Abstract
Background and Objective:
Our recent study revealed the antihyperglycemic activity of an ethanolic extract of roselle calyxes (Hibiscus sabdariffa) in diabetic rats. The present study had, therefore, an objective to investigate the mechanism underlying this activity.
Materials and Methods:
Male Sprague Dawley rats were induced to be diabetes by intraperitoneal injection of 45 mg/kg streptozotocin (STZ). Normal rats as well as diabetic rats were administered with the ethanolic extract of H. sabdariffa calyxes (HS-EE) at 0.1 and 1.0 g/kg/day, respectively, for 6 weeks. Then, blood glucose and insulin levels, at basal and glucose-stimulated secretions, were measured. The pancreas was dissected to examine histologically.
Results:
HS-EE 1.0 g/kg/day significantly decreased the blood glucose level by 38 ± 12% in diabetic rats but not in normal rats. In normal rats, treatment with 1.0 g/kg HS-EE increased the basal insulin level significantly as compared with control normal rats (1.28 ± 0.25 and 0.55 ± 0.05 ng/ml, respectively). Interestingly, diabetic rats treated with 1.0 g/kg HS-EE also showed a significant increase in basal insulin level as compared with the control diabetic rats (0.30 ± 0.05 and 0.15 ± 0.01 ng/ml, respectively). Concerning microscopic histological examination, HS-EE 1.0 g/kg significantly increased the number of islets of Langerhans in both normal rats (1.2 ± 0.1 and 2.0 ± 0.1 islet number/10 low-power fields (LPF) for control and HS-EE treated group, respectively) and diabetic rats (1.0 ± 0.3 and 3.9 ± 0.6 islet number/10 LPF for control and HS-EE treated group, respectively).
Conclusion:
The antidiabetic activity of HS-EE may be partially mediated via the stimulating effect on insulin secretion.
Keywords: Antihyperglycemic activity, insulin secretion, pancreas, roselle
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder signified by the presence of hyperglycemia. Hyperglycemia is mostly caused by a functional deficiency of insulin action which can be due to a decrease in insulin secretion by the β-cells of pancreas or a decrease in responses to insulin by target tissues. Impaired β-cell function and possibly β-cell mass appear to be reversible, particularly at early stages of diabetes where the limiting threshold for β-cell mass recovery has probably not been overstepped.[1] Strategies for how to prevent β-cell loss and how to improve β-cell function have been extensively studied and hoped to be a specific treatment of diabetes.[2]
Roselle (Hibiscus sabdariffa L., family Malvaceae) is known in Thai as Kra-Jeab Daeng. Its calyxes are used to make beverages and have been used in folk medicines and claimed effective as diuretic, stomachic, aphrodisiac, antiseptic, and antihypertensive agents. Pharmacological activities of H. sabdariffa calyx extract, such as decreased blood glucose,[3] decreased blood cholesterol,[4] decreased blood pressure in spontaneously hypertensive rats[5] and protected against tert-butyl hydroperoxide, and lipopolysaccharide-induced hepatic damage in rats have been reported.[6] Furthermore, anthocyanin, red color in roselle possessed antioxidant capacity and reduced liver lesions induced by tert-butyl hydroperoxide and paracetamol in rats.[7] Recently, sour tea of H. sabdariffa calyx has been reported to increase high-density lipoprotein cholesterol (HDL-C) in patients with diabetes.[8] We have recently reported that the ethanolic extract of H. sabdariffa (HS-EE) possesses antidiabetic activity in chronic streptozotocin (STZ)-induced diabetic rats.[9] Oral administration of the HS-EE at doses of 0.5 and 1.0 g/kg for 6 weeks decreased the blood glucose and improved the glucose tolerance of chronic diabetic rats significantly. However, the mechanism of this antihyperglycemic activity has not been elucidated yet. Since insulin has a key role in regulating blood glucose and the STZ-induced diabetic rat model causes a severe oxidative damage of the islet cells of the pancreas[10] resulting in the impairment of insulin production and secretion, we hypothesized that H. sabdariffa extract may decrease the blood glucose by enhancing insulin secretion. Therefore, this study was aimed to investigate whether H. sabdariffa extract causes an increase in insulin release and stimulates the recovery of damaged pancreas in STZ-induced diabetic rats. The experiments were designed to determine the effects of the HS-EE on serum insulin concentration and histological appearance of pancreas in STZ-induced diabetic rats.
