Abstract
Background:
While curcuminoids have been reported to possess diverse biological activities, the anti-inflammatory activity of polar extracts (devoid of curcuminoids) of Curcuma longa (C. longa) has seldom been studied. In this study, we have investigated immune-stimulatory and anti-inflammatory activities of an aqueous based extract of C. longa (NR-INF-02) and its fractions in presence and absence of mitogens.
Materials and Methods:
Effects of NR-INF-02 (Turmacin™, Natural Remedies Pvt. Ltd., Bangalore, India) on proliferation, nitric oxide (NO), monocyte chemotactic protein-1 (MCP-1), interleukins (ILs) and prostaglandin (PGE2) levels of mouse splenocytes and mouse macrophage (RAW264.7) cells were determined.
Results:
NR-INF-02 increased splenocytes number in presence and absence of lipopolysaccharide (LPS) or concanavalin A. Treatment of NR-INF-02 showed a significant increase of NO, IL-2, IL-6, IL-10, IL-12, interferon (IFN) gamma, tumor necrosis factor (TNF) alpha and MCP-1 production in unstimulated mouse splenocytes and mouse macrophages. Interestingly, NR-INF-02 showed potent inhibitory effect towards release of PGE2 and IL-12 levels in LPS stimulated mouse splenocytes. Further, NR-INF-02 was fractionated into polysaccharide fraction (F1) and mother liquor (F2) to study their immune-modulatory effects. F1 was found to be more potent than F2 toward inhibiting PGE2 and IL-12 in LPS stimulated splenocytes.
Conclusion:
Present findings revealed the novel anti-inflammatory property of NR-INF-02 and its polysaccharide fraction by inhibiting the secretion of IL-12 and PGE2 in vitro.
Keywords: Curcuma longa, immunomodulation, inflammation, pain, polysaccharides, Turmacin™
INTRODUCTION
Curcuma longa (C. longa) Linn. commonly known as turmeric, is a perennial plant belonging to the family Zingiberaceae. It is a common ingredient in many health supplements in Asia, being used in various therapeutic applications such as blood purifying, wound healing, and inflammatory disorders and holds a prominent position in traditional Indian medicinal system.[1]
Curcuminoids (mixture of curcumin, demethoxycurcumin, and bisdemethoxycurcumin) are considered as key active constituents of C. longa and are reported to possess several biological activities. Numerous lines of evidence suggested, that curcuminoids are potent anti-inflammatory agents working through multiple mechanisms viz., suppression of the activation of nuclear factor (NF)-kappa B, inhibition of cyclooxygenase (COX)-2, down-regulation of the expression of cell proliferation, anti-apoptotic, and metastatic gene products.[2–4] Curcuminoids have also been demonstrated to modulate the proliferation and cellular response of various immune cell types, such as T cells, B cells, macrophages, neutrophils, natural killer NK cells and dendritic cells.[5–7]
In addition to curcuminoids, turmerones and other sesquiterpenoids from essential oil of C. longa have been shown to possess various biological activities viz., anti-inflammatory, antioxidant, and chemo-preventive properties.[8,9]
Among the polar constituents, polysaccharides viz., ukonan A, B, C, and D from rhizome of C. longa were shown to have the activity on reticuloendothelial system.[10–14] Polysaccharides from medicinal plants have attracted attention recently in the enhancement of host defense mechanisms. Several polysaccharides isolated from different medicinal plants, such as Lentinus edodes, Schizophyllium commune, Angelica gigas, Phellinus linteus, Platycodon grandiflorum have been shown to possess immune-stimulatory activity.[15–17] Polysaccharides and polysaccharide containing plant products have been demonstrated for immune-modulatory activity in various pre-clinical and human clinical models after oral administration. In addition, polysaccharides have been shown to be bioavailable in various in vivo and human clinical models after oral administration.[18,19] Additionally, polysaccharides containing C. longa extracts have been shown to have anti-diabetic, anti-tumor, anti-depressant, anti-oxidant, anti-microbial, anti-fertility, immune-modulatory, and hepato protective properties.[9,20–26]
While anti-inflammatory and immune-modulatory activities of curcuminoids have been studied extensively, very limited reports on polar extracts (containing polysaccharides) of C. longa are available. In this context, we developed an aqueous based extract of C. longa, devoid of curcuminoids, standardized to polysaccharides (NR-INF-02) and evaluated for inflammation related health conditions.
A randomized placebo controlled study on 120 patients (37 males and 83 females) with primary osteoarthritis demonstrated the efficacy of NR-INF-02 and it could possibly be a safer and effective option for the management of primary painful knee and joint pain.[27] In the present study, we have evaluated the potential anti-inflammatory and immune-stimulatory activities of NR-INF-02 in presence and absence of mitogens in cell based systems (in vitro).
MATERIALS AND METHODS
Source of materials
Lipopolysaccharide (LPS), concanavalin A (Con A), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT), 1400 W dihydrochloride, dexamethasone, beta-mercaptoethanol (Beta-ME), histopaque 1077 and Tween-20 were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Roswell Park Memorial Institute-1640 (RPMI-1640) and Dulbecco's modified Eagle's medium were supplied by Gibco Life Technologies (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories, Inc. (Logan, UT, USA).
