Abstract
Background:
The levels of 5 minerals namely; lead, arsenic, mercury, cadmium, and aluminum were assessed in 10 medicinal plants sampled from 5 different geographical locations to determine the effect of location on the plants’ mineral content.
Materials and Methods:
Atomic absorption spectrophotometry (wet digestion) was used for the analyzes, and content of the minerals per sample was expressed as μg/g. The levels of minerals were compared to their limit specification for herbs and daily total intake of these minerals. A two-way analysis of variance, which tends to look at the effect of the location and the medicinal plant itself on the plants mineral content, was used in the statistical analysis.
Results:
Lead (Pb) was present in all plant species examined, except Ocimum gratissimum. One plant exceeded the maximum safety limit for lead. Cadmium was also detected in some of the medicinal plant species (44%) whilst majority were below the detection limit (0.002) representing 56%. 40% of the plant species exceeded the limit for cadmium. Mercury and arsenic in all the plant species were below the detection limit (0.001). Significant variation existed in mineral content for the various locations (P ≤ 0.05).
Conclusion:
The findings generally suggest the variation in mineral levels for the various locations. Thus, our study has shown that same species of medicinal plants, growing in different environments, accumulates different levels of heavy metals.
Keywords: Geographical locations, heavy metals, medicinal plants, minerals
INTRODUCTION
Herbal medicines have found extensive use in disease treatment, prevention, and management. Due to the immense benefits herbal medicines bring to bear, majority of the world's population in one way or the other depend on them for various health benefits. According to the world health organization report,[1] there is an estimated 65 to 80% of the world's population relying on traditional (alternative) medicine as their primary form of healthcare. However, the use of herbal medicines has come under scrutiny due to their perceived long term toxicity among other considerations. The causes of the toxicities, which could be attributed to the chemical and mineral contents of various plants, are also linked to the source of the material.[2]
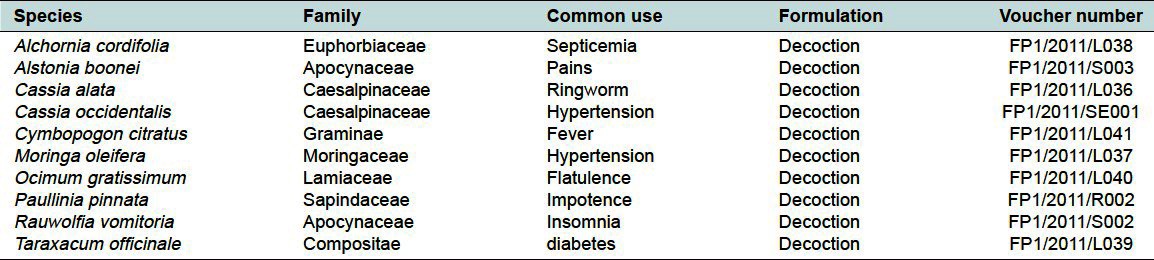
The mineral contents of medicinal plant species used in herbal formulations cannot be over-looked considering the important role these minerals play in the proper functioning of the vital organs as well as in the promotion of the general well-being of the body.[3] However, they may be toxic if consumed beyond their estimated safe daily intake. Several works have been reported on the phytochemical and biological activities of medicinal plants, although there is little report in regards to the heavy metal contents of these plants. Medicinal herbs can present health risks due to the presence of toxic metals such as Pb, Cd, Al, and Hg, which are hazardous to humans.[4] The present study was carried out to ascertain the amount of 5 minerals namely; lead (Pb), cadmium (Cd), aluminum (Al), mercury (Hg), and arsenic (As) in 10 common medicinal plants (Alchornia cordifolia, Alstonia boonei, Cassia alata, Cassia occidentalis, Cymbopogon citratus, Moringa oleifera, Ocimum gratissimum, Paullinia pinnata, Rauwolfia vomitoria, and Taraxacum officinale leaves) used in the treatment, prevention, and management of diseases and sampled from 5 different geographical locations in Ghana. This may help understand the importance of location in collection and ultimately heavy metal toxicity of medicinal plants. These plants were selected because they are found in most herbal remedies on the Ghanaian market and widely used by individuals and families as they are common in most communities.
