Abstract
Müllerian inhibiting substance (MIS) not only induces Müllerian duct regression during male sexual differentiation but also modulates Leydig cell steroidogenic capacity and differentiation. MIS actions are mediated through a complex of homologous receptors: a type II ligand-binding receptor [MIS type II receptor (MISRII)] and a tissue-specific type I receptor that initiates downstream signaling. The putative MIS type I receptors responsible for Müllerian duct regression are activin A type II receptor, type I [Acvr1/activin receptor-like kinase 2 (ALK2)], ALK3, and ALK6, but the one recruited by MIS in Leydig cells is unknown. To identify whether ALK3 is the specific type I receptor partner for MISRII in Leydig cells, we generated Leydig cell-specific ALK3 conditional knockout mice using a Cre-lox system and compared gene expression and steroidogenic capacity in Leydig cells of ALK3fx/fxCyp17cre+ and control mice (ALK3fx/fxCyp17cre− or ALK3fx/wtCyp17cre− littermates). We found reduced mRNA expression of the genes encoding P450c17, StAR, and two enzymes (17βHSD-III and 3βHSD-VI) that are expressed in differentiated adult Leydig cells and increased expression of androgen-metabolizing enzymes (3α-HSD and SRD5A2) and proliferating cell nuclear antigen (PCNA) in Leydig cells of ALK3fx/fxCyp17cre+ mice. Despite down-regulation of steroidogenic capacity in ALK3fx/fxCyp17cre+ mice, the loss of MIS signaling also stimulates Leydig cell proliferation such that plasma testosterone and androstenedione concentrations are comparable to that of control mice. Collectively, these results indicate that the phenotype in ALK3 conditional knockout mice is similar to that of the MIS-knockout mice, confirming that ALK3 is the primary type I receptor recruited by the MIS-MISRII complex during Leydig cell differentiation.
Müllerian inhibiting substance (MIS) (also called anti-Müllerian hormone) is a Sertoli cell product and a member of the TGF-β superfamily of growth and differentiation factors (1, 2). MIS not only induces Müllerian duct regression during male sexual differentiation (3, 4) but also modulates androgen biosynthesis during Leydig cell development (5–9) and inhibits Leydig cell proliferation and differentiation after birth (5–7). These actions of MIS are elicited by binding of the bioactive carboxy terminus of MIS to its constitutively active type II receptor [MIS type II receptor (MISRII)], followed by recruitment of its type I receptor partner (1). The ligand-receptor complex stimulates phosphorylation of downstream signaling molecules Smad 1, 5, or 8 (1, 10). These Smads have been identified in MIS target organs and associate with Smad4 to translocate to the nucleus to regulate transcription of downstream genes (1, 10–13). Studies by our group (9) and others (4, 14–17) have shown that deleting MIS or MISRII results in persistence of Müllerian duct structures in males and also in Leydig cell hyperplasia, focal tumors, and delayed maturation and differentiation of Leydig cells accompanied by decreased androgen biosynthetic capacity per cell. Conversely, overexpression of MIS reduces Leydig cell numbers and decreases serum testosterone concentrations (3, 7, 18).
Null deletions in several candidate type I receptor genes for MIS cause early embryonic lethality before defects in Müllerian duct regression can be appreciated (19–21). A Cre/Lox conditional deletion of activin receptor-like kinase 3 receptor (ALK3) (also known as bone morphogenetic receptor, type 1A, BmpR1a) was created using an MISRII promoter to target the deletion to the Müllerian ducts and gonads (22, 23). Male mice with this conditional deletion had retained Müllerian ducts, confirming that ALK3 plays a critical role in Müllerian duct regression. When MIS is overexpressed in the ALK3 conditional mutants, however, it can signal through other type I receptors to induce Müllerian duct regression (24). ALK2, which colocalizes with MISRII to the mesenchymal cells of the Müllerian duct and is expressed more abundantly in male than female mesenchyme, may be an alternate type 1 receptor partner for MISRII (11). This potential role for ALK2 is supported by the finding that an antisense ALK2 oligonucleotide blocked Müllerian duct regression in an organ culture system (13). In addition, ALK6 was shown to be a unique MIS type I receptor in CHO cells transfected with different type I receptors because only ALK6 coimmunoprecipitated with MISRII in a ligand-dependent fashion (12). However, in an ALK6-deletion mouse model, male animals have normal Müllerian duct regression (11). These studies suggest that recruitment of type I receptors by MIS is cellular context dependent. Another study has provided additional insights on the role of these three candidate type I receptors in MIS signaling (25). ALK2, ALK3, and ALK6 were shown to activate a Smad-1-responsive Gal4-luc reporter transfected into SMAT-1 Sertoli cells, which express type I and II MIS receptors (25). Within this cellular context, ALK3 was the primary binding partner, but in the absence of ALK3, ALK2 was able to compensate to activate the reporter gene. In contrast, ALK6 antagonized ALK3 transactivation of the reporter gene (25). These data suggest that MISRII can complex with different type I receptors, as described for bone morphogenetic protein, activin, and TGF-β in the testis (26–29).
In Leydig cells of the testis, MIS plays an important role in modulating differentiation and proliferation of progenitor Leydig cells. To identify the specific type I receptor binding partner of MISRII in Leydig cells and to explore the consequences of ALK3 deletion, we generated Leydig cell-specific ALK3 conditional knockout mice. We focused on the testicular phenotype of this conditional deletion restricted to Leydig cells by studying the expression of genes important for steroidogenesis and assessing steroidogenic capacity in these mice (ALK3fx/fxCyp17cre+) compared with control littermates (ALK3fx/fxCyp17cre− or ALK3fx/wtCyp17cre−).
