Abstract
Organophosphate nerve agents and pesticides are potent inhibitors of acetylcholinesterase (AChE). Although oxime nucleophiles can reactivate the AChE-phosphyl adduct, the adduct undergoes a reaction called aging. No compounds have been described that reactivate the aged-AChE adduct. A family of 2-methoxypyridinium species which reverse aging in a model system is presented. A kinetic study of this system, which includes an SAR analysis, demonstrates that the reaction is highly tunable based on the ring substituents.
Organophosphorus (OP) agents have been used as both pesticides and chemical warfare agents for most of the past century.1,2 Pesticides based on phosphate or thiophosphate agents (e.g. parathion, diazinon, or malathion) are or have been used in domestic and commercial agriculture to protect crops from a variety of destructive species. Despite numerous efforts to ban or limit the use of OP pesticides, their continued use results in over 200,000 fatalities and over 1 million instances of morbidity annually due to OP exposure. Phosphonate nerve agents, including sarin, soman, VX, and tabun, are extremely toxic and are considered a serious threat to national security due to their potential use in terrorist actions.1
These nerve agents elicit their acute toxicity by inhibiting the cholinergic system which results in an overstimulation of muscles due to the buildup of acetylcholine in the neuromuscular junction. The ultimate result is muscle fasciculation and subsequent cardiopulmonary arrest.1,2 Specifically, OPs inhibit acetylcholinesterase (AChE) by covalently bonding to the serine residue in the catalytic triad of the active site (Figure 1). The well documented and tremendous affinity of OP agents for the active cite of AChE is a result of an OP’s structural similarity to the tetrahedral transition state of the cognate hydrolysis reaction of acetylcholine.3
Figure 1.
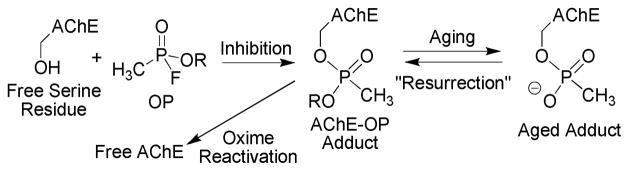
AChE Inhibition by Organophosphonates.
There are several potential treatments for both acute and chronic organophosphate poisoning. Most include a nucleophilic oxime to reactivate the enzyme along with atropine as an acetylcholine receptor agonist and diazepam to treat seizures that arise from inhibition of AChE in the CNS.1,2 If administered shortly after exposure, the oxime (i.e. 2-PAM) is able to displace the bound OP and liberate the serine residue (Figure 1).4 If administration of the oxime is delayed a process called aging occurs where the enzyme bound phosphonate experiences a solvolytic loss of an alkyl group (Figure 1: R = isopropyl for sarin, pinacoyl for soman).1,5 The aged adduct is a phosphonate ester monoanion, which is intrinsically less reactive as an electrophile than the neutral phosphonate ester of the initial phosphonyl-AChE adduct. The aged adduct is further stabilized by several key interactions with the AChE active site, and is refractory to oxime reactivation.5 No compounds have yet been discovered that are able to recover AChE activity once aging has occurred, and therefore there are no antidotes against aged AChE-OP adducts.1,4
We hypothesize that realkylation of the aged adduct could lead to a reactivatable AChE adduct and have initiated studies to demonstrate this. Because the aged complex has long been thought of as a dead enzyme, we term this concept “resurrection” of the aged adduct (Figure 1). Presented here is the description of a family of N-methyl-2-methoxypyridinium species which are effective at “resurrecting” a model phosphonate system. An analysis of the kinetics of these reactions demonstrates the highly tunable reactivity of N-methyl-2-methoxypyridinium species as alkylating agents for the methyl methanephosphonate monoanion.
Our studies began with a literature search of alkylating agents that are known to react with phosphate or phosphonate anions. Although several alkylating agents or reactive intermediates are capable of phosphonate anion alkylation, few provide practical solutions to the aging problem due to their aqueous instability, poor selectivity, or toxicity (i.e. MeOTf is not suitable).6 This led to the consideration of less traditional methyl transfer reagents. Particularly appealing were classic reports that describe the equilibration of 2-methoxypyridine to N-methylpyridone that utilized N-methyl-2-methoxypyridinium as a catalyst.7 The methyl transfer reactivity of these species and their structural similarity to the oxime AChE reactivator 2-PAM lead to the consideration of them as aged AChE-OP resurrecting agents.