Plant material
The rhizomes of C. longa Linn. were collected from different parts of Tamil Nadu State, India and authenticated at National Institute of Science Communication and Information Resources. A voucher specimen (No. 653) was deposited in our herbarium.
Preparation of NR-INF-02
Coarsely powdered rhizomes of C. longa were subjected to steam distillation and the oil was separated and collected. The rhizomes were further extracted by refluxing with water in a commercial extraction facility. The liquid water extract was concentrated by distillation under vacuum and the resultant concentrated liquid was spray dried to obtain a free flowing powder. NR-INF-02 was prepared by blending the spray dried water extract and the turmeric oil at a proportion of 99:1 (w/w) followed by sieving. NR-INF-02 was manufactured and registered as Turmacin™ by Natural Remedies Pvt. Ltd., Bangalore, India. Content of polysaccharides in NR-INF-02 were determined as 12.6% w/w by high-performance liquid chromatography as per the method described by Gomis et al.[28] Content of curcuminoids in NR-INF-02 were found to be negligible as determined by a modified USP method.[29]
Preparation of polysaccharide fraction from NR-INF-02
Polysaccharide fraction was isolated from NR-INF-02 by precipitation with alcohol as described earlier.[10] Briefly, the extract was dissolved in water and added to 5 volumes of ethanol. The above contents were centrifuged at 2000 rpm for 20 min. The supernatant obtained was concentrated under vacuum to get mother liquor (F2). The precipitate obtained after centrifugation was stirred with 5 volumes of ethanol at room temperature for 10 min and filtered. The retentate obtained after filtration was dried under vacuum at <70°C to obtain polysaccharide fraction (F1).
Characterization of polysaccharides
13C Nuclear magnetic resonance spectrum (200 MHz, Bruker, Switzerland) of F1 confirmed the presence of polysaccharides as indicated by oxygen bearing carbons at 70-80 ppm (CHOH), 60-62 ppm (CH2OH); anomeric carbons in the range of 95-111 ppm; rhamnosyl methyl carbon (20-22 ppm) and the carboxylic carbons at 170-175 ppm [Figure 1].
Figure 1.
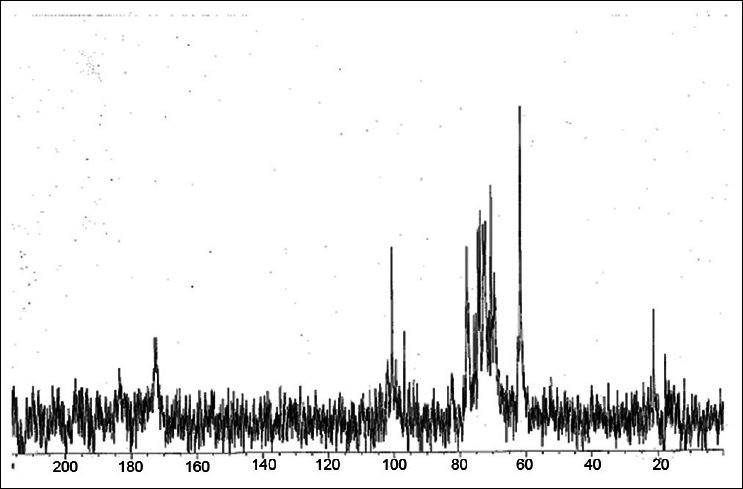
13C NMR spectrum of polysaccharide fraction (F1) of NR-INF-02 in D2O
Preparation of splenocyte culture
Swiss albino male mice 8-10 weeks old (25-30 g body weight) were taken from our experimental animal house (Natural Remedies Pvt. Ltd., Bangalore). Mice were killed by cervical dislocation and the spleen was removed from animals under aseptic conditions. This work was approved by Institutional Animal Ethics Committee (IAEC) held on 12.03.2011 (Approval Number: IAEC/CA/01/03.11).
The immune cells were isolated from the spleens by passing through 40 μm cell strainer and the resultant cell suspension was collected and centrifuged. Single splenocyte suspension was obtained by density-gradient centrifugation (Histopaque 1077). Cells were re-suspended in RPMI-1640 medium containing 10% heat inactivated FBS, and 1% antibiotics.[30] The above prepared splenocyte single cell suspension was used for further experiments. NR-INF-02, F1 and F2 was dissolved in phosphate buffered saline and filtered sterilized through 0.2 μm positively charged nylon filter before adding to cell culture treatment media.
Lymphocyte proliferation assay
Splenocytes were seeded in 48-well plates containing growth media and beta-ME (50 μM). Cells were treated with NR-INF-02 (0.8-500 μg/mL) at the indicated concentrations with or without mitogens (LPS [5 μg/mL] or Con A [2.5 μg/mL]) and further incubated for 48 h at 37°C under 5% CO2 humidified atmosphere. Post-incubation, the plates were centrifuged and cell culture supernatants were collected for quantification of cytokines (IL-2, IL-6, IL-10, IL-12, TNF alpha, interferon IFN gamma) and prostaglandin E2 (PGE2). Proliferative response of NR-INF-02 on splenic lymphocytes in presence and absence of mitogens was determined by MTT assay. Dexamethasone and celecoxib were used as an inhibitor of IL-12 and PGE2 respectively.