Materials and Methods
Animals
Leydig cell-specific ALK3 conditional null mice were obtained by first breeding cyp17cre+/− mice (gift provided by Dr. Dale Buchanan Hales, Southern Illinois University Carbondale School of Medicine, Carbondale, IL) with ALK3fx/fx mice (gift provided by Dr. Yuji Mishina, University of Michigan School of Dentistry, Ann Arbor, MI) to generate ALK3fx/wtCyp17cre+ mice and then breeding female ALK3fx/fx with male ALK3fx/wtCyp17cre+ (17-hydroxylase-cre-IRES-GFP) mice or male ALK3fx/fx with female ALK3fx/wtCyp17cre+ mice. The mice were maintained on a C57BL/6-129/SvEv mixed genetic background and all genotypes were analyzed. The presence of the loxP sites flanking the ALK3 allele (exon 2) and the successful recombination event was confirmed through genotyping using specific PCR primers and detection of GFP expression in Leydig cells. The day of birth was designated as d 1. At 65 d of age, blood and testes were collected for analysis. To prepare testicular extracts, the testes were homogenized in 900 μl Tris-buffered saline with gelatin buffer (1 g/liter gelatin, 28 mm Trizma HCl, 22 mm Trizma base, 100 mm NaCl, and 15 mm NaN3). Cellular debris was removed by centrifugation, and the supernatants were collected for steroid assays.
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School (A1619).
Isolation of primary Leydig cells
Testes were harvested, decapsulated, dispersed with collagenase (0.5 mg/ml), and fractionated in Percoll gradient as described for rat Leydig cell isolation (30, 31) with minor modifications (32). The fractionated Leydig cells were purified through a 2.5–10% gradient of BSA (Sigma Chemical Co., St. Louis, MO) to remove contaminating sperm (32). The purity of each Leydig cell preparation, assessed by 3β-hydroxysteroid dehydrogenase (3β-HSD) staining with 0.4 mm etiocholanolone, was greater than 90% (33). The average number of Leydig cells isolated per testis was compared between ALK3fx/fxCyp17cre+ and control mice (34).
In vitro cell culture
Freshly harvested Leydig cells were cultured at 2–2.5 × 104 cells/ml in serum-free media (phenol-red-free DMEM/F12) supplemented with 15 mm HEPES, 26 mm sodium bicarbonate, 0.1% BSA, 12 μg/ml gentamicin, and 100 ng/ml LH. After gentle rotatory agitation for 3 h in a 34 C water bath at 85 rpm, the adult-type Leydig cells were pelleted by centrifugation at 14,000 rpm for 8 min. Conditioned media were collected and stored at −20 C until hormone assay.
Hormonal assays
Testosterone, androstenedione, and androstanediol were measured by RIA in plasma, in conditioned medium from in vitro short-term Leydig cell cultures, and in supernatants of total testicular homogenates (35–37). All conditioned medium samples were assayed in duplicate, and each experimental group consisted of four to seven littermates. The androstenedione rabbit antiserum (Sigma) is 100% specific for androstenedione (38). Testosterone antibody was obtained from Dr. Gordon Niswender (35) and androstanediol rabbit antiserum from Dr. Gerald Barbe, University of Western Ontario, London, Canada (39). Both antibodies are highly specific and have minimal cross-reactivity with other commercial steroids. The lower limit of detection for the androgen assays was 33 pg/ml. Intra- and interassay coefficients of variation were 9.04 and 7.76%, respectively for testosterone, 6.1 and 5.76% for androstenedione, and 6.98 and 3.97% for androstanediol.
Quantitative real-time PCR (qPCR)
RNA was extracted from 5 × 105 cultured primary Leydig cells using Trizol (Invitrogen, Carlsbad, CA). For each reverse transcription reaction, after deoxyribonuclease treatment to remove contaminating DNA, cDNA was transcribed using High Capacity cDNA reverse transcription kit (AB Applied Biosystems, Foster City, CA) for qPCR amplification (Mastercycler Realplex2; Eppendorf, Westbury, NY). Primer sequences of target genes are shown in Table 1. All primers except 3βHSD-IV were designed to produce an amplicon spanning at least one intron boundary. For all experiments, murine β-actin was amplified as an internal quality control. qPCR were carried out in a 20-μl sample volume using the Quantitect SYBR green PCR kit (QIAGEN, Valencia, CA). Each reaction contained 1× reaction buffer, 2.5 mm MgCl2, 200 μm deoxynucleotide triphosphate, 150–400 nm of the specific primer, and HotStar Taq DNA polymerase. After denaturing at 95 C for 15 min, 45 cycles were run at 95 C for 20–30 sec, 52–56 C (primer-specific annealing temperature) for 20–30 sec, and 72 C for 20–45 sec. The relative amount of initial mRNA copies was determined by calculating the difference (ΔCt) in threshold cycle (Ct) value between the target gene and actin, which is then interpreted as 2−ΔCt to represent the target gene concentration. Real-time PCR analysis was repeated at least four times for each gene with mRNA from at least three different Leydig cell isolations and is expressed as the mean ± sem.
Table 1.
