/ml) in controls and cases, and baseline levels in HIV-negative reference participants.
FIG. 2.
Baseline and latest levels of serum high-sensitivity C-reactive protein (hsCRP) (μg/ml) in controls and cases, and baseline levels in HIV-negative reference participants.
Discussion
This study demonstrates that elevated IL-6 can be a strong predictor of mortality among those initiating cART in resource-limited settings. In this case-control study, patients who died during follow-up had higher levels of IL-6, D-dimer, and hsCRP at baseline compared to matched controls, although after adjustment for BMI and the baseline presence of opportunistic infections, only IL-6 remained a significant predictor of death. In these patients, IL-6 remained elevated following cART initiation and was significantly higher at the latest level (time-point closest to death), compared to matched controls at the same time-point, suggesting that persistent untreated inflammatory processes can be important contributors to all-cause mortality even in persons receiving fully suppressive cART regimens. Consistent with published data from the United States and Europe, markers of inflammation were elevated in individuals receiving cART compared to HIV-uninfected individuals.25 Our results contribute to the growing body of evidence suggesting that a chronic inflammatory state occurs during HIV infection and can persist despite viral suppression with cART.
Recent work in South Africa and Zambia has also shown the importance of inflammatory biomarkers as predictors of mortality. Work in Zambia found hsCRP was significantly associated with ART-associated mortality during the first 12 weeks of treatment.3 Similarly to the current investigation, a South African study has found IL-6, D-dimer, and hsCRP to be elevated at enrollment in patients who died on the study as compared to matched controls. However, in these patients, D-dimer and hsCRP also remained significant predictors of death after adjustment for disease state and specific laboratory measurements (hemoglobin, platelet count, white blood cell count, and serum SGOT/AST and SGPT/ALT levels).4
The biological mechanisms by which IL-6 and not D-dimer and hsCRP are related to risk for all-cause mortality are not clear, but observed differences in adjustment variables and patient characteristics (i.e., CD4+ cell counts <50 in South Africa vs. >160 in this Botswana study) may explain differences in the predictive value of D-dimer and hsCRP between our study and others. However, as serum levels of D-dimer and hsCRP trended in the same direction as IL-6 (both lower in controls), potentially our study was not large enough to detect a true association. Additionally, despite matching on sex, age, CD4+ cell count, plasma HIV-1 RNA, and date of randomization, and adjusting for BMI and OI, it is possible that residual confounding and/or other unmeasured variables may have affected the associations between serum biomarkers and mortality. Due to the potential for certain nucleoside reverse transcriptase inhibitors to cause serious toxicities (particularly the “D” drugs d4T (stavudine) and ddI (didanosine), additional analyses were performed that did not show an association between “D” drug exposure and all-cause mortality. Consistent with published studies,2,32,33 we also found that being significantly malnourished (having a baseline BMI<18) was an important predictor of all-cause mortality. All HIV-infected adults in the region should have a height and weight measurement obtained at or prior to the initiation of cART, as BMI is an inexpensive means to risk-stratify patients in settings where serum biomarker levels such as IL-6 are not routinely available.
With monthly monitoring and 98% of all scheduled follow-up visits attended,21 bias due to loss to follow-up was minimized in the Adult Antiretroviral Treatment and Drug Resistance (Tshepo) study, in which the current study is nested. In the first analysis of its kind, Wester et al.24 noted the high incidence of non-AIDS-defining events in this low-resource setting. The current study sought to investigate this further through the analysis of serum biomarkers of inflammation and coagulation. Unfortunately, a definitive cause of death could not be established for all deaths (only two physical autopsies were conducted), therefore we used “all-cause mortality” as our primary outcome.19 Our results indicate that inflammatory processes could contribute to, or be indicative of an increased risk of death and potentially NADEs in this setting, but that further investigations with larger sample sizes and careful ascertainment of cause of death are warranted.
In summary, these results from Botswana add weight to recently published data from Zambia3 and South Africa indicating that closer attention be made to posttreatment monitoring of inflammatory biomarkers and potential trials of antiinflammatory therapies for patients commencing cART in sub-Saharan Africa.
