Abstract
Context:
Bacillus Calmette-Guerin (BCG) therapy is the standard treatment for nonmuscle-invasive bladder cancer (NMIBC). However, its toxicity is a major concern.
Aim:
If we reduce the number of BCG doses by half and replace the second half with epirubicin, we may have a lower toxicity while maintaining the same efficacy of BCG. To test this hypothesis, we conducted this study as an update of our previous report.
Setting and Design:
The study included 607 patients with Ta and T1 NMIBC between January 1994 and December 2008.
Materials and Methods:
After transurethral resection of bladder tumor (TURBT), the patients received weekly doses of 120 mg BCG alternating with 50 mg epirubicin for six weeks (three weekly doses of each). Maintenance was given. Recurrence, progression rates, and toxicity were assessed. End points were progression, recurrence, and cancer-specific survival.
Results:
A total of 532 patients were eligible for evaluation (mean age: 58 years; median follow-up: 45 months). Of these, 291 (55%) were free, 157 (29.5%) showed recurrence, and 84 (15.8%) showed muscle-invasive progression. Toxicity developed in 221 patients. These were mild in the majority (167), whereas 10 developed hematuria, 30 severe cystitis, and five systemic complications. The rate of permanent therapy discontinuation was 3.8%.
Statistical Analysis Used:
SPSS package version 16 and Kaplan-Meier curves were used to evaluate survival.
Conclusions:
Reducing the frequency of BCG instillations by half and replacing the second half with epirubicin results in a similar efficacy and a lower toxicity compared with historical cases receiving BCG alone. However, further trials are required to support these results.
Keywords: Bacillus Calmette-Guerin, epirubicin, nonmuscle-invasive bladder cancer, Ta, T1, toxicity
INTRODUCTION
Bacillus Calmette-Guerin (BCG) is the adjuvant treatment of choice for recurrence prophylaxis of nonmuscle-invasive bladder cancer (NMIBC). However, its toxic local and systemic effects are limitations against its wide use by urologists.[1] Intravesical chemotherapeutic agents, such as epirubicin, are also effective in NMIBC. Although the efficacy of these agents is not comparable to that of BCG, they have the advantage of being less toxic.[2,3]
There have been many trials aimed at maintaining the efficacy and reducing the frequency and severity of toxic and side effects of BCG by dose reduction.[4–7] However, it was suggested that the standard dose may be more effective in high-risk, multiple, tumor in situ (TIS)-associated Ta and/or in cases of NMIBC with a history of previous treatment.[5,8,9] Furthermore, in some studies, dose reduction was not associated with a subsequent reduction of side effects.[10,11]
We hypothesized that if we reduced the number of BCG doses by half and replaced the second half with intravesical epirubicin, we may have a lower toxicity profile while maintaining the efficacy of BCG alone. Therefore, we tested this hypothesis in a prospective randomized trial in 1999 and found that the new therapeutic regimen (alternating immunochemotherapy) had the same efficacy and a lower toxicity than BCG alone.[12]
Herein, an update of our experience with this new regimen in NMIBC, including a larger number of patients over a longer follow-up, is presented.
MATERIALS AND METHODS
The preliminary study idea and design was approved by the local Urology Council (representing the Research Ethics Committee). This prospective study was conducted on 607 patients with histologically proven Ta and T1 bladder urothelial cancer, between January 1994 and December 2008. The inclusion criteria included grade 2 or 3, stage T1, rapid recurrence within six months of initial resection, multicentricity, tumor size equal to or more than 3 cm, associated unifocal carcinoma in situ (CIS) and/or positive postoperative urinary cytology. Exclusion criteria included follow-up less than 18 months, multifocal CIS, and patient death from a known disease unrelated to bladder cancer.
Initially the patients were evaluated by urinalysis, urine culture, serum creatinine, fasting blood sugar level, complete blood count, chest X-ray, excretory urography, and bladder wash for cytology. Complete transurethral resection of the bladder tumor (TURBT) was carried out in all patients. After TURBT, the patients received weekly doses of 120 mg BCG (Pasteur strain) alternating with 50 mg epirubicin for six weeks (single drug per week, i. e., three weeks with BCG and three weeks with epirubicin as shown in Figure 1). Maintenance was given for one year as monthly doses of BCG alternating with epirubicin.[12] As of March 2003, all the patients routinely received a single instillation of 50 mg of epirubicin in the first six hours after TURBT.
Figure 1.