Primer sequences for qPCR
| Gene (Protein) | Accession no. | Primer sequences (5′–3′) | Product size (bp) |
|---|---|---|---|
| P450scc | NM_019779.3 | 112 | |
| Forward | TCGACTCCTCAGAACTAAGACCTGGA | ||
| Reverse | TCGCTTCTGCCTTAAGTCCCAGTA | ||
| StAR | NM_011485.4 | 403 | |
| Forward | ACAACCAGGAAGGCTGGAAG | ||
| Reverse | ATGCAGGTGGGGCCGTGTTCA | ||
| P450c17 | NM_007809.3 | 257 | |
| Forward | CACAACTGCAGTGATTGTCGGTCA | ||
| Reverse | AGTCACACAGTGAGTTGGCTTCCT | ||
| 17βHSD-III | NM_008291.3 | 67 | |
| Forward | GCCAACTCAAATGAATAGGCTTTC | ||
| Reverse | TGGGACAATGGGCAGTGAT | ||
| 3βHSD-VI | NM_013821.3 | 117 | |
| Forward | AAGCCTCTCAGCACCTCTTG | ||
| Reverse | TGTTTCTTCCATTGGGTTTCA | ||
| 5α-reductase | NM_175283.3 | 68 | |
| Forward | GCGCTAGTCTACCTGGAGGGT | ||
| Reverse | GAAGAGCCCACCATCTGGAG | ||
| 3α-HSD | NM_134072.1 | 76 | |
| Forward | GGAAAACTGTTGGCTGAAGCA | ||
| Reverse | CCAGTCCGGCATCTTTACACTT |
Immunohistochemistry
Testes from a minimum of three mice at d 65 from both ALK3fx/fxCyp17cre+ and control mice were collected, fixed in Bouin's fixative overnight, and then embedded in paraffin and sectioned at 5 μm. For immunohistochemistry, tissue sections were blocked for 1 h in 1.5% normal goat serum and then incubated overnight at 4 C with aromatase antibody at a concentration of 1:2000 (Research Diagnostics, Flanders, NJ). The sections were incubated with biotinylated antirabbit IgG secondary antibody to aromatase followed by the avidin-biotin immunoperoxidase system (Vectastain ABC kit; Vector Laboratories, Inc., Burlingame, CA) and 3,3′-diaminobenzidine tetrachloride (Roche Molecular Biochemicals, Indianapolis, IN) and then counterstained with hematoxylin or with Alexa Fluor 568 secondary antibody and Alexa Fluor 488 (Molecular Probes, Life Technology, Grand island, NY) for single [P450scc, P450c17α, and proliferating cell nuclear antigen (PCNA)] and double (PCNA and P450scc) staining. Alternate sections were processed with PBS instead of the primary antibody for a negative primary antibody control. To compare aromatase, cholesterol side-chain cleavage enzyme (P450sccc) 17α-hydroxylase P450c17α, and PCNA staining of sections from ALK3fx/fxCyp17cre+ and control mice, 500 cells were counted and the intensity compared in random sections.
Statistical analysis
All computations and data analyses were performed using Student's t test and mixed-model ANOVA in SAS 9.3 (1997). Androgen concentrations below the lower limits of the assays were considered undetectable and assigned values of 0.01 ng/ml for statistical analysis. Differences were regarded as significant if P < 0.05. All hormonal concentrations, weights, and Leydig cell numbers are expressed as mean ± sem.
Results
Body and testicular weight
The mean body weights were similar in ALK3fx/fxCyp17cre+ (26.12 ± 0.62 g; n = 13) and control mice (26.75 ± 0.30 g; n = 13) at 65 d of age. The mean testis weight (99.38 ± 2.60 mg/testis) of ALK3fx/fxCyp17cre+ mice (n = 13) was 5% lighter than that of control mice (105.45 ± 2.19 mg/testis; n = 13) at 65 d of age (P < 0.05) (Table 2).
Table 2.
Comparison of body and testis weight and Leydig cell numbers in d-65 ALK3fx/fxCyp17cre+ and control mice (mean ± sd)
| ALK3fx/fxCyp17cre+ | Control | |
|---|---|---|
| Body weight (g) | 26.8 ± 0.7 | 26.9 ± 0.4 |
| Testis weight (mg) | 99.50 ± 2.01 | 105.35 ± 1.83 |
| Isolated Leydig cells (cells/mg testis) | 570.81 ± 60.46a | 439.01 ± 33.55 |
α P < 0.05.
Proliferation and number of Leydig cells
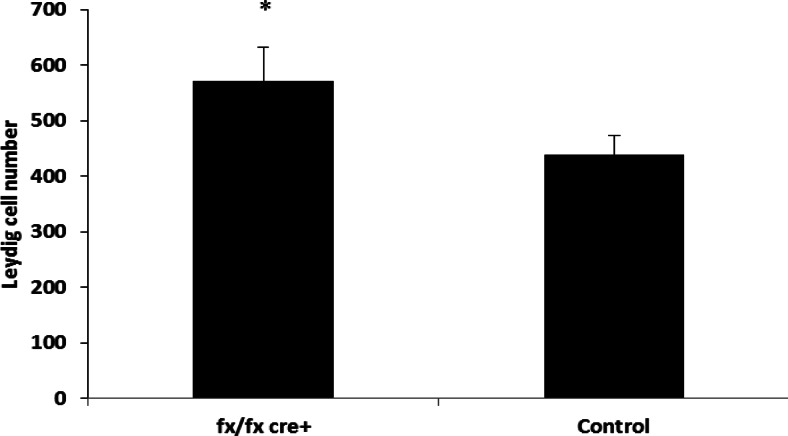
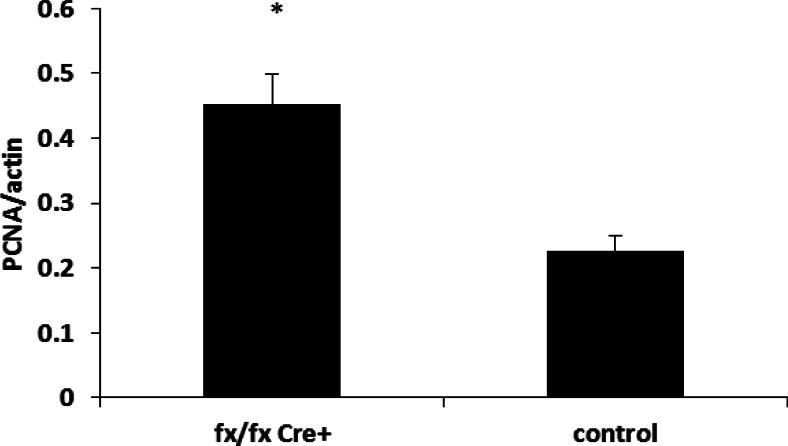
The mean number of isolated Leydig cells in ALK3fx/fxCyp17cre+ mice (570.81 ± 60.46 cells/g testis) was significantly higher than that of control mice (439.01.3 ± 33.55 cells/g testis) (P < 0.05) (Fig. 1). The mRNA expression of PCNA (a representative marker for proliferation) in Leydig cells of ALK3fx/fxCyp17cre+ mice was almost 2-fold greater than that of control (Fig. 2).