Acknowledgments
We would like to formally acknowledge and thank all study participants. We would also like to acknowledge the Botswana–Harvard HIV Reference Laboratory, especially the research laboratory team for their input and discussions of this work, and the specimen repository department and clinical chemistry department for their assistance in conducting this study. We would like to thank Baitshepi Mokaleng and Emmanuel Kedisitse for their assistance. We would like to thank Lendsey Melton for editorial assistance.
The study was funded in part by the NIH Fogarty International Center AIDS International Training and Research Program, D43 TW000004 (S.M.), and by research grants from the National Institute of Allergy and Infectious Diseases, K23 AI073141 and P30AI 060354 (PI: C.W. Wester), and the Harvard Center for AIDS Research (CFAR), P30 AI060354, an NIH funded program (P30 AI060354) that is supported by the following NIH co-funding and participating institutes and centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, and OAR. No funder had any role in the study design, data collection, analysis, interpretation, writing, or submission of this article for publication.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Walensky RP. Paltiel AD. Losina E. Mercincavage LM. Schackman BR. Sax PE. Weinstein MC. Freedberg KA. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 2.Johannessen A. Naman E. Ngowi BJ. Sandvik L. Matee MI. Aglen HE. Gundersen SG. Bruun JN. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koethe JR. Blevins M. Nyirenda C. Kabagambe EK. Shepherd BE. Wester CW. Zulu I. Chiasera JM. Mulenga LB. Mwango A. Heimburger DC. Nutrition and inflammation serum biomarkers are associated with 12-week mortality among malnourished adults initiating antiretroviral therapy in Zambia. J Int AIDS Soc. 2011;14:19. doi: 10.1186/1758-2652-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledwaba L. Tavel JA. Khabo P. Maja P. Qin J. Sangweni P. Liu X. Follmann D. Metcalf JA. Orsega S. Baseler B. Neaton JD. Lane HC. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS ONE. 2012;7:e24243. doi: 10.1371/journal.pone.0024243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawat R. Humphrey JH. Mutasa K. Ntozini R. Stoltzfus RJ. Short communication: Predicting adverse HIV-related outcomes in a resource-limited setting: Use of the inflammation marker alpha(1)-acid glycoprotein. AIDS Res Hum Retroviruses. 2010;26:1171–1174. doi: 10.1089/aid.2010.0053. [DOI] [PubMed] [Google Scholar]
- 6.Wester CW. Eden SK. Bussmann H. Shepherd BE. Moyo S. Makhema J. Novitsky V. Essex M. Marlink RG. Risk Factors for Mortality among HIV+ Adults Receiving cART in Botswana: Results from a Clinical Trial. 19th Conference on Retroviruses and Opportunistic Infections; Seattle: Washington State Convention Center; 2012. Poster #625. [Google Scholar]
- 7.Lawn SD. Myer L. Orrell C. Bekker LG. Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: Implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ., Jr. Baker RK. Moorman AC. Chmiel JS. Wood KC. Brooks JT. Holmberg SD. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 9.Egger M. May M. Chene G. Phillips AN. Ledergerber B. Dabis F. Costagliola D. D'Arminio Monforte A. de Wolf F. Reiss P. Lundgren JD. Justice AC. Staszewski S. Leport C. Hogg RS. Sabin CA. Gill MJ. Salzberger B. Sterne JA. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 10.Antiretroviral Therapy Cohort Collaboration: Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: Collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks SG. Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 12.Martinez E. Milinkovic A. Buira E. de Lazzari E. Leon A. Larrousse M. Lonca M. Laguno M. Blanco JL. Mallolas J. Garcia F. Miro JM. Gatell JM. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007;8:251–258. doi: 10.1111/j.1468-1293.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 13.Calza L. Mosca L. Pocaterra D. Piergentili B. Colangeli V. Manfredi R. Erario A. Grossi G. Verucchi G. Viale P. Assessing the impact of hepatitis C virus coinfection on lopinavir/ritonavir trough concentrations in HIV-infected patients. Eur J Clin Pharmacol. 2011;67:143–149. doi: 10.1007/s00228-010-0904-4. [DOI] [PubMed] [Google Scholar]