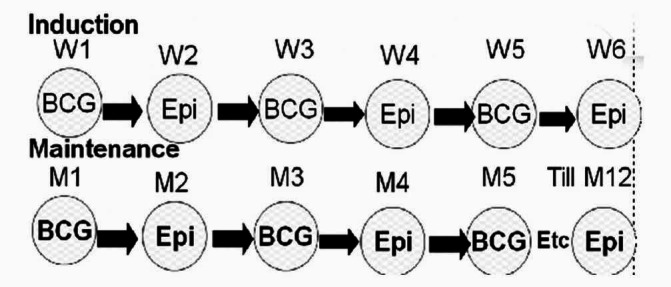
Diagrammatic representation of the induction and maintenance schedules of sequential immunochemotherapy with BCG and epirubicin (Epi). W1, W2, etc refer to week 1, week 2, etc; M1 refers to month 1
The patients were followed every three months during the first two years and every six months thereafter. Clinical manifestations of disease recurrence and/or progression and complications of the intravesical therapy were noted. Evaluation at each visit is detailed in our previous report.[12] In cases of recurrence of the tumor while still in the NMIBC stage, the same protocol was repeated. If there was a failure for the second time in six months, cystectomy was indicated.
The number of patients developing recurrence or muscle-invasive progression during follow-up is referred to as recurrence or progression rate, respectively. It is expressed as a percentage of the total number of patients. The recurrence rate per 100-patient months is defined as the number of recurrences divided by the number of follow-up in months and multiplied by 100.
Recurrence, muscle-invasive progression, time to recurrence or progression, and/or death from bladder cancer were considered as the end points of the study. Kaplan-Meier survival curves were used to estimate disease-free survival.
RESULTS
Seventy-five patients were excluded from the evaluation. Of these, 60 had a follow-up in less than 18 months and 15 died from a definite disease unrelated to bladder cancer. Therefore, 532 patients (454 males, 78 females) were eligible for evaluation. Mean age ± standard deviation (range) in this cohort was 58 ± 12 years (23-85). Follow-up ranged from 18 to 177 months (median 45).
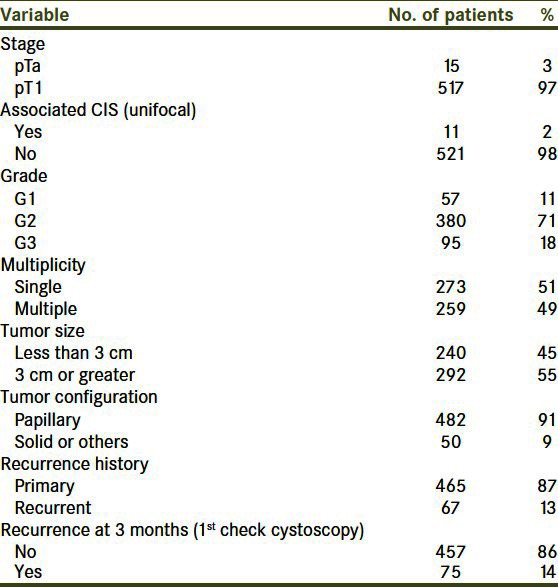
The tumor characteristics in this cohort are shown in Table 1. The stage of the tumor was T1 in 517 patients and unifocal CIS was present in only 11 patients.
Table 1.
Tumor characteristics of the 532 patients eligible for evaluation
During follow-up, recurrence developed in 157 patients (29.5%) and muscle-invasive progression was noted in 84 patients (15.8%). The five-year disease-free survival (no recurrence or progression) and progression-free survival ± standard error values were 57% ± 2% and 83% ± 2%, respectively. The recurrence rate per 12-patient months was 0.1. The mean intervals to first recurrence and to progression were 18 and 20 months, respectively. The disease-free survival in the study group is shown in Figure 2. The actuarial five-year progression-free survival rate was higher for grades 1 and 2 than grade 3 (100, 86, and 58%, respectively; log rank P ≤ 0.001).
Figure 2.