Fig. 1.
Leydig cells in ALK3fx/fx Cyp17cre+ and control mice. Leydig cells were isolated from testes of 17 littermates and the number of isolated Leydig cells per milligram testis calculated. Data are represented as mean ± sem. *, Significant differences (P < 0.05).
Fig. 2.
The mRNA expression of PCNA in Leydig cells of ALK3fx/fx Cyp17cre+ and control mice. Total mRNA was extracted from Leydig cells of adult mouse testes (eight littermates). The data (mean ± sem) are shown as mRNA expression of PCNA normalized by β-actin. *, Significant differences (P < 0.05).
Plasma hormone concentrations
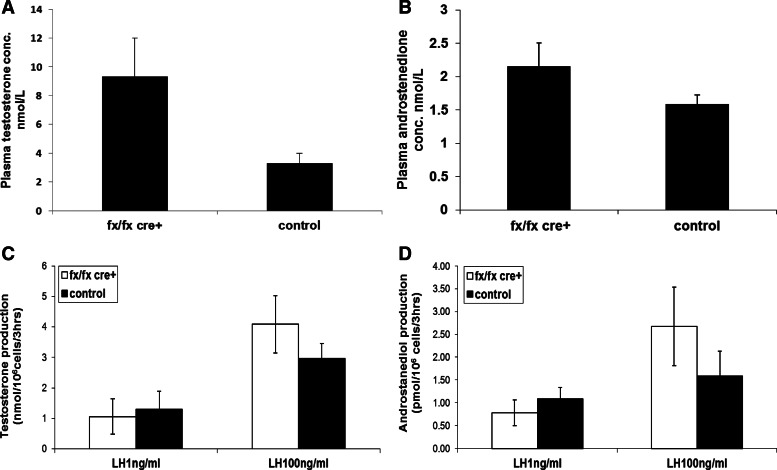
LH concentration (0.094 ± 0.05 nmol/liter; n = 14) in plasma of ALK3fx/fxCyp17cre+ mice was 5.9-fold higher than that of control littermates (0.038 ± 0.02 nmol/liter; n = 25), but this was not significant. Testosterone concentration in plasma of ALK3fx/fxCyp17cre+ mice (8.76 ± 4.79 nmol/liter, n = 12) was not significantly higher than that of control littermates (2.57 ± 0.53 nmol/liter, n = 9) (Fig. 3A). Androstenedione concentration in plasma of ALK3fx/fxCyp17cre+ mice (2.16 ± 0.35 nmol/liter; n = 12) was comparable to that in control littermates (1.59 ± 0.14 nmol/liter; n = 9) (Fig. 3B).
Fig. 3.
Hormone studies. A and B, Plasma testosterone (A) and androstenedione (B) concentrations (conc.); C and D, in vitro testosterone (C) and androstanediol production (D) under both basal and LH-stimulated conditions are similar in adult ALK3fx/fxCyp17cre+ and control mice.
In vitro androgen production
In vitro production of androgens by cultured primary Leydig cells was examined under both basal (1 ng/ml LH) and LH-stimulated (100 ng/ml LH) conditions. In vitro basal testosterone production was similar in Leydig cells from ALK3fx/fxCyp17cre+ and control mice (1.06 ± 0.58 vs. 1.30 ± 0.59 nmol/million cells per 3 h; P > 0.05) as was LH-stimulated testosterone production (4.09 ± 0.94 vs. 2.97 ± 0.49 nmol/million cells per 3 h; P > 0.05) (Fig. 3C). The in vitro production of androstanediol was also similar in Leydig cells from ALK3fx/fxCyp17cre+ and control mice (basal, 0.78 ± 0.28 vs. 1.07 ± 0.25 pmol/million cells per 3 h, P > 0.05; LH-stimulated, 2.67 ± 0.86 vs. 1.59 ± 0.54 nmol/million cells per 3 h, P > 0.05) (Fig. 3D).
Expression of steroidogenic acute regulatory protein (StAR) and steroidogenic and metabolic enzymes ALK2 and ALK6
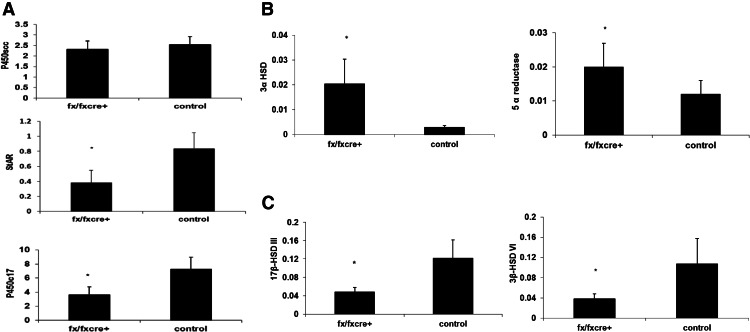
The expression of genes encoding androgen biosynthetic and metabolizing enzymes and StAR was examined in mRNA extracted from 5 × 105 Leydig cells. mRNA expression of P450c17 and StAR in Leydig cells of ALK3fx/fxCyp17cre+ mice was 50 and 54% lower than that of control littermates, respectively (Fig. 4A), whereas mRNA expression of P450scc and aromatase was not significantly different. Furthermore, the expression of 5α-reductase and 3α-HSD in Leydig cells of ALK3fx/fxCyp17cre+ mice was 66 and 606% higher than that of control littermates, respectively (Fig. 4B), whereas Leydig cell expression of 17βHSD-III and 3βHSD-VI was 60 and 65% lower in ALK3fx/fxCyp17cre+ mice than control littermates, respectively (Fig. 4C).