- 14.Torriani FJ. Komarow L. Parker RA. Cotter BR. Currier JS. Dube MP. Fichtenbaum CJ. Gerschenson M. Mitchell CK. Murphy RL. Squires K. Stein JH. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friis-Moller N. Thiebaut R. Reiss P. Weber R. Monforte AD. De Wit S. El-Sadr W. Fontas E. Worm S. Kirk O. Phillips A. Sabin CA. Lundgren JD. Law MG. Predicting the risk of cardiovascular disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 16.Obel N. Thomsen HF. Kronborg G. Larsen CS. Hildebrandt PR. Sorensen HT. Gerstoft J. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 17.Triant VA. Lee H. Hadigan C. Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Sadr WM. Lundgren JD. Neaton JD. Gordin F. Abrams D. Arduino RC. Babiker A. Burman W. Clumeck N. Cohen CJ. Cohn D. Cooper D. Darbyshire J. Emery S. Fatkenheuer G. Gazzard B. Grund B. Hoy J. Klingman K. Losso M. Markowitz N. Neuhaus J. Phillips A. Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 19.Kuller LH. Tracy R. Belloso W. De Wit S. Drummond F. Lane HC. Ledergerber B. Lundgren J. Neuhaus J. Nixon D. Paton NI. Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tien PC. Choi AI. Zolopa AR. Benson C. Tracy R. Scherzer R. Bacchetti P. Shlipak M. Grunfeld C. Inflammation and mortality in HIV-infected adults: Analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wester CW. Thomas AM. Bussmann H. Moyo S. Makhema JM. Gaolathe T. Novitsky V. Essex M. deGruttola V. Marlink RG. Non-nucleoside reverse transcriptase inhibitor outcomes among combination antiretroviral therapy-treated adults in Botswana. AIDS. 2010;24(Suppl 1):S27–36. doi: 10.1097/01.aids.0000366080.91192.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Health Botswana: Botswana Guidelines on Antiretroviral Treatment 2002 version. Gaborone; Botswana: 2002. [Google Scholar]
- 23.Ministry of Health Botswana: Botswana Guidelines on Antiretroviral Treatment 2005 version. Gaborone; Botswana: 2005. [Google Scholar]
- 24.Wester CW. Koethe JR. Shepherd BE. Stinnette SE. Rebeiro PF. Kipp AM. Hong H. Bussmann H. Gaolathe T. McGowan CC. Sterling TR. Marlink RG. Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in resource-replete versus resource-limited urban setting. AIDS. 2011;25:1471–1479. doi: 10.1097/QAD.0b013e328347f9d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhaus J. Jacobs DR., Jr Baker JV. Calmy A. Duprez D. La Rosa A. Kuller LH. Pett SL. Ristola M. Ross MJ. Shlipak MG. Tracy R. Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzardini G. Piconi S. Ruzzante S. Fusi ML. Lukwiya M. Declich S. Tamburini M. Villa ML. Fabiani M. Milazzo F. Clerici M. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS. 1996;10:1535–1542. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Tuomisto K. Jousilahti P. Sundvall J. Pajunen P. Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemostas Stuttgart. 2006;95:511. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 28.Tien PC. Choi AI. Zolopa AR. Benson C. Tracy R. Scherzer R. Bacchetti P. Shlipak M. Grunfeld C. Inflammation and mortality in HIV-infected adults: Analysis of the FRAM study cohort. JAIDS J Acquir Immune Defic Syndr. 2010;55:316. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastburn A. Scherzer R. Zolopa AR. Benson C. Tracy R. Do T. Bacchetti P. Shlipak M. Grunfeld C. Tien PC. Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS ONE. 2011;6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasieni P. A note on the presentation of matched case‐control data. Stat Med. 1992;11:617–620. doi: 10.1002/sim.4780110506. [DOI] [PubMed] [Google Scholar]
- 31.Wester CW. Eden SK. Shepherd BE. Bussmann H. Novitsky V. Samuels DC. Hendrickson SL. Winkler CA. O'Brien SJ. Essex M. Risk factors for symptomatic hyperlactatemia and lactic acidosis among combination antiretroviral therapy-treated adults in Botswana: Results from a clinical trial. AIDS Res Hum Retroviruses. 2012;28:759–765. doi: 10.1089/aid.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringer JS. Zulu I. Levy J. Stringer EM. Mwango A. Chi BH. Mtonga V. Reid S. Cantrell RA. Bulterys M. Saag MS. Marlink RG. Mwinga A. Ellerbrock TV. Sinkala M. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 33.Zachariah R. Fitzgerald M. Massaquoi M. Pasulani O. Arnould L. Makombe S. Harries AD. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]