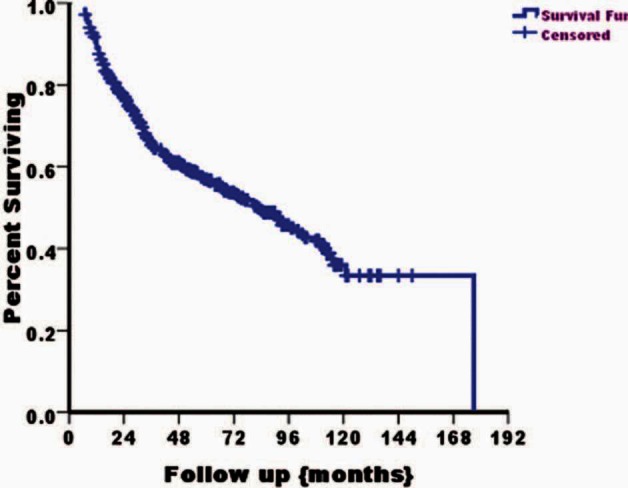
Kaplan-Meier curve showing disease-free (recurrence- and progression-free) survival in the study cohort
The complications and side effects of therapy are shown in Table 2. These side effects were noted in 221 patients (41.5%). Toxic episodes developed in only 4.5% of the instillations. Most of these complications (167; 31.4%) were mild irritative voiding symptoms (mild frequency, urgency, burning micturition) and/or low grade fever and malaise and did not necessitate therapy discontinuation. Ten patients developed hematuria without the need for blood transfusion, 12 had reduced bladder capacity, and five systemic complications. Of the 12 patients with reduced bladder capacity, seven improved on conservative treatment with antituberculous drugs for six months and regained normal capacity, whereas five developed fibrotic contracted bladder and were treated with cystectomy.
Table 2.
Toxicity and side effects of alternating bacillus calmette-guerin and epirubicin in the study cohort
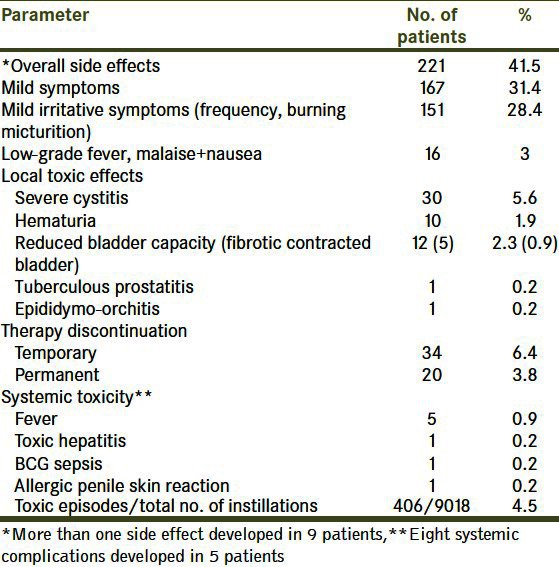
Side effects developed after the fourth dose in 190 patients. Temporary and permanent therapy discontinuation due to toxicity was observed in 34 and 20 patients, respectively.
DISCUSSION
In the current study as well as in our previous trial, we proved that this alternating therapy has a lower toxicity and a similar efficacy as compared with historical controls receiving BCG alone.[1,12,13] Since 1994, this alternating therapy has become the standard of care for Ta and T1 NMIBC in our hospital.
Efficacy
It has been proved in both randomized controlled trials (RCTs) and in meta-analyses that intravesical BCG is the most effective agent for recurrence prophylaxis of NMIBC.[14,15] In the study of Han and Pan, the recurrence rate in 2,342 patients was 40.5% compared with 49.7% in patients not receiving BCG.[14] In addition, Malmstrom et al. found that BCG with maintenance was more effective than mitomycin C both in patients previously treated and those not previously treated with chemotherapy.[15] In these studies, the recurrence rates in BCG arms are comparable to those observed in our trial and this further proves that the efficacy of this alternating therapy is as effective as BCG alone.