MATERIALS AND METHODS
Plant material and extraction
The HS-EE was employed in this study. One-year-old H. sabdariffa was collected from Khoun Meed District, Songkhla Province, in September 2010. The plant specimen (No. SKP 1090819) was deposited in the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkhla University, Songkhla, Thailand. The dried calyxes of H. sabdariffa were minced and immersed in absolute ethanol for 3 days, and then filtered. The left fiber was repeatedly extracted by ethanol for three times. Alcohol was removed from the filtrate using the rotary vacuum evaporator. The remaining alcohol was further evaporated at a controlled temperature of 50°C. By this procedure, the dry powder (HS-EE) was obtained and the yield was 21.5%. The amount of markers, gallic acid and quercetin, of these extracts was determined by HPLC method which are 0.26 and 1.05 mg/g extracts, respectively.
Induction of diabetic rats
Male Sprague Dawley rats (200-250 g) were obtained from the Laboratory Animal Unit, Faculty of Medicine, Khon Kaen University, Khon Kaen. They were maintained in air-conditioned room (25 ± 1°C) under a 12 h light: 12 h dark cycle. They received standard rat chow (C.P. mice feed, Thailand) and tap water ad libitum. All procedures were complied with the standards for the care and use of experimental animals and approved by Animal Ethics Committee of Khon Kaen University, Khon Kaen, Thailand (AEKKU 26/2551). Rats were induced to be diabetes by a single intraperitoneal injection of STZ, 45 mg/kg. After 14 days of STZ injection, fasting blood glucose (FBG) level was determined using glucometer (Accu-check Advantage II; Roche). The animals with FBG over 200 mg/dL were included in the experiment.
Investigations of the effect of HS-EE on FBG, oral glucose tolerance test (OGTT), and serum insulin in normal and STZ-induced diabetic rats
Normal rats were divided into three groups, with 5-6 animals in each group, and were orally administered with distilled water (DW), HS-EE 0.1, and HS-EE 1.0 g/kg/day for 6 weeks, respectively. STZ-induced diabetic rats were also divided and treated in the same pattern as described for normal rats.
After 6 weeks of treatments, both normal and STZ-induced diabetic rats were fasted for 12 h. Then, the tail vein blood was collected before glucose loading for measuring FBG and basal insulin level, and at 30, 60, and 120 min after glucose loading (2 g/kg, orally) for measuring blood glucose and glucose-stimulated insulin secretions.
Blood glucose was determined using glucometer (Accu-check Advantage II, Roche). The concentration of insulin in serum was examined in triplicate for each sample using Rat insulin enzyme-linked immunosorbent assay (ELISA) Kit (Millipore MA, USA).
Investigation of the effect of the HS-EE on the histological changes of pancreas
The normal and STZ-induced diabetic rats of the above investigation, which received DW or HS-EE 1.0 g/kg, were euthanized by pentobarbitone sodium injection (60 mg/kg, i.p.). The pancreas was dissected and fixed in 10% buffered formalin. The organ was processed in graded series of alcohol and embedded in paraffin wax. Serial sections of 5 μm were cut using a microtome (American Optical, Model 82), mounted on glass slides and stained with hematoxylin and eosin and photographed by Leitz microscope. The number and size of islets of Langerhans in pancreas were measured in 10 low-power fields (LPF) per section using Olympus DP25 and Image-Pro plus program.
Statistical analysis
Results were presented as mean ± S.E.M. The effect of HS-EE on the levels of FBG or serum insulin within the same group and between groups was analyzed by Student's paired and unpaired t-test, respectively. The effect of HS-EE on the number and area of islets of Langerhans was analyzed by Student's unpaired t-test.