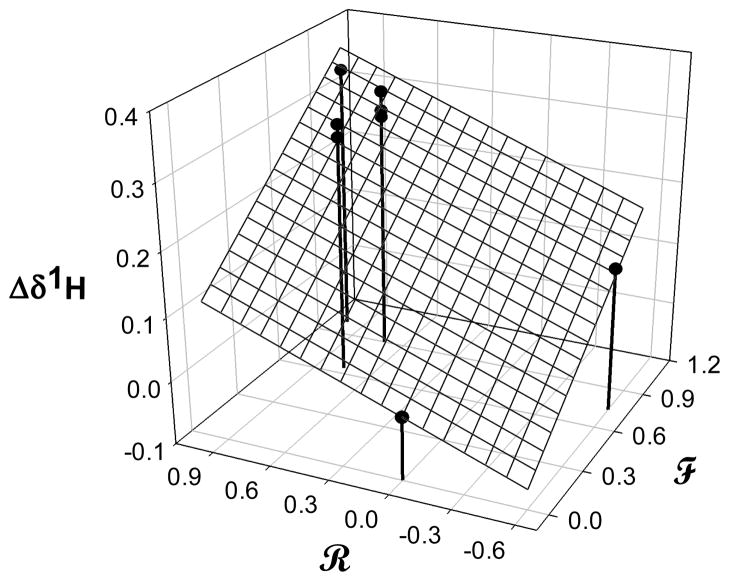
The synthesis of N-methyl-2-methoxypyridinium species was conducted by exposure of various commercial pyridines to trimethoxonium tetrafluoroborate (Scheme 1). This resulted in a family of nine pyridinium tetrafluoroborates with varied substituents. Other work demonstrated the utility of 1H NMR is measuring constants associated with this family of compounds.8 Previous reports have evaluated the 1H NMR chemical shift of the pyridinium N-methyl as a measure of electron density.8a In an expansion of this, Figure 2 shows a multiple linear correlation of the methoxy methyl shift against the Swain-Lupton field and resonance parameters9,10 of the ring substituents. The correlation shows that the chemical shift of the methoxy moiety, and hence its electron density, is markedly dependent on both field and resonance effects of substituents. Further correlations with reactivity are discussed below.
Scheme 1.
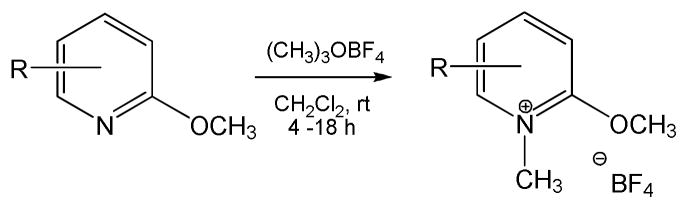
Synthesis
Figure 2.
Multiple Linear Correlation of OCH3 Chemical Shift. Parameters of the fit are field = 0.25 ± 0.02, res = 0.11 ± 0.03, r2 = 0.93, n = 8. See text for elaboration.
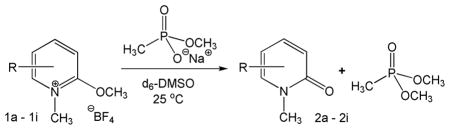
The effectiveness of these compounds as methyl transfer agents was determined by using sodium methyl methanephosphonate as an analogue of the aged AChE-OP adduct. Model reactions were conducted by mixing equimolar concentrations of phosphonate salt with the pyridinium species (27 mM in d6-DMSO) and the reaction was monitored by 1H NMR spectroscopy (see header of Table 1).
Table 1.
Methylation of Model Systema
| ||||
|---|---|---|---|---|
| entry | R = | kMe | khyd | kMe/kMeH |
| 1a | H | 4.6 ± 0.1 × 10−5 | 2.8 ± 0.2 × 10−6 | 1 |
| 1b | 3-F | 3.1 ± 0.1 × 10−2 | 1.1 ± 0.1 × 10−3 | 670 |
| 1c | 5-F | 1.6 ± 0.2 × 10−4 | NDb | 3.5 |
| 1d | 5-CF3 | 2.83 ± 0.03 × 10−3 | 6.0 ± 0.1 × 10−4 | 60 |
| 1e | 6-CF3 | 4.63 ± 0.08 × 10−3 | 8.7 ± 0.2 × 10−4 | 100 |
| 1f | 5-NO2 | 5.6 ± 0.1 × 10−3 | 2.2 ± 0.1 × 10−2 | 120 |
| 1g | 4-CN | 2.47 ± 0.06 × 10−3 | 3.3 ± 0.2 × 10−4 | 50 |
| 1h | 5-CN | 5.13 ± 0.07 × 10−3 | 3.9 ± 0.1 × 10−3 | 110 |
| 1i | 6-CN | 5.8 ± 0.2 × 10−3 | 2.1 ± 0.1 × 10−3 | 125 |
Reactions were conducted in d6-DMSO at 25.0 ± 0.5 °C and were monitored by 1H NMR spectroscopy at 500 MHz.