MATERIALS AND METHODS
Plant collection
The samples of medicinal plants, which comprised of selected parts of plants commonly used in phytotherapy included; Alchornia cordifolia leaves locally known as ‘ogyama,’ Alstonia boonei stem bark locally known as ‘onyame dua,’ Cassia alata leaves locally known as ‘osempe,’ Cassia occidentalis seeds locally known as ‘mmofra brodie,’ Cymbopogon citratus leaves locally known as ‘ti-ahaban,’ Moringa oleifera leaves, Ocimum gratissimum leaves locally known as ‘nunum,’ Paullinia pinnata roots locally known as ‘toa-ntini,’ Rauwolfia vomitoria stem bark locally known as ‘kakapenpen,’ and Taraxacum officinale leaves. The various plant samples were obtained from their specified locations in Kumasi, the Ashanti regional capital in January, 2011 and these were physique garden (PG, faculty of pharmacy and pharmaceutical sciences, Kwame Nkrumah University of Science and Technology (KNUST); located on 6° 40’ 26” N, 1° 33’ 57” W), around KNUST campus (AC; located on 6° 41’ 08” N, 1° 33’ 47” W), by roadside (RS; located on 6° 40’ 11” N, 1° 34’ 22” W), refuse dump (RD; located on 6° 41’ 13” N, 1° 34’ 12” W), and Bobiri forest (FO; located on 6° 43’ 54” N, 1° 25’ 56” W) [Figure 1].
Figure 1.
Map of KNUST and Bobiri forest showing location of sample points
The plant samples were authenticated at the department of herbal medicine, faculty of pharmacy and pharmaceutical sciences, KNUST where voucher specimens are kept [Table 1].
Table 1.
List of plant species under experimentation and their voucher numbers
Acid digest and atomic absorption spectrophotometer analysis
An acid digest of each plant species was prepared by oxidizing 0.2 g of the powdered plant sample with nitric/perchloric acid (2:1) mixture (10 ml). One milliliter of the acid digests of each sample was further diluted to 20 ml due to the corrosive nature of the acid used.[3] Aliquots of the mixture were used to estimate lead (Pb), mercury (Hg), cadmium (Cd), aluminum (Al), and arsenic (As) by the use of atomic absorption spectrophotometer (model AA240FS). The blank and working standards were first run followed by the samples. Each sample was analyzed twice, and the data reported as a mean of the analyzed samples in μg/g.
Statistical analysis
The data were based on two replicates and subjected to a two-way analysis of variance to bring out the effects of the plants location on the plants mineral content as well the effect the plant itself has on its mineral content. Variations among the locations and plant samples were evaluated by mean of least significance difference (LSD) at 5% level of probability (P ≤ 0.05). Data analysis was conducted using statgraphics centurion XV I statistical software.
RESULTS
From the study, lead (Pb) was present in all plant species examined with the exception of O. gratissimum (AC), which was below the detection limit (<0.010). Cadmium was also detected in some of the medicinal plant species (44%) whilst majority were below the detection limit (0.002) representing 56%. Aluminum (Al) was found in all of the plant species, except two (4%). Mercury and arsenic in all the plant species were below the detection limit (0.001), representing 100% [Tables 2–5].
Table 2.
Concentration of heavy metals (μg/g) in Cassia alata and Cassia occidentalis
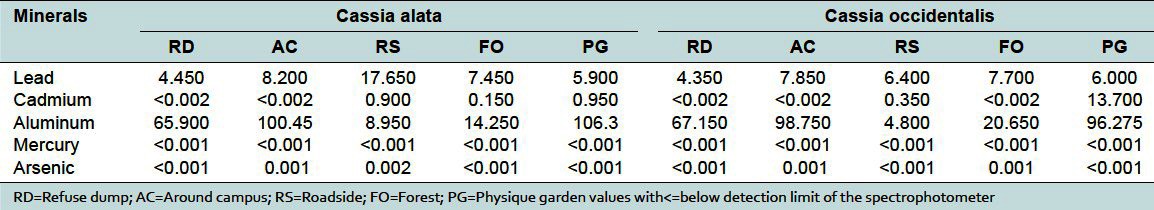
Table 5.
Concentration of heavy metals (μg/g) in Ocimum gratissum and Alstonia boonei

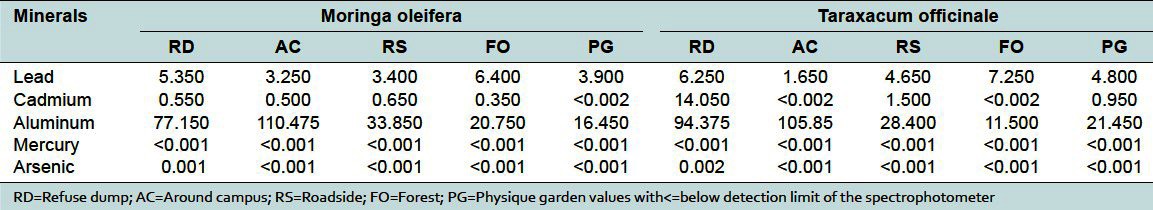
Table 4.