The mean interval to first recurrence and all recurrence and progression parameters reported in this study are actually a continuation of the same results of the previous trial.[12]
The rationale for choosing intravesical epirubicin alternating with BCG has been stated before.[12] The toxic effects of epirubicin are less frequent and less severe than those of BCG and it is also effective, though not comparable to BCG, in NMIBC.[16]
Toxicity
The most important result of the current therapeutic regimen is the lower rate of toxic and side effects compared with those of BCG alone.[13] Furthermore, the overall toxicity rate, the rate of severe cystitis, and hematuria in this study are lower compared with some studies using a reduced BCG dose.[17,18] Overall toxic effects may develop in more than 90% of the cases.[13] However, they are mostly in the form of reversible mild irritative bladder symptoms or low-grade fever and malaise, and only 5% of the patients show severe toxicity.[1,13] Severe systemic BCG reactions may be explained by exaggerated immune response or active BCG tuberculous infection, and life-threatening reactions such as BCG sepsis occur most probably due to systemic absorption. The rate of systemic toxicity in our series is low (<1%) and compares favorably with other trials using BCG alone.[1,13,19]
One of the most important indicators of BCG toxicity is the necessity to stop the drug temporarily or permanently because of severe toxicity. Therapy discontinuation due to severe toxicity has been reported by Koga et al. in nine of 123 patients treated with BCG, a rate which is comparable to that found in the current study.[13] Furthermore, the discontinuation rate in the current study is lower than the rates reported in some trials using BCG alone.[20,21] Saint et al. and Takeda et al., respectively, reported 39 and 13% rates of BCG discontinuation.[20,21] Toxicity is a cumulative effect that usually occurs after the first two doses, and if it occurs once, the probability becomes higher in the subsequent doses.[22] This observation goes hand in hand with our findings with only one exception, that is, most of the side effects in our series developed after the fourth dose.
Dose and schedule
In principle, the mechanism of action of BCG and BCG-induced local immune response are not known exactly, and therefore improvements in the rational choice of the dose and schedule are not yet well defined.
The usual dose of 120 mg of Frappier (Pasteur) strain and the six weekly instillations were empirically determined by Morales and associates.[23] Strategies to decrease the side effects while maintaining the same efficacy of BCG therapy included reduction of the dose, dwell time, or number of doses.[5–11,24–26]
Limitations of reduction of bacillus calmette-guerin dose
Although reduction of BCG dose is an accepted method to reduce BCG toxicity, some limitations have been noted.[4–9,24] Reduction of BCG dose to a third of the usual dose was as effective as and less toxic than the classical dose in the study of the Spanish (CUETO) group, in both intermediate and high-risk NMIBC.[5,24] However, these authors stated, in their earlier report, that patients with multifocal tumors fared better with the standard dose and a trend toward better recurrence rates in patients with high-risk tumors was observed.[5] Therefore, in this study and others, it was suggested that the standard dose may be more optimal in high-risk, multiple, TIS-associated Ta and/or in cases of NMIBC with history of previous treatment.[5,8,9] Furthermore, at least in some trials, dose reduction did not achieve a subsequent reduction in toxicity.[10,11]
Reduction in dwell time
Reducing the dwell time to 30 minutes or less has been advocated as an alternative to dose reduction in patients showing severe BCG toxicity.[25] However, the maintained efficacy of the BCG may be related to the earlier standard doses, which were associated with pronounced side effects. Furthermore, prospective randomized trials are needed to support this issue.
Decrease in the number of bacillus calmette-guerin doses
There have been a few clinical trials that investigated the use of fewer BCG instillations or alternation of BCG with a chemotherapeutic agent.[26–29] The results of these trials support the results of the current trial. A modified induction BCG course with a two-week interval between instillations has been tried and shown to be effective with a lower toxicity profile.[26] In addition, Zlotta et al. stated that the maximal peripheral immune response to BCG therapy had already manifested after four-weekly instillations in most patients.[27]
Based on the observation that a high BCG-induced Th1/Th2 cytokine ratio was associated with effective antitumor activity, De Boer and associates stated, in a mouse model, that a modified schedule composed of only two BCG instillations, administered in week 1 and week 6, showed at least the same level of Th1 cytokines, compared to the six-week course.[28] At the same time, lower responses for the Th2 cytokines and a positive effect on the Th1/Th2 ratio were observed.[28]
Rintala and associates tried an alternating combination of BCG and mitomycin C for prophylaxis against recurrence of stages Ta and T1 bladder cancer and stated that this combination was superior to historical controls treated with BCG alone in terms of toxic effects.[29]
Maintenance bacillus calmette-guerin
We elected to use monthly maintenance rather than the three-weekly schedule because the latter was more toxic in our experience.[30]
Limitations
The study did not include an epirubicin-free arm with the same reduced number of BCG doses (half the number of the standard doses) and did not include a pure BCG arm (six weekly doses). However, in the first study, a pure BCG arm was included. In addition, the follow-up was relatively short in some patients (18 months), although the median follow-up was 45 months.