ND = not determined.
All reactants and products in the header of Table 1 could be simultaneously monitored. Moreover, a broad resonance in the 1H NMR spectra centered at 3.4 ppm was interpreted as low levels of water, which gives rise to a competing hydrolysis reaction of the N-methyl-2-methoxypyridinium reactants to generate methanol. Indeed, methanol was detected in product spectra through its methyl resonance at 3.2 ppm. Consequently, the overall kinetics of the reaction system can be described by the following component differential equations:
| (1) |
| (2) |
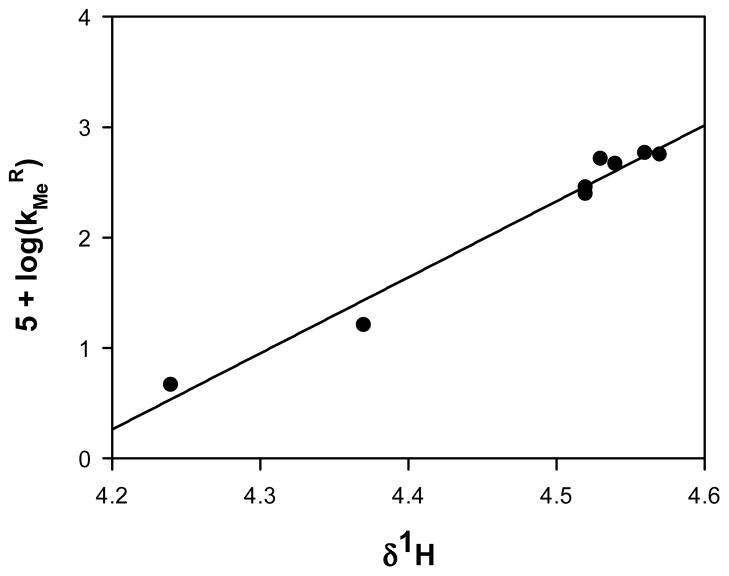
In these equations A, and B are the methoxypyridinium and phosphonate monoanion reactants, respectively, P and Q are the pyridone and neutral phosphonate products, respectively, and kMe and khyd are the respective second-order rate constants for the methyl transfer and hydrolysis reactions. Since time-dependent changes in the concentrations of all four species A, B, P and Q can be measured, one can write from equations 1 and 2 four independent differential equations, one each for -d[A]/dt, d[P]/dt, -d[B]/dt and d[Q]/dt. For each of the nine methyl transfer reactions, these four differential equations were numerically integrated by fourth-order Runge-Kutta integration10 (see Supplemental Information for details), which provided least-squares estimates of the rate constants kMe for all nine N-methyl-2-methoxypyridinium reactants, and khyd values for all but the compound for which R = 5-F (see Table 1 for rate constants). Nearly three orders of magnitude distinguish the values of the fastest and slowest rate constants of methyl transfer amongst the nine compounds evaluated. Interestingly, an excellent linear free energy correlation, plotted in Figure 3, is noted between methyl transfer rate constants and the methoxy methyl chemical shifts that are plotted in the multiple linear correlation of Figure 2. This correlation and that in Figure 2 suggest in turn that there will be a good correlation between log(kMeR) and the Swain-Lupton parameters, and this is indeed the case (see SI for plot); the corresponding parameters are field = 1.4 ± 0.2, res = 1.0 ± 0.2, r2 = 0.93, n = 8. These data indicate that kMe is markedly sensitive to both the field and resonance effects of substituents, as one would expect if the transition state for methyl transfer from N-methyl-2-methoxypyridinium species is relatively late and the leaving group strongly resembles the N-methylpyridone product. Moreover, the correlation between log(kMe) and methoxy methyl chemical shift indicates that additional methyl donor substrates can be readily screened by simple 1H NMR spectroscopy.
Figure 3.
Linear Free-Energy Correlation Between Methyl Transfer Rate Constants and Methoxy Methyl Chemical Shifts. Parameters of the fit are slope = 6.9 ± 0.4, intercept = −29 ± 2, r2 = 0.98, n = 8.