Concentration of heavy metals (μg/g) in Moringa oleifera and Taraxacum officinale
DISCUSSION
According to the world health organization (WHO),[1] about 80% of the world population use plant-based medicines. Most of these plants are collected in the wild, only few are cultivated. A number of mineral elements important to nutrition accumulate in these plants. Other elements such as Cd, Co, and Pb, which are not used directly by the plant and are detrimental to human health, also accumulate in these plants.[5,6] The environmental impact of these metals as well as their health effects has been a source of major concern.[7] Their accumulation in plants highly depends on the availability in the soil.[7] Heavy metal contamination of medicinal herbal products occurs during cultivation, cross-contamination during processing or their deliberate introduction as therapeutic ingredients. Cultivation in soils containing high concentrations of heavy metals is one mechanism by which heavy metal contamination of herbal products has been documented.[8]
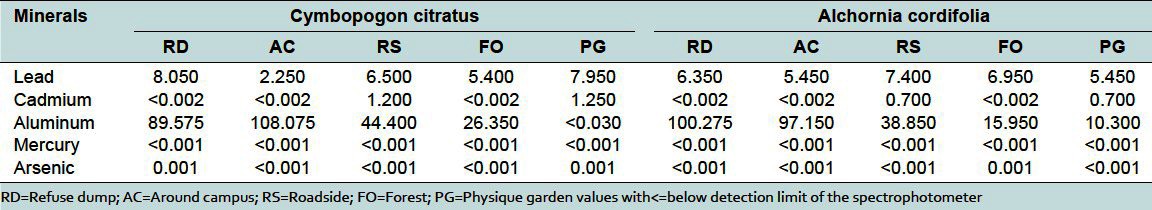
Medicinal plants have been cited as a potential source of heavy metal toxicity to both man and animals.[9] The most common heavy metals implicated in human toxicity include lead, mercury, arsenic, and cadmium, although aluminum and cobalt may also cause toxicity. Therefore, the world health organization recommends that medicinal plants, which form the raw materials for most herbal remedies, should be checked for the presence of heavy metals. However, majority of people, living in areas where these plants grow, harvest them locally for personal or family use without checking for heavy metal accumulation. The general notion that medicinal plants are safe and devoid of heavy metal toxicity could be misconstrued. From the study, the levels of these metals differed in the same plant collected from different geographical locations. For example, the levels of lead in Cassia alata varied from 17.7 to 4.45 μg/g for the 5 collection sites [Table 2]. That for Cassia occidentalis and Rauwolfia vomitoria varied between 7.85-4.35 and 9.25-1.55 μg/g, respectively (Table 2 and 3). Similarly, that of aluminum varied between 105.53-23.3 for Rauwolfia vomitoria and 104.25-12.4 μg/g for Paullinia pinnata [Table 3]. The levels of heavy metals also varied for different plants collected from the same location. Uptake of metals by plants is influenced by a number of factors including metal concentrations in soils, cation exchange capacity, soil pH, organic matter content, types and varieties of plants, and plant age. However, the prevailing factor is the concentration of the metal in the soil[10] and thus the existing environmental conditions.
Table 3.
Concentration of heavy metals (μg/g) in Rauwolfia vomitoria and Paulinia pinnata
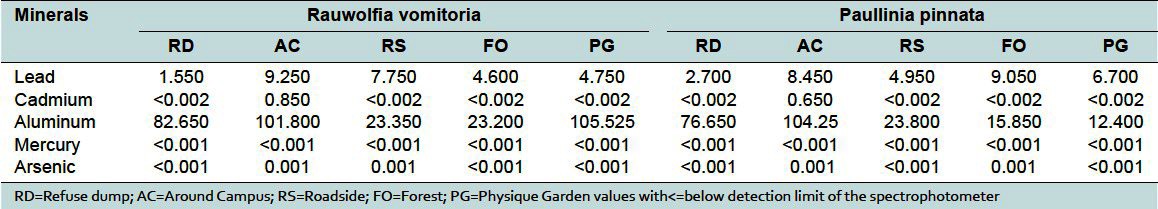
Lead is considered very harmful for plants, animals, and particularly for microorganisms.[7] It has no physiologic role. The highest level of lead occurred in Cassia alata harvested from the roadside (17.65 μg/g) [Figure 2]. This exceeded the WHO standard of 10 μg/g for lead in raw materials for herbal medicines.[1] This could possibly be due to its accumulation in the soil from vehicular exhaust fumes. In a large number of the samples, the level of lead was just below the allowable limit, suggesting that toxicity could arise from long term use of these plants. Levels of lead beyond the permissible values or long term use of these plants could lead to toxicity characterized by colic, anemia, headache, convulsions and chronic nephritis of the kidneys, brain damage, and central nervous system disorders.[7,11]
Figure 2.