Fig. 4.
mRNA expression of P450scc, StAR, and P450c17α (A); 5α reductase and 3α-HSD (B); and 17-βHSDIII and 3β-HSDVI (C) in Leydig cells of adult ALK3fx/fxCyp17cre+ and control mice. β-Actin was used as an internal control. Results are mean ± sem of RT-PCR analysis of different preparations of primary Leydig cells from at least six littermates. *, Significant differences (P < 0.05).
To verify whether a compensatory increase in expression of ALK2 and/or ALK6 occurs in Leydig cells of ALK3 conditional knockout mice, we examined their mRNA expression and found that ALK2 and ALK6 expression was similar among ALK3fx/fxCyp17cre+ mice and control littermates
Immunochemistry
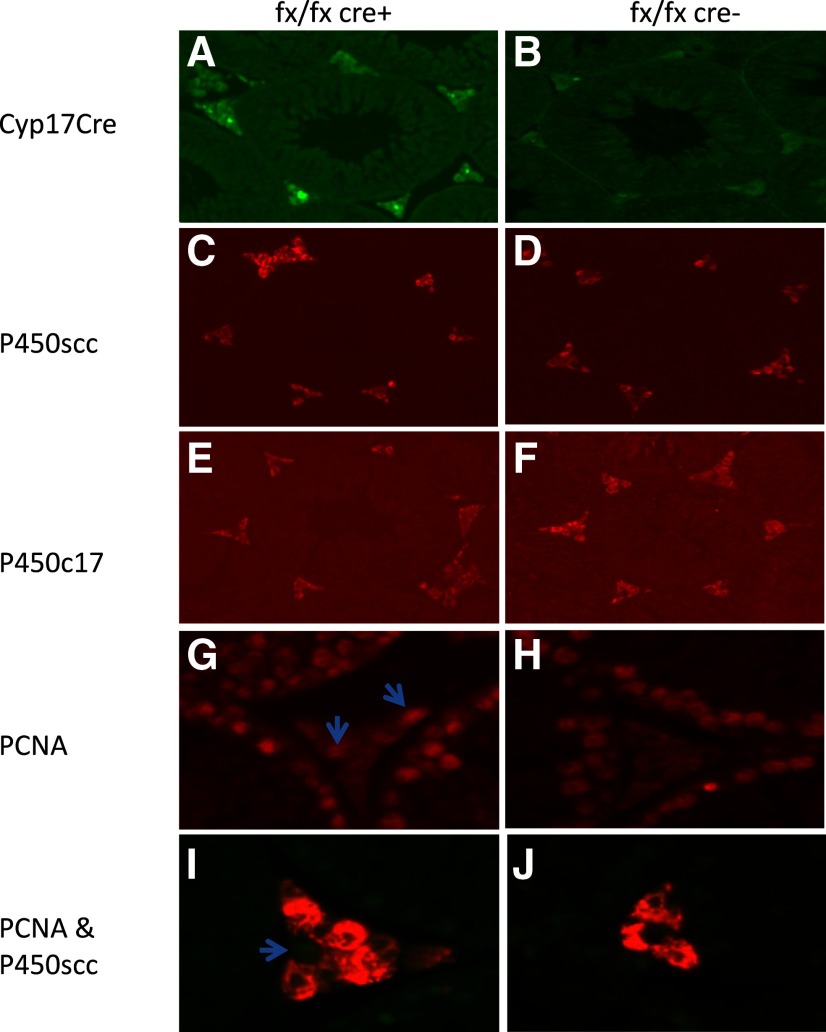
The expression of 17-hydroxylase-cre-IRES-GFP in testicular sections was found only in Leydig cells of ALK3fx/fxCyp17cre+ mice but not control mice (Fig. 5, A and B), confirming Leydig cell-specific deletion of ALK3. To determine whether loss of ALK3 in Leydig cells of ALK3fx/fxCyp17cre+ mice altered aromatization of testosterone to estrogen, expression of aromatase in testicular sections was examined by immunohistochemistry. These studies showed low expression of aromatase with no difference in the number of aromatase-positive Leydig cells in the ALK3fx/fxCyp17cre+ mice compared with control mice (data not shown). We also examined the expression of P450scc, P450c17α, and PCNA (conjugated with Alexa Fluor 568 secondary antibody) in Leydig cells. P450scc staining intensity was similar in Leydig cells from ALK3fx/fxCyp17cre+ (Fig. 5C) and control mice (Fig. 5D). P450c17α staining intensity was lower in Leydig cells of ALK3fx/fxCyp17cre+ (Fig. 5E) than control mice (Fig. 5F), whereas that of Leydig cell PCNA was higher in ALK3fx/fxCyp17cre+ mice (Fig. 5, G–J).
Fig. 5.
Immunohistochemistry for P450scc, StAR, P450c17α, and PCNA in Leydig cells. A and B, 17-Hydroxylase-cre-IRES-GFP is expressed in Leydig cells of ALK3fx/fxCyp17cre+ mice (A) but not control mice (B); C–F, although P450scc expression is similar (C and D), P450c17α staining intensity is lower in ALK3fx/fxCyp17cre+ (E) than control (F) mice.; G–J, PCNA expression (arrows) is significantly higher in ALK3fx/fxCyp17cre+ (G and I) than control (H and J) mice.
Discussion
Our study shows that deletion of ALK3 in Leydig cells alters the developmental profile of androgen synthesis and metabolism as well as the maturation and differentiation of Leydig cells. In this study, we found that the expression of two key regulatory proteins for steroidogenesis CYP17A1 (P450c17) and STAR (StAR) were significantly lower in Leydig cell-specific ALK3 conditional knockout (ALK3fx/fxCyp17cre+) mice than in control mice. We also found that Leydig cells from adult ALK3fx/fxCyp17cre+ mice were less differentiated, as reflected by decreased expression of 17βHSD-III and 3βHSD-VI (40, 41), markers of terminally differentiated Leydig cells. Except for the lower expression of P450c17 (an androgen-biosynthetic enzyme) and difference in in vitro steroidogenesis (9), these results are consistent with our previous data in Leydig cells of MIS-knockout mice (28), indicating that deletion of ALK3 in Leydig cells has a similar but not identical phenotype as deletion of MIS. Either loss of MIS or loss of its candidate type 1 receptor, ALK3, leads to less differentiated adult Leydig cells.