In a practical sense, the fastest compound (R = 3-F) was capable of 40% methyl transfer to the methyl methanephosphonate monoanion in less than 10 min. This is within the expected time frame for the treatment of OP exposure. The marked sensitivity of the methyl transfer rate constant to substituent provides a temporal tuneability that will likely be important in eventual design of resurrection agents for aged OP-AChE adducts.
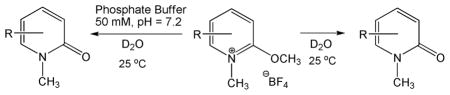
The hydrolytic stability of the N-methyl-2-methoxypyridinium species is an issue of considerable significance, since antidotes against aged OP-AChE adducts must survive the aqueous media of the bloodstream to reach their target. Consequently, initial rates of hydrolysis were obtained by observing pseudo-first order hydrolysis in D2O by 1H NMR spectroscopy (Table 2). Since the initial rate is Vi = khyd[A], the corresponding first-order rate constant is calculated as khyd = Vi/[A]. No hydrolysis was observed for compounds R = H, 5-F, and 6-CF3 over one week in D2O. The rate constants of hydrolysis for all compounds were significantly slower than those of methyl transfer in DMSO, typically by two orders of magnitude. The 5-NO2 compound showed the lowest selectivity for methyl transfer over hydrolysis. Another and perhaps more germane measure of hydrolytic stability versus methyl transfer activity was gauged by measuring rate constants of hydrolysis in phosphate buffer. Table 2 shows that the rate constants of hydrolysis in buffer at near physiological pH are significantly faster than those in pure D2O, but are still markedly slower than methyl transfer in DMSO. The only compound that showed methyl transfer to the phosphate buffer was that with R = 3-F, which showed slower kinetics of methyl transfer in aqueous media than hydrolysis. The kinetic selectivity of methyl transfer from pyridinium compounds in DMSO, as a model of the AChE active site, versus aqueous hydrolysis suggests that blood serum lifetimes of select pyridinium compounds will be sufficient to reach the aged OP-AChE targets at neuromuscular junctions. On the other hand, the kinetic selectivity of methyl transfer in DMSO versus that to phosphate buffer components in aqueous media suggests that unselective alkylation of other biological phosphorous molecules (e.g. DNA, RNA, or ATP) will be minimal.
Table 2.
Rate Constants of Hydrolysisa
| |||
|---|---|---|---|
| entry | R = | khyd in D2O | khyd in bufferb |
| 1a | H | NDc | NDc |
| 1b | 3-F | 2.3 ± 0.2 × 10−5 | 7.1 ± 0.3 × 10−5 |
| 1c | 5-F | NDc | NDc |
| 1d | 5-CF3 | 1.10 ± 0.05 × 10−6 | 7.0 ± 0.5 × 10−5 |
| 1e | 6-CF3 | NDc | 1.20 ± 0.06 x10−4 |
| 1f | 5-NO2 | 6.4 ± 0.4 × 10−5 | 2.40 ± 0.07 × 10−3 |
| 1g | 4-CN | 1.40 ± 0.03 × 10−6 | 1.60 ± 0.04 × 10−4 |
| 1h | 5-CN | 1.40 ± 0.05 × 10−5 | 8.3 ± 0.2 × 10−4 |
| 1i | 6-CN | 4.1 ± 0.3 × 10−6 | 4.10 ± 0.04 × 10−4 |
Rate constants given in units of min−1 were determined by 1H NMR spectroscopy.
50 mM phosphate buffer pH = 7.2.
Not determined, the reaction was too slow to observe hydrolysis.
In conclusion, a family of N-methyl-2-methoxypyridinium species has been shown capable of methylating a weakly basic phosphonate anion, which is a model for an aged AChE-OP adduct. This reaction is highly tunable based on substituent dependent electronic factors and gratifyingly high rates of methyl transfer have been observed.11 On the other hand, these compounds are relatively stable in aqueous solution. The resurrection activity of these compounds versus aged AChE-OP adducts is being explored and will be reported in due course.
Supplementary Material
Acknowledgments
Mr. Alexander Lodge (U-Iowa) is acknowledged for the preparation of sodium methyl methanephosphonate. Supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number 5 R21 NS076430.
Footnotes
Supporting Information Available Experimental preparation, 1H and 13C NMR for all compounds, and expanded kinetic analysis. This material is available free of charge via the Internet at http://pubs.acs.org.”