Interleukins and PGE2 quantification
Supernatant collected from above experiments was used to determine IL-2, IL-6, IL-10, IL-12, TNF alpha and IFN gamma by enzyme linked immunosorbent assay (ELISA Kit, OptEIA™ from Becton, Dickinson (BD) biosciences, USA). PGE2 levels were quantified by Homogenous Time Resolved Fluorescence (HTRF) method (HTRF kit, CisBio, France).
Nitric oxide (NO) assay and monocyte chemotactic protein-1 (MCP-1) assay
RAW264.7 macrophages, obtained from American Type Culture Collection were cultured in Dulbecco's Modified Eagle Medium DMEM supplemented with 10% FBS at 37°C using a mixture of 95% air and 5% CO2. RAW264.7 cells were adjusted in cell culture medium to a density of 1 × 105 cells per well in a 96 well plate. The cells were pre incubated with indicated concentrations of test substances for 30 min and treated with and without LPS (1 μg/mL) for 24 h. 1400 W dihydrochloride (iNOS inhibitor) and dexamethasone were used as reference standards for NO and MCP-1 assays respectively. Post treatment, cell culture supernatant was quantified for NO and MCP-1 levels using Griess reaction and ELISA (ELISA Kit, OptEIA™ from BD biosciences, USA) method, respectively.
All the above experiments were standardized using respective positive and negative controls. Assay performance measures like Z’, S/N ratio and % CV were calculated and fulfilled to the prescribed limits.[31]
Statistical analysis
Pooled data are represented as mean ± standard deviation (S.D) from three independent experiments with three replicates in each experiment. Statistical significance between groups was arrived using one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego CA). In all data analysis, P < 0.05 was considered as significant. Dose response curves were constructed manually to determine EC50, the effective concentration of test material causing 50% inhibition or activation was estimated from these dose response curves by fixing untreated control, mitogen (LPS) values as 0 and 100%, respectively. (a) significant (P < 0.05) difference between zero control and NR-INF-02 treated cells; (b) significant (P < 0.05) difference between LPS control and NR-INF-02 or reference standard (if applicable) + LPS treated cells; (c) significant (P < 0.05) difference between NR-INF-02 treated cells and NR-INF-02 + LPS treated cells; (d) significant (P < 0.05) difference between Con A control and NR-INF-02 + Con A treated cells; (e) significant (P < 0.05) difference between NR-INF-02 treated cells and NR-INF-02 + Con A treated cells.
RESULTS
Effect of NR-INF-02 on splenocyte proliferation
In this study, we have focused on cellular basis of the immune-modulating property of NR-INF-02. To study this, the effect of NR-INF-02 on unstimulated murine splenocytes was investigated. NR-INF-02 alone showed significant concentration dependent increase on proliferation of murine splenocytes within the experimental concentration range of 0.8-500 μg/mL [Figure 2a]. The next step was to examine whether the association of NR-INF-02 and mitogens influence the proliferation of splenocytes. LPS was used at 5 μg/mL as B cell mitogen, and Con A was used at 2.5 μg/mL as T cell mitogen. For splenic lymphocytes, the two mitogens produced a significant increase in cell proliferation. NR-INF-02 produced statistically significant dose dependent increase in both LPS and Con A stimulated splenocyte proliferation at the indicated concentration range of 0.8-500 μg/mL [Figure 2a].
Figure 2a.

Effect of NR-INF-02 on lymphocyte proliferation in unstimulated, concanavalin A (Con A) and lipopolysaccharide (LPS) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without Con A or LPS and further incubated for 48 h. Values are expressed as cell number)
Effect of NR-INF-02 on production of Th1, Th2 cytokines and PGE2
As reflected in graphical representation, production of Th1 and Th2 related cytokines were found to be very low in untreated splenic lymphocytes culture supernatants after 48 h culture. However, NR-INF-02 treated splenocytes produced significant concentration dependent increase of IL-2, IL-6, IL-10, IL-12, IFN gamma, TNF alpha and PGE2 levels [Figures 2b-e, and 3a-c]. Treatment with LPS resulted in a significant increase of IL-6 [Figure 3a], IL-10 [Figure 2d], IL-12 [Figure 3b], TNF alpha [Figure 2e] and PGE2 [Figure 3c]. NR-INF-02 produced distinct concentration dependent decrease of IL-12 [Figure 3b] and PGE2 [Figure 3c] levels in LPS stimulated splenocytes. NR-INF-02 at the indicated concentrations exhibited a mild significant decrease of IL-6 [Figure 3a] levels however, it did not alter the levels of IL-10 [Figure 2d] and TNF alpha [Figure 2e] in LPS stimulated murine splenocytes. Con A treatment produced significant increase of IL-2 [Figure 2b], IL-10 [Figure 2d] and IFN gamma [Figure 2c]. NR-INF-02 produced significant increase of IL-2 and IFN gamma in Con A stimulated splenocytes [Figure 2b and c]. NR-INF-02 at a concentration of 500 μg/mL, exhibited mild significant decrease of IL-10 production in Con A stimulated splenocytes [Figure 2d].