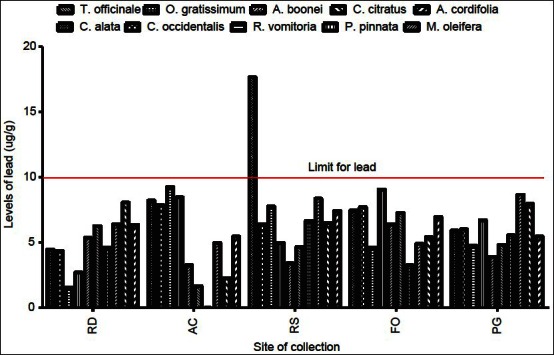
Levels of lead in plant samples in different geographical locations. RD = Refuse dump, AC = Around campus, RS = Roadside, FO = Forest, PG = Physique garden values with<=below detection limit of the spectrophotometer
The levels of mercury and arsenic, in all the medicinal plant samples in different geographical locations, were found to be below the minimum detectable limit (<0.001 μg/g) [Table 2 and 6]. All values were below the permissible values of 5 and 0.2 μg/g for arsenic and mercury, respectively.[1] Thus, human activities in the areas, where these plants were collected, do not lead to the accumulation of arsenic and mercury in the soils. Mercury levels in humans above the allowable values have been associated with male infertility, inhibition of endogenous antioxidant enzymes, and brain damage among others.[12] Supraoptimal levels of arsenic are often fatal. Other toxic symptoms include periphereal polyneuropathy, liver cirrhosis, visual disturbance, and blindness.[13] Similarly, aluminum levels in all plant samples from the same area in different geographical locations varied from 4.8 to 108 μg/g [Tables 2–6]. All values were within allowable limits for aluminum, which is not used in any biochemical process in living systems. It accumulates in the brain and, to a lesser extent, in the bones.[14] The levels of aluminum in medicinal plants in different geographical locations must be checked as long term use could lead to toxicity.
Table 6.
Concentration of heavy metals (μg/g) in Cymbopogon citratus and Alchornia cordifolia
The level of cadmium, in all 10 plant samples, exceeded the WHO recommended value of 0.3 μg/g for some geographical locations, whereas for other collection sites, the levels were below the minimum detectable values (<0.001 μg/g) [Figure 3]. The levels of cadmium from plant samples collected from the roadside exceeded the maximum safety limit, indicated by the red line in Figure 3, possibly due to contamination from vehicular activities. Also, most medicinal plants collected around the university campus and the physic garden accumulated cadmium above the recommended levels in medicinal plants [Figure 3] with the highest occurring in C. occidentalis (13.7 μg/g). About 70% of medicinal plants harvested from the physic garden (within KNUST campus) exceeded the maximum safety limit for cadmium. The most common sources for cadmium in soil and plants are phosphate fertilizers, sewage sludge application, and combustion of fossil fuels. Thus, there is the need for a proper and efficient waste disposal system in and around the university community as cadmium levels above permissible values may result in irreversible kidney damage.[15]
Figure 3.