CONCLUSIONS
The efficacy and toxicity of alternating intravesical BCG and epirubicin in the current study have maintained and supported the results of our initial report. Reduction of the frequency of intravesical BCG instillations by half and replacement of the second half with epirubicin result in a similar efficacy as compared to historical cases treated with BCG alone. In addition, the frequency and severity of toxic and side effects have been lower in this regimen. These results further support the fact that the ideal dose and regimen of intravesical BCG for the treatment of NMIBC is yet to be well defined. However, these results need to be consolidated by other trials performed at multiple centers.
ACKNOWLEDGMENT
All the residents of the Urology and Nephrology Center who have shared in the follow up of these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Lamm DL, Van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 2.Melekos MD, Chionis HS, Paranychianakis GS, Dauaher HH. Intravesical 4-epidoxorubicin (epirubicin) versus bacillus Calmette-Guerin: A controlled prospective study on the prophylaxis of superficial bladder cancer. Cancer. 1993;72:1749–55. doi: 10.1002/1097-0142(19930901)72:5<1749::aid-cncr2820720539>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, Brausi MA, Kirkels WJ, Hoeltl W, Calais Da Silva F, Powell PH, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus calmette-guérin, and bacillus calmette-guérin plus isoniazid in patients with intermediate-and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57:766–73. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez-Piñeiro JA, Solsona E, Flores N, Isorna S. Improving the safety of BCG immunotherapy by dose reduction. Cooperative Group CUETO. Eur Urol. 1995;27:13–8. doi: 10.1159/000475203. [DOI] [PubMed] [Google Scholar]
- 5.Martínez-Piñeiro JA, Flores N, Isorna S, Solsona E, Sebastian JL, Pertusa C, et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int. 2002;89:671–80. doi: 10.1046/j.1464-410x.2002.02722.x. [DOI] [PubMed] [Google Scholar]
- 6.Ojea A, Nogueira JL, Solsona E, Flores N, Gomez JM, Molina JR, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol. 2007;52:1398–406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 7.Yoneyama T, Ohyama C, Imai A, Ishimura H, Hagisawa S, Iwabuchi I, et al. Low-dose instillation therapy with bacille Calmette-Guérin Tokyo 172 strain after transurethral resection: historical cohort study. Urology. 2008;71:1161–5. doi: 10.1016/j.urology.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 8.Morales A, Nickel JC, Wilson JW. Dose-response of bacillus Calmette-Guerin in the treatment of superficial bladder cancer. J Urol. 1992;147:1256–8. doi: 10.1016/s0022-5347(17)37532-8. [DOI] [PubMed] [Google Scholar]
- 9.Takashi M, Wakai K, Ohno Y, Murase T, Miyake K. Evaluation of a low-dose intravesical bacillus Calmette-Guérin (Tokyo strain) therapy for superficial bladder cancer. Int Urol Nephrol. 1995;27:723–33. doi: 10.1007/BF02552138. [DOI] [PubMed] [Google Scholar]
- 10.Mack D, Frick J. Low-dose bacille Calmette-Guérin (BCG) therapy in superficial high-risk bladder cancer: A phase II study with the BCG strain Connaught Canada. Br J Urol. 1995;75:185–7. doi: 10.1111/j.1464-410x.1995.tb07308.x. [DOI] [PubMed] [Google Scholar]
- 11.Yalçinkaya F, Kamiş L, Ozteke O, Gunlusoy B, Yigitbasi O, Unal S. Prospective randomized comparison of intravesical BCG therapy with standard dose versus low doses in superficial bladder cancer. Int Urol Nephrol. 1998;30:41–4. doi: 10.1007/BF02550276. [DOI] [PubMed] [Google Scholar]
- 12.Ali-EI-Dein B, Nabeeh A, Ismail EH, Ghoneim MA. Sequential bacillus Calmette-Guerin and epirubicin versus bacillus Calmette-Guerin alone for superficial bladder tumors: A randomized prospective study. J Urol. 1999;162:339–42. [PubMed] [Google Scholar]