RESULTS
Effect of HS-EE on FBG and OGTT in normal and STZ-induced diabetic rats
HS-EE at doses of 0.1 and 1.0 g/kg did not affect the FBG and OGTT of normal rats [Table 1, Figure 1a]. The administration of HS-EE at dose of 1.0 g/kg for 6 weeks significantly decreased the FBG of diabetic rats by 38 ± 12% [Table 1] and improved glucose tolerance in diabetic rats [Table 1, Figure 1b].
Table 1.
The effect of ethanolic extract of H. sabdariffa on fasting blood glucose of normal and diabetic rats
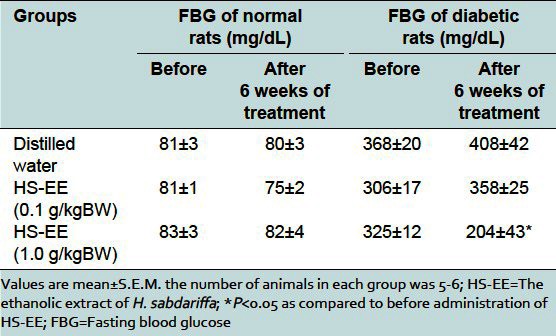
Figure 1.
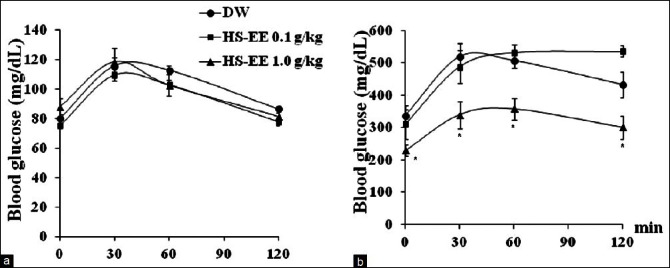
Effect of ethanolic extract of H. sabdariffa on oral glucose tolerance test (OGTT) in normal (a) and diabetic rats (b). The tail vein blood was collected before and at 30, 60, and 120 min after glucose loading (2 g/kg, orally) for measuring blood glucose at 0, 30, 60, and 120 min, respectively. HS-EE 1.0 g/kg significantly improved OGTT in diabetic rats, but not in normal rats. Values are mean±S.E.M. *P<0.05 as compared to DW receiving group. DW: Distilled water, HS-EE: Ethanolic extract of H. sabdariffa
Effect of HS-EE on serum insulin level in normal and STZ-induced diabetic rats
In normal rats receiving DW, the basal level serum insulin was 0.58 ± 0.05 ng/ml. At 30 min after the stimulation by glucose loading, the level of insulin was increased significantly to 1.32 ± 0.05 ng/ml and began to decrease at 60 min [Table 2]. It is interesting that the basal insulin level of normal rats receiving HS-EE 1.0 g/kg, but not 0.1 g/kg, was significantly higher than that of normal rat receiving DW [Table 2]. However, insulin secretion at 30 min after glucose loading was not significantly higher than the basal level.
Table 2.
Effect of ethanolic extract of H. sabdariffa on serum insulin levels in normal and streptozotocin-induced diabetic rats
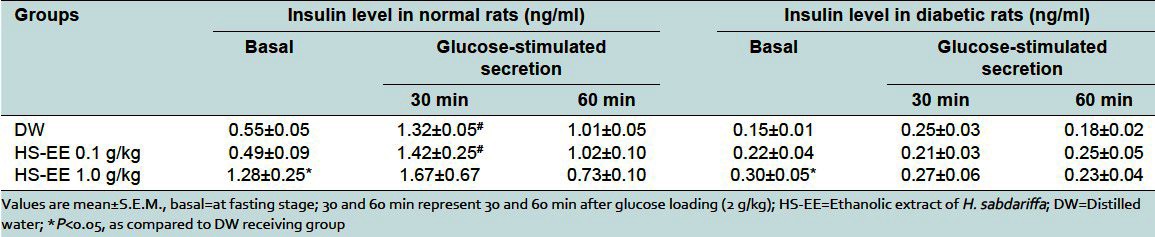
In diabetic rats receiving DW, the basal insulin level was about one-fourth of normal rats [Table 2]. At 30 min after the stimulation by glucose loading, the insulin secretion was slightly increased but not significantly different from the basal level [Table 2]. Interestingly, the basal level of insulin of diabetic rats receiving HS-EE 1.0 g/kg was significantly higher than that of diabetic rats receiving DW. However, the level of glucose-stimulated insulin secretion was still not increased from basal level [Table 2].