Figure 2b.
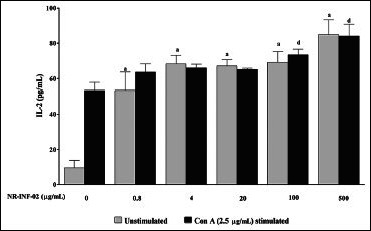
Effect of NR-INF-02 on interleukin (IL)-2 release in unstimulated and concanavalin A (Con A) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without Con A and further incubated for 48 h. After 48 h, the cells were centrifuged and the supernatants were collected for IL-2 estimation and the values are expressed as pg/mL)
Figure 2e.
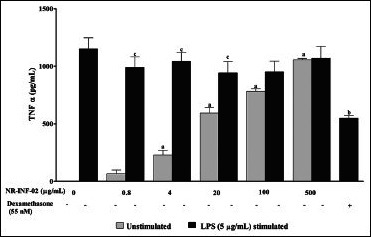
Effect of NR-INF-02 on tumor necrosis factor (TNF) alpha release in unstimulated and lipopolysaccharide (LPS) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without LPS and further incubated for 48 h. After 48 h, the cells were centrifuged and the supernatants were collected for TNF-alpha estimation and the values are expressed as pg/mL. Dexamethasone (55 nM) was used as a reference control)
Figure 3a.
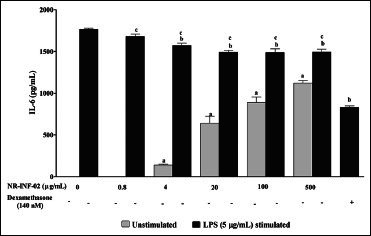
Effect of NR-INF-02 on interleukin (IL).6 release in unstimulated and lipopolysaccharide (LPS) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without LPS and further incubated for 48 h. After 48 h, the cells were centrifuged and the supernatants were collected for IL-6 estimation and the values are expressed as pg/mL. Dexamethasone (140 nM) was used as a reference control)
Figure 3c.
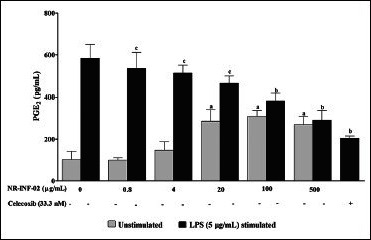
Effect of NR-INF-02 on prostaglandin E2 (PGE2) release in unstimulated and lipopolysaccharide (LPS) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without LPS and further incubated for 48 h. After 48 h, the cells were centrifuged and the supernatants were collected for PGE2 quantification and the values are expressed as pg/mL. Celecoxib (33.3 nM) was used as a reference control)
Figure 2d.
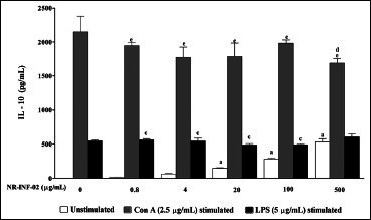
Effect of NR-INF-02 on interleukin (IL)-10 release in unstimulated, concanavalin A (Con A), and lipopolysaccharide (LPS) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without Con A or LPS and further incubated for 48 h. After 48 h, the cells were centrifuged and the supernatants were collected for IL-10 estimation and the values are expressed as pg/mL)
Figure 3b.
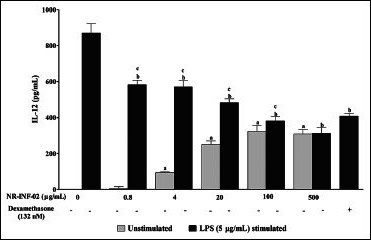
Effect of NR-INF-02 on interleukin (IL).12 release in unstimulated and lipopolysaccharide (LPS) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without LPS and further incubated for 48 h. After 48 h, the tcells were centrifuged and the supernatants were collected for IL-12 estimation and the values are expressed as pg/mL. Dexamethasone (132 nM) was used as a reference control)
Figure 2c.
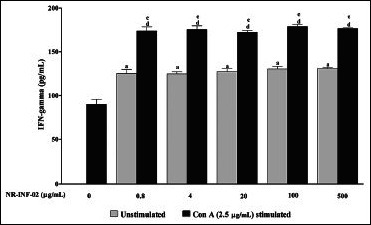
Effect of NR-INF-02 on interferon (IFN) gamma release in unstimulated and concanavalin A (Con A) stimulated murine splenocytes at 48 h of cell treatment (Mouse splenocytes were treated with NR-INF-02 at the indicated concentrations with and without Con A and further incubated for 48 h. After 48 h, the cells were centrifuged and the supernatants were collected for IFN gamma estimation and the values are expressed as pg/mL)
Effect of NR-INF-02 on NO and MCP-1 production
NR-INF-02 was found to significantly increase the NO and MCP-1 levels in RAW264.7 cells. A clear concentration dependent increase in the levels of NO and MCP-1 was observed upon treatment with NR-INF-02. Significant decrease of NO at lesser concentrations (0.8-20 μg/mL) and no decrease or increase in MCP-1 levels were observed in LPS stimulated RAW264.7 cells at the tested concentrations of NR-INF-02 [Figure 3d and e].