Leydig cell differentiation is accompanied by characteristic maturational changes in the expression of androgen-metabolizing enzymes (5α-reductase and 3α-HSD) that degrade and inactivate testosterone. With onset of puberty, the androgen-metabolizing enzymes are induced to their highest concentrations during midpuberty, and then their expression declines as Leydig cells mature, resulting in increased concentrations of testosterone in the late pubertal and adult stage (42–44). Our study shows that the expression of 5α-reductase and 3α-HSD was significantly higher in Leydig cells of adult ALK3fx/fxCyp17cre+ mice on postnatal d 65 than that of control littermates, indicating that MIS signaling might play a role in the normal maturational decline in the expression of metabolic enzymes. Consequently, Leydig cells in adult ALK3fx/fxCyp17cre+ mice with absent MIS signaling have increased androgen metabolic activity, possibly reflecting a more immature state (9, 44). In parallel with these changes in the metabolic enzymes, the young adult ALK3fx/fxCyp17cre+ mice have lower expression of steroidogenic enzymes. Cumulatively, these data provide evidence that Leydig cells in the ALK3fx/fxCyp17cre+ mouse model are relatively immature and less differentiated than that of control mice. The Leydig cell conditional deletion of ALK3 has effects on Leydig cell steroid pathways that are similar to that of mice with a targeted deletion of MIS (9).
Based on the net changes in expression of the steroidogenic and metabolic enzymes, we expected to find lower plasma and/or intratesticular androgen concentrations in the ALK3 conditional knockout mice. However, plasma concentrations of both testosterone and androstenedione in ALK3fx/fxCyp17cre+ mice were comparable to those in control littermates despite their reduced steroidogenic capacity, similar to the MIS-knockout mice (9). To explain these findings, we speculate that the increase in cell numbers caused by the loss of ALK3 expression compensated for the reduced per-cell capacity for androgen biosynthesis.
Progenitor Leydig cells are highly proliferative and then become less mitotically active as they differentiate from the prepubertal to the pubertal stage and become quiescent when terminally differentiated. Leydig cell proliferation and differentiation are stimulated by LH and IGF-I (45, 46), but we have shown that MIS is also an important factor in modifying the proliferation of progenitor Leydig cells and the differentiation of immature pubertal Leydig cells (7–9). During normal Leydig cell development, Leydig cell proliferation gradually declines, and the expression of androgen biosynthetic enzymes increases, whereas the expression of androgen-metabolizing enzymes declines (36, 43, 47, 48). That MIS has a critical role in this process is supported by several lines of evidence. First, transgenic mice that overexpress MIS have fewer Leydig cells (3, 7); second, MIS-null and MISRII-null mice develop Leydig cell hyperplasia (7, 9); and third, MIS inhibits Leydig cell proliferation in vitro. Therefore, if ALK3 is the crucial type I receptor partner of MIS for Leydig cell differentiation, deleting ALK3 disrupts MIS signaling, which then permits the proliferation of Leydig cell progenitors. Indeed, we previously showed that targeted deletion of the MIS gene promotes continued proliferation of progenitor Leydig cells and induces their maturational delay (9). Similarly, in the current study, we found that we were able to harvest approximately 30% more Leydig cells from ALK3fx/fxCyp17cre+ mice than from control littermates and that PCNA expression in ALK3fx/fxCyp17cre+ mice was 2-fold higher than that of control littermates, suggesting that deletion of ALK3 results in a greater number of immature Leydig cells that remain actively proliferating. Therefore, we speculate that ALK3 is the primary candidate for the type I receptor recruited to the MIS signaling complex during Leydig cell development.
Circulating androgen concentration is dependent on the net balance between androgen biosynthesis and metabolism and the total number of Leydig cells. The increased Leydig cell numbers in ALK3fx/fxCyp17cre+ mice may offset the decreased androgen biosynthetic capacity and increased androgen-metabolizing activity of individual Leydig cells in these mice. Our data show that plasma and intratesticular testosterone and androstenedione concentrations in ALK3fx/fxCyp17cre+ mice were similar to that of control mice. The comparable plasma and intratesticular testosterone concentrations in these mouse models are consistent with our previous results in adult MIS-knockout and wild-type mice (9). One discrepancy between the two models is the in vitro testosterone production of cultured primary Leydig cells. Leydig cells from MIS-knockout mice produce less testosterone than controls, whereas those from ALK3 conditional deletion mice are similar to controls. Although both genetic models result in an increase in Leydig cell numbers that compensates for the decreased biosynthetic capacity of individual Leydig cells, the increase in Leydig cell numbers did not dramatically alter testis weight of ALK3fx/fxCyp17cre+ mice, which is similar to that of control mice, whereas testes of MIS-knockout mice were 17.5% heavier than controls (9).
We speculate that the severity of the phenotype might be modified by the ability of other type 1 receptors to be recruited by the MISRII. This hypothesis is consistent with the report that ALK2 can compensate for ALK3 in in vitro systems to transactivate reporter constructs (25). Although we found no compensatory increase in the expression of ALK2 or ALK6 mRNA in ALK3fx/fxCyp17cre+ Leydig cells, it is possible that in the absence of ALK3, the endogenous ALK2 could form a complex with MISRII and elicit downstream actions. In contrast, MISRII is highly specific for MIS and does not bind other related ligands; therefore, the MIS-null mice will have complete loss of function, whereas other type I receptors might partially compensate for the ALK3 deletion, leading to the phenotypic differences described above.