Effect of HS-EE on the histological changes of pancreas of normal and STZ-induced diabetic rats
As shown in Figure 2a and b and Table 3, the average size of islets of Langerhans in the pancreas of normal rats receiving HS-EE 1.0 g/kg was comparable to that of normal rats receiving DW. However, the average number of islets (per 10 LPF) was significantly more than that of normal rats receiving DW, which seemed to correspond to the higher level of basal insulin level in normal rat treated with HS-EE.
Figure 2.
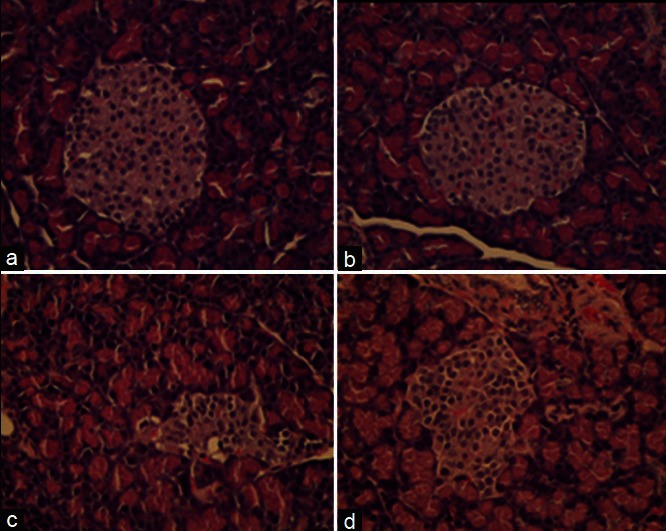
Photomicrograph of pancreas stained with (H and E, ×40). (a) Normal rat receiving distilled water. (b) Normal rat receiving ethanolic extract of H. sabdariffa 1.0 g/kg. (c) Diabetic rat receiving distilled water. (d) Diabetic rat receiving HS-EE 1.0 g/kg. The islets of Langerhans of streptozotocin-induced diabetic rat were irregular in shape and size, and were mostly smaller than that of normal rats. The islets of Langerhans of diabetic rats receiving HS-EE 1.0 g/kg appeared to be larger than that of diabetic rats receiving distilled water
Table 3.
Effect of ethanolic extract of H. sabdariffa on histological changes of islets of Langerhans in pancreas of normal and diabetic rats
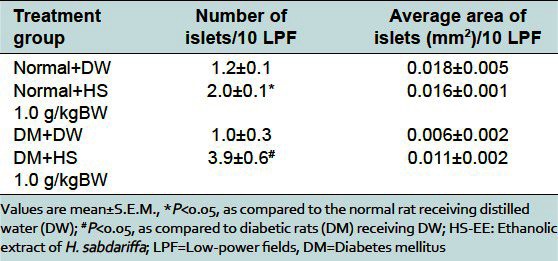
As shown in Figure 2c and d, the islets of Langerhans of STZ-induced diabetic rats were irregular in shape and size, and most of them were smaller than that of normal rats [Table 3]. The average size of islets of Langerhans of diabetic rats receiving HS-EE 1.0 g/kg showed a tendency to be larger than that of diabetic rats receiving DW, but not statistically significant. However, the average number of islets (per 10 LPF) of diabetic rats treated with HS-EE 1.0 g/kg was significantly more than that of diabetic rats receiving DW [Table 3]. The increase in number of islets tended to correspond to the higher level of basal insulin as compared to DW receiving group.
DISCUSSION
The results demonstrated that HS-EE 1.0 g/kg decreased FBG and improved glucose tolerance of STZ-induced diabetic rats. In addition, HS-EE 1.0 g/kg enhanced the basal release of insulin and stimulated the regeneration of islets of Langerhans in pancreas of both normal and STZ-induced diabetic rats.