Figure 3d.
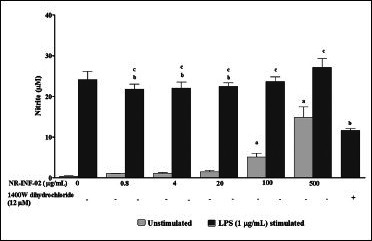
Effect of NR-INF-02 on nitric oxide release in unstimulated and lipopolysaccharide (LPS) stimulated RAW264.7 cells at 24 h of cell treatment (RAW264.7 cells were pre incubated at the indicated concentrations of NR-INF-02 for 30 min and treated with and without LPS for 24 h. After 24 h, the nitrite content in culture supernatant was analyzed by Griess reaction assay and expressed in μM. 1400 W dihydrochloride (12 μM) was used as reference control)
Figure 3e.
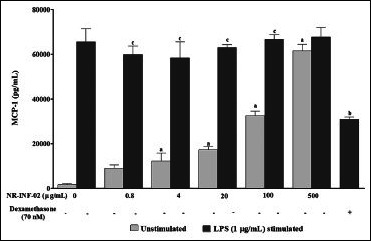
Effect of NR-INF-02 on monocyte chemotactic protein-1 (MCP-1) release in unstimulated and lipopolysaccharide (LPS) stimulated RAW 264.7 cells at 24 h of cell treatment (RAW264.7 cells were pre incubated at the indicated concentrations of NR-INF-02 for 30 min and treated with and without LPS for 24 h. After 24 h, the culture supernatant was analyzed for MCP-1 levels by ELISA and expressed as pg/mL. Dexamethasone (70 nM) was used as a reference control)
Effect of NR-INF-02 fractions, F1 and F2 on lymphocyte proliferation, interleukins and PGE2
F1 and F2 significantly stimulated the proliferation of mouse splenocytes after 48 h of treatment. The relative EC50 of NR-INF-02 and F1 were found to be 18.6 and 9 μg/mL respectively. F2 fraction showed less potent (EC50 = 162 μg/mL) activity than the NR-INF-02 and F1. Polysaccharide fraction (F1) showed almost two times more potent activity than NR-INF-02 [Figure 4a]. As shown in [Figure 4b], F1 and F2 dose dependently increased the cell number of LPS stimulated splenocytes. F1 showed very potent stimulatory activity with an EC50 of 0.072 μg/mL. F1 exhibited almost 10 times more potent stimulatory activity than NR-INF-02. Furthermore, F1 could significantly stimulated the IL-10 levels. NR-INF-02 and F1 showed equipotent activity towards production of IL-10 with an EC50 value of 42 and 36 μg/mL respectively. However, F2 significantly stimulated IL-10 production only at the highest concentration of 500 μg/mL [Figure 4c].
Figure 4a.
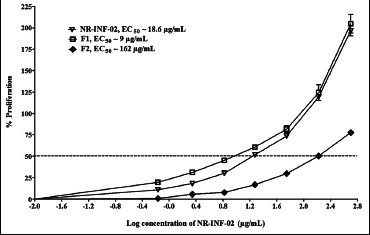
Dose-response curve generated for NR-INF-02, F1 and F2 on proliferation of unstimulated mouse splenocytes
Figure 4b.
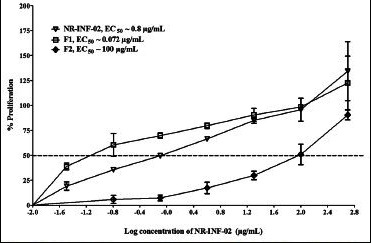
Dose-response curve generated for NR-INF-02, F1 and F2 on proliferation of lipopolysaccharide stimulated mouse splenocytes
Figure 4c.

Dose-response curve generated for NR-INF-02, F1 and F2 on interleukin (IL).10 secretion in unstimulated mouse splenocytes
Results also indicated that NR-INF-02, F1 and F2 concentration dependently decreased the production of IL-12 in LPS stimulated splenocytes. F1 showed remarkable inhibitory effect (EC50 = 0.1 μg/mL) towards LPS stimulated IL-12 production after 48 h of treatment. NR-INF-02 and F2 showed dose dependent inhibition of IL-12 with an EC50 of 2 and 54 μg/mL respectively [Figure 4d]. Similar trend was observed on inhibition of LPS stimulated PGE2 levels. NR-INF-02, F1 and F2 significantly ameliorated the PGE2 production in LPS stimulated mouse splenocytes [Figure 4e]. However, F1 showed stronger inhibitory potential than NR-INF-02 and F2. F1 showed almost 20 times more potent activity than NR-INF-02 towards inhibition of PGE2 and IL-12 in LPS treated splenocytes [Figure 4d and e].
Figure 4d.