In summary, deletion of ALK3 compromises the androgen-biosynthetic capacity of individual Leydig cells and results in inhibition of Leydig cell maturation and differentiation. Despite these effects, plasma testosterone concentrations were unaffected, but measures of proliferation were increased. Therefore, the concentrations of plasma testosterone and androstenedione in the ALK3 conditional knockout mouse model were similar to controls, as we observed in MIS-knockout and control mice (9). This study reveals that conditional deletion of ALK3 in a mouse model is similar to the Leydig cell phenotype of MIS-knockout mice, verifying that ALK3 is the specific type I receptor partner for MIS in Leydig cells and plays a key role in the regulation of Leydig cell development and differentiation.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD01367 and HD36768 to M.M.L.). LH was assayed at the Ligand Assay and Analysis Core of the University of Virgina Center for Research in Reproduction through NICHD (Specialized Cooperative Centers Program in Reproductive Research) Grant U54-HD28934. M.M.L. is a member of the University of Massachusetts Diabetes Research Center P3DK032520.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALK 3
- Activin receptor-like kinase 3
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- MIS
- Müllerian inhibiting substance
- MISRII
- MIS type II receptor
- PCNA
- proliferating cell nuclear antigen
- qPCR
- quantitative real-time PCR
- StAR
- steroidogenic acute regulatory protein.
References
- 1. Massagué J. 1998. TGF-β signal transduction. Annu Rev Biochem 67:753–791 [DOI] [PubMed] [Google Scholar]
- 2. Massagué J, Wotton D. 2000. Transcriptional control by the TGF-beta/Smad signaling system. EMBo J 19:1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. 1990. Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature 345:167–170 [DOI] [PubMed] [Google Scholar]
- 4. Behringer RR, Finegold MJ, Cate RL. 1994. Mullerian-inhibiting substance function during mammalian sexual development. Cell 79:415–425 [DOI] [PubMed] [Google Scholar]
- 5. Lee MM. 2000. MIS actions in the developing testis. In: Goldberg E, ed. The testis: from stem cell to sperm function. New York: Springer-Verlag; 30–42 [Google Scholar]
- 6. Lee MM, Seah CC, Masiakos PT, Sottas CM, Preffer FI, Donahoe PK, Maclaughlin DT, Hardy MP. 1999. Mullerian inhibiting substance type II receptor expression and function in purified rat Leydig cells. Endocrinology 140:2819–2827 [DOI] [PubMed] [Google Scholar]
- 7. Racine C, Rey R, Forest MG, Louis F, Ferré A, Huhtaniemi I, Josso N, di Clemente N. 1998. Receptors for anti-Mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc Natl Acad Sci USA 95:594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salva A, Hardy MP, Wu XF, Sottas CM, MacLaughlin DT, Donahoe PK, Lee MM. 2004. Mullerian-inhibiting substance inhibits rat Leydig cell regeneration after ethylene dimethanesulphonate ablation. Biol Reprod 70:600–607 [DOI] [PubMed] [Google Scholar]
- 9. Wu X, Arumugam R, Baker SP, Lee MM. 2005. Pubertal and adult Leydig cell function in Mullerian inhibiting substance-deficient mice. Endocrinology 146:589–595 [DOI] [PubMed] [Google Scholar]
- 10. Kretzschmar M, Massagué J. 1998. SMADs: mediators and regulators of TGF-β signaling. Curr Opin Genet Dev 8:103–111 [DOI] [PubMed] [Google Scholar]
- 11. Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. 2001. Mullerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol 15:946–959 [DOI] [PubMed] [Google Scholar]
- 12. Gouédard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, Josso N, Massagué J, di Clemente N. 2000. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J Biol Chem 275:27973–27978 [DOI] [PubMed] [Google Scholar]
- 13. Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, Ingraham HA. 2001. The serine/threonine transmembrane receptor ALK2 mediates Mullerian inhibiting substance signaling. Mol Endocrinol 15:936–945 [DOI] [PubMed] [Google Scholar]
- 14. Matzuk MM, Finegold MJ, Mishina Y, Bradley A, Behringer RR. 1995. Synergistic effects of inhibins and Mullerian-inhibiting substance on testicular tumorigenesis. Mol Endocrinol 9:1337–1345 [DOI] [PubMed] [Google Scholar]
- 15. Mishina Y. 2002. The in vivo function of Mullerian-inhibiting substance during mammalian sexual development. In: Matzuk MM, Brown CW, Kumar TR, eds. Transgenics in endocrinology (contemporary endocrinology). Totowa, NJ: Human Press; 41–59 [Google Scholar]
- 16. Mishina Y, Crombie R, Bradley A, Behringer RR. 1999. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol 213:314–326 [DOI] [PubMed] [Google Scholar]
- 17. Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR. 1996. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 10:2577–2587 [DOI] [PubMed] [Google Scholar]
- 18. Lyet L, Louis F, Forest MG, Josso N, Behringer RR, Vigier B. 1995. Ontogeny of reproductive abnormalities induced by deregulation of anti-Mullerian hormone expression in transgenic mice. Biol Reprod 52:444–454 [DOI] [PubMed] [Google Scholar]
- 19. Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. 1999. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126:2551–2561 [DOI] [PubMed] [Google Scholar]
- 20. Mishina Y, Suzuki A, Ueno N, Behringer RR. 1995. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9:3027–3037 [DOI] [PubMed] [Google Scholar]
- 21. Mishina Y, Whitworth DJ, Racine C, Behringer RR. 1999. High specificity of Mullerian-inhibiting substance signaling in vivo. Endocrinology 140:2084–2088 [DOI] [PubMed] [Google Scholar]