A lack of insulin secretion and also function play a significant role in the pathogenesis of both types 1 and 2 diabetes. Although insulin resistance is an important pathogenic factor in the initiating of type 2 DM, however, β-cell failure in the second step of pathogenesis is also accountable for progression of impaired glucose tolerance to overt Type 2 DM.[11]
Strategies to prevent β-cell loss and to improve β-cell function have been extensively studied to imply in the management of diabetic patients. The most widely used insulin secretagogues are the sulfonylureas: Glimepiride, glipizide, and glyburide. It has also been shown that lifestyle modification, such as diet control and physical activity, may improve β-cell function.[12] Nevertheless, many diabetic patients have reported the difficulty to maintain their life style modification. Therefore, various plant extracts and herbal biomolecules may be appropriate as supplemental alternative medicine.[13] The most usual substances used to induce diabetes in the experimental animals are alloxan[14] and STZ.[15] The STZ has a high specific cytotoxic action on the β cells of the islets of Langerhans causing a necrotic damage.[16] Some medicinal plants with antihyperglycemic properties such as an ethanolic seed extract of Swietenia macrophylla[17] and chloroform leaf extract of Azadirachta indica[18] were shown to increase insulin secretion in STZ-induced diabetic rats, and fruit juice of Momordica charantia (bitter melon)[19] and stem of Tinospora cordifolia[20] were shown to increase insulin secretion in in-vitro experiment using rat pancreatic β-cell line. We also found that ethanolic extract of dried roselle calyxes (HS-EE) at 1.0 g/kg causes the significant decrease in FBG of chronic diabetic rats and the significant increase in the basal insulin levels of normal rats and chronic diabetic rats. It is important to note that we also found the regeneration of islets (increase in the number of islets) in both normal and chronic diabetic rats treated with HS-EE. However, the size of islet of diabetic rats receiving HS-EE showed only a nonsignificant tendency to be larger than that of the control diabetic rats. Because the β-cells of islet have a regenerative property to maintain their mass,[21] it is likely that the islets of diabetic rats which were partially destroyed by STZ still did not fully recover. For the mechanism by which HS-EE increased the basal insulin release, it is probably the result of islet regeneration as indicated by the increased number of islets in HS-EE treated animals. The pancreatic tissue is able to regenerate in several species of mammals after surgical insult and is also known to have the potential to maintain or increase its β-cell mass in response to metabolic demands during pregnancy and obesity. The general mechanisms of regeneration include replication of existing mature β-cells and differentiation (or neogenesis) by ductal or intra-islet pancreatic precursor cells.[21]
Sign of regeneration of islets has been reported following consumption of some medicinal plant extracts. A flavonoid fraction extracted from Pterocarpus marsupium caused a decrease in blood glucose and increase in the number of β-cells.[22] Nigella sativa and gliclazide were also reported to increase β-cells.[23] Two components extracted from Gymnema sylvestre were reported to decrease blood glucose and increase number of β-cells and islets in diabetic rats.[24] Islets of Langerhans are composed of β-cells producing insulin and α-cells producing glucagon. Unfortunately, it was difficult in our experiment to differentiate β-cells from α-cells on H and E staining tissue. However, our results showed the increase in number of islets which corresponded to the increase in insulin levels in this experiment.
CONCLUSION
The antidiabetic activity of HS-EE calyxes may be partly mediated via the increase in insulin releasing effect. This study supports the use of roselle calyx extract as an alternative medicine for diabetic patients.
ACKNOWLEDGMENTS
This work was supported by National Research Council of Thailand and Khon Kaen University Graduate Research Fund, Khon Kaen University, Thailand.