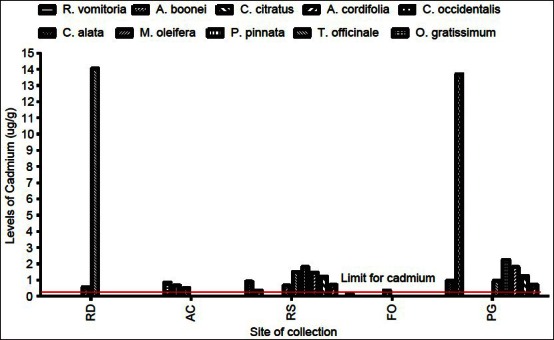
Levels of cadmium in plant samples in different geographical locations. RD=Refuse dump, AC=Around xampus, RS=Roadside, FO=Forest, PG=Physique garden values with<=below detection limit of the spectrophotomete
CONCLUSION
Our study has shown that same species of medicinal plants, growing in different geographical locations, cumulates different levels of heavy metals. Concentration of heavy metals also differed for different plant species collected from the same geographical location. Amounts of heavy metals detected in plants collected from the Bobiri forest reserve were within permissible limits, whereas the levels in other geographical locations exceeded the recommended values. Thus, medicinal plants for the formulation of herbal remedies should be harvested from pollution-free natural habitat. Our findings further indicate that the medicinal plants, used for local or pharmaceutical purposes, should be collected from areas not contaminated with heavy metals. It is, therefore, advised that the metal content in medicinal plants be checked for levels of heavy metals before their use for local and pharmaceutical purposes.
ACKNOWLEDGEMENT
The authors are grateful to the technical staff of Atomic Energy Commission, Accra, Ghana for their assistance in this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.WHO. WHO: Geneva Switzerland; 1998. Quality control methods for medicinal plant materials. available at http://whqlibdoc.who.int/publications/1998/9241545100.pdf . [Google Scholar]
- 2.Lekouch N, Sedki A, Nejmeddine A, Gamon S. Lead and traditional Moroccan pharmacopoeia. Sci Total Environ. 2001;280:39–43. doi: 10.1016/s0048-9697(01)00801-4. [DOI] [PubMed] [Google Scholar]
- 3.Annan K, Kojo AI, Cindy A, Samuel AN, Tunkumgnen BM. Profile of heavy metals in some medicinal plants from Ghana commonly used as components of herbal formulations. Pharmacognosy Res. 2010;2:41–4. doi: 10.4103/0974-8490.60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap C K, Fitri M, Mazyhar Y, Tan SG. Effects of Metalcontaminated Soils on the accumulation of heavy metals in different parts of Centella asiatica: A Laboratory Study. Sains Malaysiana. 2010;39:347–52. [Google Scholar]
- 5.Baker AJM, Brooks RR. Terrestrial higher plants, which hyperaccumulate metallic elements–A review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- 6.Lasisi AA, Yusuff AA, Ejelonu BC, Nwosu EO, Olayiwola MA. Heavy metals and macronutrients content in selected herbal plants of Nigeria. International Jour Chern. 2005;15:147–54. [Google Scholar]
- 7.Khan MA, Ahmad I, Rahman I. Effect of environmental pollution on heavy metals content of Withania somnifera. Journal of the Chinese Chemical Society. 2007;54:339–43. [Google Scholar]
- 8.Quig D. Cysteine metabolism and metal toxicity. Altern Med Rev. 1998;3:262–70. [PubMed] [Google Scholar]
- 9.Dwivedi SK, Dey S. Medicinal herbs: A potential source of toxic metal exposure for man and animals in India. Arch Environ Health. 2002;57:229–31. doi: 10.1080/00039890209602941. [DOI] [PubMed] [Google Scholar]
- 10.Jun MC. Heavy metal concentrations in soils and factors affecting metal uptake by plants in the vicinity of a Korean Cu-W Mine. Sensors. 2008;8:2413–23. doi: 10.3390/s8042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheljazkov VD. Amhers, USA: International Society for Horticultural Science; 1995. Heavy metal content in some essential oils and plant extracts; p. 429. [Google Scholar]
- 12.Choy CM, Lam CW, Cheung LT, Briton-Jones CM, Cheung LP, Haines CJ. Infertility, blood mercury concentrations and dietary seafood consumption: A case-control study. BJOG. 2002;109:1121–5. doi: 10.1111/j.1471-0528.2002.02084.x. [DOI] [PubMed] [Google Scholar]
- 13.Ling LJ, Clark RF, Erickson TB, Trestrail JH. philadelphia: Hanley and Belfus Inc; 2001. Toxicology secrets; pp. 153–69. [Google Scholar]
- 14.Cassel I. Aluminum Toxicity: A misdiagnosed epidemic (Part 1) Idaho Observer. 2007 [Google Scholar]
- 15.Saied S, Zahir H, Siddique A. Heavy metal levels in commonly used traditional medicinal plants. J chem Soc Pak. 2010;32:737–43. [Google Scholar]