- 22. Jamin SP, Arango NA, Mishina Y, Behringer RR. 2002. Genetic studies of MIS signalling in sexual development. Novartis Found Symp 244:157–164, discussion 164–168, 203–206, 253–257 [PubMed] [Google Scholar]
- 23. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. 2002. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32:408–410 [DOI] [PubMed] [Google Scholar]
- 24. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. 2003. Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol Cell Endocrinol 211:15–19 [DOI] [PubMed] [Google Scholar]
- 25. Belville C, Jamin SP, Picard JY, Josso N, di Clemente N. 2005. Role of type I receptors for anti-Mullerian hormone in the SMAT-1 Sertoli cell line. Oncogene 24:4984–4992 [DOI] [PubMed] [Google Scholar]
- 26. Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K. 2001. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci 114:1483–1489 [DOI] [PubMed] [Google Scholar]
- 27. Bernard DJ. 2003. SMAD expression in the testis predicts age- and cell-specific responses to activin and TGFβ. J Androl 24:201–203 [DOI] [PubMed] [Google Scholar]
- 28. Xu J, Beyer AR, Walker WH, McGee EA. 2003. Developmental and stage-specific expression of Smad2 and Smad3 in rat testis. J Androl 24:192–200 [DOI] [PubMed] [Google Scholar]
- 29. Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. 2005. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci USA 102:5062–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klinefelter GR, Hall PF, Ewing LL. 1987. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod 36:769–783 [DOI] [PubMed] [Google Scholar]
- 31. Klinefelter GR, Kelce WR, Hardy MP. 1993. Isolation and culture of Leydig cells from adult rats. In: Heindel J, Chaplin RE, eds. Methods in reproductive toxicology. New York: Academic Press; 166–181 [Google Scholar]
- 32. Salva A, Klinefelter GR, Hardy MP. 2001. Purification of rat Leydig cells: increased yields after unit-gravity sedimentation of collagenase-dispersed interstitial cells. J Androl 22:665–671 [PubMed] [Google Scholar]
- 33. Payne AH, Downing JR, Wong KL. 1980. Luteinizing hormone receptors and testosterone synthesis in two distinct populations of Leydig cells. Endocrinology 106:1424–1429 [DOI] [PubMed] [Google Scholar]
- 34. Wang ZJ, Jeffs B, Ito M, Achermann JC, Yu RN, Hales DB, Jameson JL. 2001. Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc Natl Acad Sci USA 98:7988–7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cochran RC, Ewing LL, Niswender GD. 1981. Serum levels of follicle stimulating hormone, luteinizing hormone, prolactin, testosterone, 5α-dihydrotestosterone, 5α-androstane-3α,17β-diol, 5α-androstane-3β,17β-diol, and 17β-estradiol from male beagles with spontaneous or induced benign prostatic hyperplasia. Invest Urol 19:142–147 [PubMed] [Google Scholar]
- 36. Cochran RC, Schuetz AW, Ewing LL. 1979. Age-related changes in conversion of 5α-androstan-17β-ol-3-one to 5α-androstane-3α,17β-diol and 5α-androstane-3β,17β-diol by rat testicular cells in vitro. J Reprod Fertil 57:143–147 [DOI] [PubMed] [Google Scholar]
- 37. Coffey JC, French FS, Nayfeh SN. 1971. Metabolism of progesterone by rat testicular homogenates. IV. Further studies of testosterone formation in immature testis in vitro. Endocrinology 89:865–872 [DOI] [PubMed] [Google Scholar]
- 38. Weinstein A, Lindner HR, Friedlander A, Bauminger S. 1972. Antigenic complexes of steroid hormones formed by coupling to protein through position 7: preparation from 4-3-oxosteroids and characterization of antibodies to testosterone and androstenedione. Steroids 20:789–812 [DOI] [PubMed] [Google Scholar]
- 39. Zamecnik J, Barbe G, Moger WH, Armstrong DT. 1977. Radioimmunoassays for androsterone, 5α-androstane-3α,17β-diol and 5α-androstane-3β,17β-diol. Steroids 30:679–689 [DOI] [PubMed] [Google Scholar]
- 40. O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. 2002. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci 115:3491–3496 [DOI] [PubMed] [Google Scholar]
- 41. O'Shaughnessy PJ, Willerton L, Baker PJ. 2002. Changes in Leydig cell gene expression during development in the mouse. Biol Reprod 66:966–975 [DOI] [PubMed] [Google Scholar]
- 42. Dong L, Jelinsky SA, Finger JN, Johnston DS, Kopf GS, Sottas CM, Hardy MP, Ge RS. 2007. Gene expression during development of fetal and adult Leydig cells. Ann NY Acad Sci 1120:16–35 [DOI] [PubMed] [Google Scholar]
- 43. Ge RS, Hardy MP. 1998. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology 139:3787–3795 [DOI] [PubMed] [Google Scholar]
- 44. Wu X, Arumugam R, Zhang N, Lee MM. 2010. Androgen profiles during pubertal Leydig cell development in mice. Reproduction 140:113–121 [DOI] [PubMed] [Google Scholar]
- 45. Sriraman V, Niu E, Matias JR, Donahoe PK, MacLaughlin DT, Hardy MP, Lee MM. 2001. Mullerian inhibiting substance inhibits testosterone synthesis in adult rats. J Androl 22:750–758 [PubMed] [Google Scholar]
- 46. Wu X, Wan S, Lee MM. 2007. Key factors in the regulation of fetal and postnatal Leydig cell development. J Cell Physiol 213:429–433 [DOI] [PubMed] [Google Scholar]
- 47. Ge RS, Shan LX, Hardy MP. 1996. Pubertal development of Leydig cells. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig cell. 1st ed Vienna, Cache River Press; 159–174 [Google Scholar]
- 48. Hardy MP, Gelber SJ, Zhou ZF, Penning TM, Ricigliano JW, Ganjam VK, Nonneman D, Ewing LL. 1991. Hormonal control of Leydig cell differentiation. Ann NY Acad Sci 637:152–163 [DOI] [PubMed] [Google Scholar]