Footnotes
Source of Support: National Research Council of Thailand and Khon Kaen University Graduate Research Fund, Khon Kaen University, Thailand
Conflict of Interest: None declared
REFERENCES
- 1.Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 2.Gong Z, Muzumdar RH. Pancreatic function, type 2 diabetes, and metabolism in aging. Int J Endocrinol. 2012;2012:320482. doi: 10.1155/2012/320482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng CH, Chyau CC, Chan KC, Chan TH, Wang CJ, Huang CN. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J Agric Food Chem. 2011;59:9901–9. doi: 10.1021/jf2022379. [DOI] [PubMed] [Google Scholar]
- 4.Hirunpanich V, Utaipat A, Morales NP, Bunyapraphatsara N, Sato H, Herunsale A, et al. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J Ethnopharmacol. 2006;103:252–60. doi: 10.1016/j.jep.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Onyenekwe PC, Ajani EO, Ameh DA, Gamaniel KS. Antihypertensive effect of roselle (Hibiscus sabdariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in Wistar rats. Cell Biochem Funct. 1999;17:199–206. doi: 10.1002/(SICI)1099-0844(199909)17:3<199::AID-CBF829>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Tseng TH, Wang CJ, Kao ES, Chu HY. Hibiscus protocatechuic acid protects against oxidative damage induced by tert-butylhydroperoxide in rat primary hepatocytes. Chem Biol Interact. 1996;101:137–48. doi: 10.1016/0009-2797(96)03721-0. [DOI] [PubMed] [Google Scholar]
- 7.Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411–6. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffari-Khosravi H, Jalali-Khanabadi BA, Afkhami-Ardekani M, Fatehi F. Effects of sour tea (Hibiscus sabdariffa) on lipid profile and lipoproteins in patients with type II diabetes. J Altern Complement Med. 2009;15:899–903. doi: 10.1089/acm.2008.0540. [DOI] [PubMed] [Google Scholar]
- 9.Wisetmuen E, Pannangpetch P, Kongyingyoes B, Kukongviriyapan U, Itharat A. Antidiabetic effect of ethanolic extract of roselle (Hibiscus sabdariffa) in chronic streptozotocin-induced diabetic rats. Thai J Pharmacol. 2008;29:69–73. [Google Scholar]
- 10.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–32. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–9. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 12.Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care. 2010;33:1561–6. doi: 10.2337/dc09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandel HS, Pathak AK, Tailang M. Standardization of some herbal antidiabetic drugs in polyherbal formulation. Pharmacognosy Res. 2011;3:49–56. doi: 10.4103/0974-8490.79116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kujur RS, Singh V, Ram M, Yadava HN, Singh KK, Kumari S, et al. Antidiabetic activity and phytochemical screening of crude extract of Stevia rebaudiana in alloxan-induced diabetic rats. Pharmacognosy Res. 2010;2:258–63. doi: 10.4103/0974-8490.69128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 16.Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967;126:201–5. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- 17.Kalaivanan K, Pugalendi KV. Antihyperglycemic effect of the alcoholic seed extract of Swietenia macrophylla on streptozotocin-diabetic rats. Pharmacognosy Res. 2011;3:67–71. doi: 10.4103/0974-8490.79119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez RM, Gómez YG, Guzman MD. Attenuation of nonenzymatic glycation, hyperglycemia, and hyperlipidemia in streptozotocin-induced diabetic rats by chloroform leaf extract of Azadirachta indica. Pharmacogn Mag. 2011;7:254–9. doi: 10.4103/0973-1296.84243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller AC, Ma J, Kavalier A, He K, Brillantes AM, Kennelly EJ. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine. 2011;19:32–7. doi: 10.1016/j.phymed.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine. 2011;18:1045–52. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee M, Kanitkar M, Bhonde RR. Approaches towards endogenous pancreatic regeneration. Rev Diabet Stud. 2005;2:165–76. doi: 10.1900/RDS.2005.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarthy B, Gambhir S, Gode K. Pancreatic beta-cell regeneration: A novel antidiabetic mechanism of Pterocarpus marsupium. Indian J Pharmacol. 1980;12:123–7. [Google Scholar]
- 23.Moneim AA, El-Feki M, Salah E. Effect of Nigella sativa, fish oil and gliclazide on alloxan diabetic rats, 1-biochemical and histopathological studies. J Egy Ger Soci Zool. 1997;23:237–65. [Google Scholar]
- 24.Shanmugasundaram ER, Gopinath KL, Radha Shanmugasundaram K, Rajendran VM. Possible regeneration of the islets of Langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol. 1990;30:265–79. doi: 10.1016/0378-8741(90)90106-4. [DOI] [PubMed] [Google Scholar]


