Abstract
Objective:
Low testosterone, with or without symptoms, reported in diabetic men in some studies. We investigated the prevalence of hypogonadism in Iranian type 2 diabetic men.
Materials and Methods:
Total testosterone (TT) and sex hormone binding globulin (SHBG) concentrations were measured in 247 diabetic men >30 years who had symptoms of androgen deficiency, according to ADAMs questionnaire. The correlation between some parameters and total, free and bioavailable testosterone levels was determined using Pearson correlation coefficient. Free and bioavailable testosterone were calculated by electronic calculator. Four patients were excluded because of high testosterone level, due to unreported androgen use. Overt hypogonadism was defined as total testosterone ≤8 nmol/l or calculated bioavailable testosterone (cBT)≤2.5 nmol/l and borderline hypogonadism was considered as TT 8-12 nmol/l or cBT 2.5-4nmol/l.
Results:
The mean and SD of age was 59 (9.3) years. The mean TT, calculated free testosterone (cFT), and cBT and SHBG levels were 4.81 (1.7) nmol/l, 0.11 (0.06) nmol/l, 2.42 (1.17) nmol/l and 36.15 (18.3) nmol/l, respectively. According to TT and cBT, overt hypogonadism observed in 7.4% and 61.6% of men, respectively, and the prevalence of borderline hypogonadism was 9.9% and 36%, respectively. cFT ≤0.16 nmol/l found in 227 diabetic men (96%). Hypogonadism (TT ≤12 nmol/l) was not correlated with obesity, smoking, age,duration of diabetes, blood pressure, and HbA1c.
Conclusion:
Hypogonadism is highly prevalent in type 2 diabetes men.
Keywords: Hypogonadism, male, Iran, prevalence, Type 2 diabetes mellitus
INTRODUCTION
The most important epidemic disease in aged adults of the 21st century is diabetes,[1,2] particularly in Asian developing countries.[3] The overall prevalence of type 2 diabetes in Isfahan, a centrally located city in Iran with a population of almost two million (1,986,542 in 2006 (men 1,017,940)), was reported as high as 7.76% in subjects aged over 40 years and 7.54% in male group.[4] Many studies have reported that men with type 2 diabetes, have significantly lower levels of testosterone compared with nondiabetic people as a part of diabetic complications.[5–14] Grossman et al, reported that 43% of diabetic men had low total testosterone and 57% had low calculated free testosterone.[14] Notably, low free testosterone (FT) level, measured by equilibrium dialysis, the gold standard for measuring FT has been reported in 25% of 57 men with type 2 diabetes.[12] Inverse correlation of testosterone and fasting plasma glucose has been shown in large population study.[13] However, both symptoms or signs and biochemical evidence of testosterone deficiency are needed to define hypogonadism.[15] The Food and Drug Administration (FDA) and the Endocrine Society recommend to consider hypogonadism in men, if total testosterone (TT) concentration is less than or equal to 300 ng/dl (10.4 nmol/l) in addition to related signs and symptoms of androgen deficiency.[12] Testosterone in the circulation is in the three major fractions: free (2%–3%), albumin-bound (20%-40%), and sex hormone-binding globulin (SHBG)-bound (60%-80%). Both the free and albumin-bound fractions comprise bioavailable testosterone because they are biologically active component that is readily available to the tissues, while SHBG-bound testosterone is strongly bound and inactive. Bound serum testosterone is regulated by SHBG. Obesity, waist circumference, aging, diabetes, and other comorbidities may change SHBG levels and influence TT levels.[16] Hence, measuring bioavailable or free testosterone is essential in men with diabetes.[12] Hypertension, body mass index, waist circumference, advanced age, diabetes, current smoking, general health status, and physical activity are considered as the risk factors of low testosterone levels.[17,18]
We studied male patients older than 30 years of age with type 2 diabetes mellitus who had been registered in Isfahan Endocrine and Metabolism Research Center (IEMRC) to investigate the prevalence of hypogonadism in type 2 diabetes. The results will help us for future health programming, as the prevalence has a wide range in different areas of the world. On the other hand, no similar study has already done in Iran.