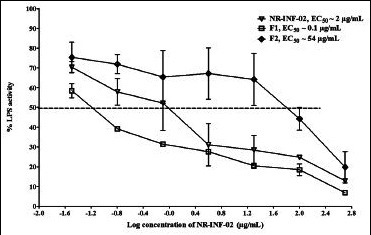
Dose-response curve generated for NR-INF-02, F1 and F2 on interleukin (IL)-12 secretion in lipopolysaccharide stimulated mouse splenocytes
Figure 4e.
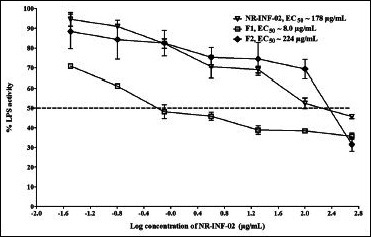
Dose-response curve generated for NR-INF-02, F1 and F2 on prostaglandin E2 (PGE2) secretion in lipopolysaccharide stimulated mouse splenocytes
DISCUSSION
Number of medicinal plant extracts and their constituents are known to alter immune function and display array of immune-modulatory effects. In various in vitro and in vivo studies, herbal medicines have been reported to modulate cytokine secretion, immunoglobulin secretion, lymphocyte expression and phagocytosis.[32] In the present study, the immune stimulatory and anti-inflammatory properties of an aqueous based extract of C. longa (NR-INF-02) were evaluated in vitro.
Lymphocytes are the key constituents of the immune system as they recognize the foreign antigens and mount an immune response; a rise or fall in the concentration of these cells affects the health/immune constitution of the body.[33]
The present results showed mitogenic activity of NR-INF-02 which was comparable to classical mitogens like LPS and Con A. Also, the effects of NR-INF-02 on production of T cell cytokines including Th1 (IL-2 and IFN gamma) and Th2 cytokines (IL-10) were measured. Results showed that NR-INF-02 increased both Th1 (IL-2 and IFN gamma) and Th2 (IL-10) cytokines indicating its dual immune functions. NR-INF-02 significantly increased the IL-2 and IFN gamma levels in Con A stimulated splenic lymphocytes. The above results indicated that NR-INF-02 showed a specific immunity response by stimulating both Th1 and Th2 cells.
Polysaccharide fraction (F1) derived from NR-INF-02 showed potent immune stimulatory activity towards proliferation of splenocytes cell number and IL-10 secretion than F2. In concordance to our results, the recent report on polysaccharide fraction of the rhizome of C. longa revealed the proliferative response and cytokine production in peripheral blood mononuclear cells (PBMC) in vitro.[9] Hence, we hypothesize that polysaccharides present in this extract might be contributing to this proliferative and cytokine release property in murine splenocytes.
Macrophages are important as a first line of defense against infections. NR-INF-02 increased the NO levels in mouse macrophages (RAW264.7) which plays an important role as cytotoxic agents against invading pathogens. Increased synthesis of NO can induce immune-stimulating activity of macrophages.
NR-INF-02 increases the release of MCP-1 from murine macrophages in a concentration dependent manner indicating its role in the recruitment of monocytes to sites of injury and infection. Four polysaccharides namely ukonan A, ukonan B, ukonan C and ukonan D have been isolated from C. longa and proved to have remarkable reticulo endothelial system potentiating activity in carbon clearance test.[10–14] Macrophage activation of NR-INF-02 might be due to presence of these ukonan polysaccharides.
Activated macrophages in turn inhibit the invasion of microorganisms by releasing cytokines. As a result of direct macrophage activation by NR-INF-02, PGE2 levels from macrophages of murine splenocytes were increased as that of LPS stimulation. PGE2 is involved in diverse functions, including nerve growth, wound healing, and the immune response.
NR-INF-02 activated the macrophages which secreted many inflammatory mediators such as PGE2, IL-6, IL-12 and TNF alpha. The present findings are in concordance with previous findings of Kim et al. who demonstrated enhanced phagocytic activity in Gram positive and negative bacteria and also augmented the oxygen burst response with purified polysaccharide fraction of one another species, Curcuma zeodoaria.[34] Additionally, macrophage stimulating activity of C. zeodoaria was confirmed by a significant increase of H2 O2, NO, and TNF alpha production in RAW264.7 cells. Similar to this report, crude polysaccharide extract prepared from rhizome of Curcuma xanthorrhiza significantly increased the phagocytosis activity of macrophages, release of NO, H2O2, TNF alpha and PGE2 in a dose dependent manner. Increase of NO and PGE2 was mediated in part by specific activation of NF-kappa B.[35] Enhanced release of pro-inflammatory cytokines, including IFN gamma and TNF alpha by aqueous extract of C. longa treatment led to an expression of cell adhesion molecule such as inter-cellular adhesion molecule-1, vascular-cell adhesion molecule-1 and E-selectin mediate the extravasation of leukocytes from blood vessels to the sites of injury or infection.[36]
However, inflammatory processes subsequently need to be down regulated to allow healing. These divergent and at times seemingly contradictory effects reflect the dichotomy of macrophages as both pro and anti-inflammatory effectors in response to host environmental changes.[37]
NR-INF-02 exerted strong inhibition on LPS stimulated PGE2 and IL-12 production by macrophages. Furthermore, polysaccharide fraction derived from NR-INF-02 showed potent inhibitory effect toward LPS stimulated IL-12 and PGE2 secretion.