- 13.Koga H, Kuroda M, Kudo S, Yamaguchi A, Usami M, Suzukiet T, et al. Adverse drug reactions of intravesical bacillus Calmette-Guerin instillation and risk factors of the development of adverse drug reactions in superficial cancer and carcinoma in situ of the bladder. Int J Urol. 2005;12:145–51. doi: 10.1111/j.1442-2042.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 14.Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216–23. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–56. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 16.de Reijke TM, Kurth KH, Sylvester RJ, Hall RR, Brausi M, van de Beek K, et al. Bacillus Calmette-Guerin versus epirubicin for primary, secondary or concurrent carcinoma in situ of the bladder: Results of a European Organization for the Research and Treatment of Cancer-Genito-Urinary Group Phase III Trial (30906) J Urol. 2005;173:405–9. doi: 10.1097/01.ju.0000150425.09317.67. [DOI] [PubMed] [Google Scholar]
- 17.Mack D, Frick J. Five-year results of a phase II study with low-dose bacille calmette-guérin therapy in high-risk superficial bladder cancer. Urology. 1995;45:958–61. doi: 10.1016/s0090-4295(99)80115-0. [DOI] [PubMed] [Google Scholar]
- 18.Yalçinkaya F, Kamiş L, Özteke O, Günlüsoy B, YigitbaşiO, Unal S. Prospective randomized comparison of intravesical BCG therapy with standard dose versus low doses in superficial bladder cancer. Int Urol Nephrol. 1998;30:41–4. doi: 10.1007/BF02550276. [DOI] [PubMed] [Google Scholar]
- 19.Shelley MD, Wilt TJ, Court J, Coles B, Kynaston H, Mason MD. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: A meta-analysis of randomized trials. BJU Int. 2004;93:485–90. doi: 10.1111/j.1464-410x.2003.04655.x. [DOI] [PubMed] [Google Scholar]
- 20.Saint F, Irani J, Patard JJ, Salomon L, Hoznek A, Zammattio S, et al. Tolerability of bacille Calmette-Guérin maintenance therapy for superficial bladder cancer. Urology. 2001;57:883–8. doi: 10.1016/s0090-4295(00)01117-1. [DOI] [PubMed] [Google Scholar]
- 21.Takeda T, Kikuchi E, Yuge K, Matsumoto K, Miyajima A, Nakagawa K, et al. Discontinuance of bacille Calmette-Guérin instillation therapy for nonmuscle-invasive bladder cancer has negative effect on tumor recurrence. Urology. 2009;73:1318–22. doi: 10.1016/j.urology.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Berry DL, Blumenstein BA, Magyary DL, Lamm DL, Crawford ED. Local toxicity patterns associated with intravesical bacillus Calmette-Guérin: A southwest oncology group study. Int J Urol. 1996;3:98–100. doi: 10.1111/j.1442-2042.1996.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 23.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumours. J Urol. 1976;116:180–3. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Piñeiro JA, Martínez-Piñeiro L, Solsona E, Rodriguez RH, Gomez JM, Martin MG, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol. 2005;174:1242–7. doi: 10.1097/01.ju.0000173919.28835.aa. [DOI] [PubMed] [Google Scholar]
- 25.Andius P, Fehrling M, Holmäng S. Intravesical bacillus Calmette-Guèrin therapy: Experience with a reduced dwell-time in patients with pronounced side-effects. BJU Int. 2005;96:1290–3. doi: 10.1111/j.1464-410X.2005.05817.x. [DOI] [PubMed] [Google Scholar]
- 26.Bassi P, Spinadin R, Carando R, Balta G, Pagano F. Modified induction course: A solution to side-effects? Eur Urol. 2000;37:31–2. doi: 10.1159/000052380. [DOI] [PubMed] [Google Scholar]
- 27.Zlotta AR, van Vooren JP, Huygen K, Drowart A, Decock M, Pirson M, et al. What is the optimal regimen for BCG intravesical therapy? Are six weekly instillations necessary. Eur Urol. 2000;37:470–7. doi: 10.1159/000020170. [DOI] [PubMed] [Google Scholar]
- 28.de Boer EC, Rooyakkers SJ, Schamhart DH, de Reijke TM, Kurth KH. BCG dose reduction by decreasing the instillation frequency: Effects on local Th1/Th2 cytokine responses in a mouse model. Eur Urol. 2005;48:333–8. doi: 10.1016/j.eururo.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Rintala E, Jauhiainen K, Kaasinen E, Nurmi M, Alfthan O Finnbladder Group. Alternating mitomycin C and bacillus Calmette-Guerin instillation prophylaxis for recurrent papillary (Stages Ta to T1) superficial bladder cancer. J Urol. 1996;156:56–9. [PubMed] [Google Scholar]
- 30.Ali-El-Dein B, Sarhan O, Nabeeh A, Abu-Eideh RH, Ibrahiem EI. Maintenance intravesical BCG therapy for superficial bladder tumors: 3-week multiple courses versus monthly doses. J Urol. 2007;174:522. abstract No. 1579. [Google Scholar]