References
- 1.Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Acc Chem Res. 2012;45:756. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- 2.Jokanovic M, Prostran M. Curr Med Chem. 2009;16:2177. doi: 10.2174/092986709788612729. [DOI] [PubMed] [Google Scholar]
- 3.Quinn DM. Chem Rev. 1987;87:955. [Google Scholar]
- 4.The synthesis of improved oximes is an ongoing field of research: Kalisiak J, Ralph EC, Zhang J, Cashman JR. J Med Chem. 2011;54:3319. doi: 10.1021/jm200054r.Kalisiak J, Ralph EC, Cashman JR. J Med Chem. 2012;55:465. doi: 10.1021/jm201364d.Mercey G, Verdelt T, Saint-Andre G, Gillon E, Wagner A, Baati R, Jean L, Nachon F, Renard PY. Chem Commun. 2011;47:5295. doi: 10.1039/c1cc10787a.Sit RK, Radic Z, Gerardi V, Zhang L, Garcia E, Katalinic M, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. J Bio Chem. 2011;286:19422. doi: 10.1074/jbc.M111.230656.
- 5.Characterization of aged OP-AChE adducts: Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Biochem. 1999;38:7032. doi: 10.1021/bi982678l.Sanson B, Nachon F, Colletier J, Froment M, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. J Med Chem. 2009;52:7593. doi: 10.1021/jm900433t.Carletti E, Colletier J, Dupeux F, Trovaslet M, Masson P, Nachon F. J Med Chem. 2010;53:4002. doi: 10.1021/jm901853b.Masson P, Nachon F, Lockridge O. Chem Bio Interact. 2010;187:157. doi: 10.1016/j.cbi.2010.03.027.
- 6.Phosphponate or phosphate anion alkylation with bio-incompatible reagents: Moriarty RM, Condeiu C, Tao A, Prakash O. Tetrahedron Lett. 1997;38:2401.Di Raddo P, Chan TH. J Chem Soc, Chem Commun. 1983:16.Schole J, Schole C, Eikemeyer J. Tetrahedron. 1994;50:1125.Andre V, Lahrache H, Robin S, Rousseau G. Tetrahedron. 2007;63:10066.Pitsch S, Pombo-Villar E, Eschenmoser A. Helvetica Chimica Acta. 1994;77:2251.Jankowski S, Kovacs J, Huben K, Blaszczyk M, Glowka M, Keglevich G. Hetroatom Chem. 2006;17:369.Ayukawa H, Ohuchi S, Ishikawa M, Hata T. Chem Lett. 1995:81.Chavez MR, Zhao P, Kovacs Z, Sherry AD. Lett Org Chem. 2004;1:194.
- 7.(a) Beak P, Bonham J, Lee JT., Jr J Am Chem Soc. 1968;90:1569. [Google Scholar]; (b) Beak P, Bonham J. Chem Commun. 1966:631. [Google Scholar]; (c) Beak P, Lee JT., Jr J Org Chem. 1969;34:2125. [Google Scholar]
- 8.(a) Huang S, Wong JCS, Leung AKC, Chan YM, Wong L, Fernendez MR, Miller AK, Wu W. Tetrahedron Lett. 2009;50:5018. doi: 10.1016/j.tetlet.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wong FM, Capule CC, Chen DX, Gronert S, Wu W. Org Lett. 2008;10:2757. doi: 10.1021/ol800892d. [DOI] [PubMed] [Google Scholar]; (c) Titsky GD, Mitchenko ES, Dereza LI. Ukrainskii Khimicheskii Zhurnal. 1993;59:2077. [Google Scholar]
- 9.(a) Swain CG, Lupton EC. J Am Chem Soc. 1968;90:4328. [Google Scholar]; (b) Swain CG, Unger SH, Rosenquist NR, Swain MS. 1983;105:492. [Google Scholar]; (c) Ewing DF. Correlation of nmr chemical shifts with Hammett sigma values and analogous parameters. In: Chapman NB, Shorter J, editors. Correlation analysis in chemistry-recent advances. Plenum Press; New York: 1978. pp. 357–392. [Google Scholar]; (d) Hamer GK, Peat IR, Reynolds WF. Can J Chem. 1973;51:897. [Google Scholar]
- 10.Carpenter BK. Determination of Organic Reaction Mechanisms. 1. John Wiley & Sons; New York: 1984. pp. 76–82. [Google Scholar]
-
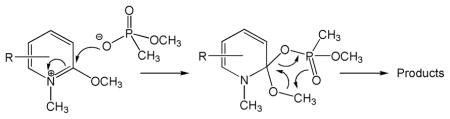
11.An SN2 reaction is depicted in the abstract. The absence of observable intermediates in these reactions would support this. However, a reviewer suggested an alternative mechanism, which is shown below.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.