NR-INF-02 showed mild inhibitory effect towards NO, and IL-6 production in the presence of LPS. Interestingly, NR-INF-02 neither increased nor decreased the levels of IL-10, TNF alpha, and MCP-1 cytokines in the presence of LPS.
According to our knowledge, for the first time, we have demonstrated the anti-inflammatory activity of polysaccharide containing C. longa extract by down regulating the PGE2 and IL-12 levels in LPS stimulated mouse splenocytes. Furthermore, NR-INF-02 increased IL-10 in unstimulated splenocytes and is one of the best-known cytokine that inhibit various types of inflammatory responses. An in vivo study by Yegnarayan et al. proved that water extract of C. longa had potent anti-inflammatory activity in carrageenan induced oedema, granuloma pouch and cotton pellet implantation method in male albino mice.[38] The above study concluded that water extract of C. longa is superior than curcuminoids containing C. longa extract.
CONCLUSION
In conclusion, the present experiment shows that NR-INF-02 exhibits potent in vitro immune-stimulatory activity by macrophage activation, splenocytes proliferation and cytokine release. Interestingly, NR-INF-02 and polysaccharide fraction showed prominent anti-inflammatory activity by down regulating PGE2 and IL-12 secretion. Based on the data presented here on the polysaccharide fraction of NR-INF-02, we hypothesize that polysaccharides of C. longa contribute to the anti-inflammatory and immune-stimulatory activities of NR-INF-02. Further studies are directed to understand the molecular mechanism of action of NR-INF-02 and polysaccharide fraction towards inhibition of LPS induced IL-12 and PGE2 secretion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Khanna NM. Turmeric, Nature's precious gift. Curr Sci. 1999;76:1351–6. [Google Scholar]
- 2.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 3.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 5.Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78–82. doi: 10.1016/s0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- 6.Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J Surg Res. 2000;89:169–75. doi: 10.1006/jsre.2000.5826. [DOI] [PubMed] [Google Scholar]
- 7.Jagetia GC, Aggarwal BB. Spicing up of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 8.He XG, Lin LZ, Lian LZ, Lindenmaier M. Liquid chromatographyelectrospray mass spectrometric analysis of curcuminoids and sesquiterpenoids in turmeric (Curcuma longa) J Chromatogr. 1998;818:127–32. [Google Scholar]
- 9.Yue GG, Chan BC, Hon PM, Kennelly EJ, Yeung SK, Cassileth BR, et al. Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int J Biol Macromol. 2010;47:342–7. doi: 10.1016/j.ijbiomac.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonda R, Tomoda M, Shimizu N, Kanari M. Characterization of polysaccharides having activity on the reticuloendothelial system from the rhizome of Curcuma longa. Chem Pharm Bull (Tokyo) 1990;38:482–6. doi: 10.1248/cpb.38.482. [DOI] [PubMed] [Google Scholar]
- 11.Gonda R, Takeda K, Shimizu N, Tomoda M. Characterization of a neutral polysaccharide having activity on the reticuloendothelial system from the rhizome of Curcuma longa. Chem Pharm Bull (Tokyo) 1992;40:185–8. doi: 10.1248/cpb.40.185. [DOI] [PubMed] [Google Scholar]
- 12.Gonda R, Tomoda M, Takada K, Ohara N, Shimizu N. The core structure of ukonan A, a phagocytosis-activating polysaccharide from the rhizome of Curcuma longa and immunological activities of degradation products. Chem Pharm Bull (Tokyo) 1992;40:990–3. doi: 10.1248/cpb.40.990. [DOI] [PubMed] [Google Scholar]
- 13.Gonda R, Tomoda M, Ohara N, Takada K. Arabinogalactan core structure and immunological activities of ukonan C, an acidic polysaccharide from the rhizome of Curcuma longa. Biol Pharm Bull. 1993;16:235–8. doi: 10.1248/bpb.16.235. [DOI] [PubMed] [Google Scholar]
- 14.Tomoda M, Gonda R, Shimizu N, Kanari M, Kimura M. A reticuloendothelial system activating glycan from the rhizomes of Curcuma longa. Phytochemistry. 1990;29:1083–6. [Google Scholar]
- 15.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–74. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 16.Jeon YJ, Kim HM. Experimental evidences and signal transduction pathways involved in the activation of NF-kappa B/ Rel by angelan in murine macrophages. Int Immunopharmacol. 2001;1:1331–9. doi: 10.1016/s1567-5769(01)00065-0. [DOI] [PubMed] [Google Scholar]
- 17.Yoon YD, Kang JS, Han SB, Park SK, Lee HS, Kang JS, et al. Activation of mitogen-activated protein kinases and AP-1 by polysaccharide isolated from the radix of Platycodon grandiflorum in RAW 264.7 cells. Int Immunopharmacol. 2004;4:1477–87. doi: 10.1016/j.intimp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Ramberg JE, Nelson ED, Sinnott RA. Immunomodulatory dietary polysaccharides: A systematic review of the literature. Nutr J. 2010;9:54. doi: 10.1186/1475-2891-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trnovec T, Hrmová M. Immunomodulator polysaccharides: Chemistry, disposition and metabolism. Biopharm Drug Dispos. 1993;14:187–98. doi: 10.1002/bdd.2510140302. [DOI] [PubMed] [Google Scholar]
- 20.Mohankumar S, McFarlane JR. An aqueous extract of Curcuma longa (turmeric) rhizomes stimulates insulin release and mimics insulin action on tissues involved in glucose homeostasis in vitro. Phytother Res. 2011;25:396–401. doi: 10.1002/ptr.3275. [DOI] [PubMed] [Google Scholar]
- 21.Deshpande SS, Ingle AD, Maru GB. Chemopreventive efficacy of curcumin-free aqueous turmeric extract in 7,12-dimethylbenz a anthracene-induced rat mammary tumorigenesis. Cancer Lett. 1998;123:35–40. doi: 10.1016/s0304-3835(97)00400-x. [DOI] [PubMed] [Google Scholar]
- 22.Yu ZF, Kong LD, Chen Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol. 2002;83:161–5. doi: 10.1016/s0378-8741(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 23.Selvam R, Subramanian L, Gayathri R, Angayarkanni N. The anti-oxidant activity of turmeric (Curcuma longa) J Ethnopharmacol. 1995;47:59–67. doi: 10.1016/0378-8741(95)01250-h. [DOI] [PubMed] [Google Scholar]
- 24.Anbu Jeba Sunilson J, Suraj R, Rejitha G, Anandarajagopal K, Anita Gnana Kumari AV, Promwichit P. In vitro antimicrobial evaluation of Zingiber officinale, Curcuma longa and Alpinia galanga extracts as natural food preservatives. Am J Food Technol. 2009;4:192–200. [Google Scholar]
- 25.Mishra RK, Singh SK. Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception. 2009;79:479–87. doi: 10.1016/j.contraception.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian L, Selvam R. Prevention of C4-induced hepatotoxicity by aqueous extract of turmeric. Nutr Res. 1999;19:429–41. [Google Scholar]
- 27.Madhu K, Chanda K, Saji MJ. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: A randomized placebo-controlled trial. Inflammopharmacology. 2013;21:129–36. doi: 10.1007/s10787-012-0163-3. [DOI] [PubMed] [Google Scholar]
- 28.Gomis DB, Tamayo DM, Alonso JM. Determination of monosaccharides in cider by reversed-phase liquid chromatography. Anal Chim Acta. 2001;436:173–80. [Google Scholar]
- 29.United States Pharmacopeia (USP) 35-NF30. 2012;1:957. [Google Scholar]
- 30.Chandrasekaran CV, Sundarajan K, David K, Agarwal A. In vitro efficacy and safety of poly-herbal formulations. Toxicol in vitro. 2010;24:885–97. doi: 10.1016/j.tiv.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasekaran CV, Edwin Jothie R, Kapoor P, Gupta A, Agarwal A. Optimization of cell-based assays to quantify the anti-inflammatory/allergic potential of test substances in 96-well format. Inflammopharmacology. 2011;19:169–81. doi: 10.1007/s10787-010-0065-1. [DOI] [PubMed] [Google Scholar]
- 32.Plaeger SF. Clinical immunology and traditional herbal medicines. Clin Diagn Lab Immunol. 2003;10:337–8. doi: 10.1128/CDLI.10.3.337-338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayathirtha MG, Mishra SH. Preliminary immunomodulatory activities of methanol extracts of Eclipta alba and Centella asiatica. Phytomedicine. 2004;11:361–5. doi: 10.1078/0944711041495236. [DOI] [PubMed] [Google Scholar]
- 34.Kim KI, Shin KS, Jun WJ, Hong BS, Shin DH, Cho HY, et al. Effects of polysaccharides from rhizomes of Curcuma zedoaria on macrophage functions. Biosci Biotechnol Biochem. 2001;65:2369–77. doi: 10.1271/bbb.65.2369. [DOI] [PubMed] [Google Scholar]
- 35.Kim AJ, Kim YO, Shim JS, Hwang JK. Immunostimulating activity of crude polysaccharide extract isolated from Curcuma xanthorrhiza Roxb. Biosci Biotechnol Biochem. 2007;71:1428–38. doi: 10.1271/bbb.60241. [DOI] [PubMed] [Google Scholar]
- 36.Madan B, Gade WN, Ghosh B. Curcuma longa activates NF-kappaB and promotes adhesion of neutrophils to human umbilical vein endothelial cells. J Ethnopharmacol. 2001;75:25–32. doi: 10.1016/s0378-8741(00)00386-x. [DOI] [PubMed] [Google Scholar]
- 37.Zhai Z, Liu Y, Wu L, Senchina DS, Wurtele ES, Murphy PA, et al. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J Med Food. 2007;10:423–34. doi: 10.1089/jmf.2006.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yegnanarayan R, Saraf AP, Balwani JH. Comparison of anti-inflammatory activity of various extracts of Curcuma longa (Linn.) Indian J Med Res. 1976;64:601–8. [PubMed] [Google Scholar]