MATERIALS AND METHODS
Diabetic men (n = 325), aged >30 years, who were registered in IEMRC were selected by consecutive patient selection method to enter this cross-sectional study, from June to November 2009.
All patients gave written informed consent, and the ethics committee of Isfahan University of Medical Sciences approved the protocol. The study complied with the current version of the Declaration of Helsinki.
Those patients who had overt nephrotic syndrome or cirrhosis, breast, and prostate cancer, type1 diabetes, were single or divorced, or were already receiving androgen did not enroll the study (n = 37).
All enrolled patients (n = 288) were asked about their medical history, smoking, marriage status, and medications by one endocrinologist (MRM). Blood pressure, height and weight, waist circumference were measured, and BMI was calculated. Waist was defined as the middle point between the iliac crest and the lower rib.[19]
Patients (n = 288) filled in an Androgen Deficiency in the Aging Male (ADAM) questionnaire.[20] This questionnaire is a screening tool which assesses androgen deficiency males through 10 items. Decreased sexual desire or erectile dysfunction or any three out of eight items of loss of energy, deterioration of work performance, decrease in muscle strength, and sleepiness after dinner, difficulty to sustain erection during sexual intercourse, depressed mood, decreased enjoyment of life, and nontraumatic loss of height, were considered positive ADAM's response. Symptomatic patients with positive ADAM's questionnaire, 265 out of 288 patients (92%), were referred to getting 20 ml venous blood sample between 7:30 Am and 9:30 A.M. Serum samples were separated by centrifugation and immediately freezed at -20 °C and stored before analysis. Total testosterone (TT) was measured by the ELISA assay technique (DRG, Germany) with inter- and intra-assay coefficient variation (CV) of 3.59% and 7.13%, respectively. SHBG was measured by the ELISA assay method (DiaMetra, Italy) with both inter and intra assay coefficient variation (CV) of 4.4%. Free and bioavailable testosterone were calculated by using total testosterone and SHBG via the calculator introduced by www.issam.ch/freetesto.htm.[21] The serum albumin concentration was assumed fixed (in the normal range of 43 g/l), since, patients with marked plasma protein abnormalities, such as in nephrotic syndrome or liver cirrhosis was not initially enrolled.[21] A total testosterone level ≤8 nmol/l was considered to be low, and it was considered as borderline while it was between 8 and 12 nmol/l. Total testosterone level >12 nmol/l was considered as normal.[19] A calculated free testosterone (cFT) level ≤0.16 nmol/l was postulated low.[22] Bioavailable concentration ≤2.5nmol/l was defined as low, values between 2.5 nmol/l to 4 nmol/l as borderline, and concentrations more than 4 nmol/l as normal.[22] Overt hypogonadism was defined as positive ADAM's questionnaire and total testosterone level ≤8 nmol/l or bioavailable concentration ≤2.5nmol/l. Borderline hypogonadism was defined as positive ADAM's questionnaire and total testosterone between 8 nmol/l and 12 nmol/l or bioavailable testosterone between 2.5 nmol/l to 4 nmol/l.
Waist circumference more than 89 cm consider high based on local cutoff point.[23]
Statistical analysis
Data were analyzed using the SPSS package. Values are expressed as percentage or as mean (SD), unless otherwise stated. The effects of clinical variables including decreased sexual desire, difficulty to maintain erection during sexual intercourse, erectile dysfunction, loss of energy, deterioration of work performance, decrease in muscle strength, sleepiness after dinner, depressed mood, decrease enjoyment of life, and nontraumatic loss of height on total, free, bioavailable testosterone and SHBG levels were determined. Means of total, free, bioavailable testosterone and SHBG were compared between different categories of age groups, duration of diabetes, BMI, cholesterol, triglyceride, and LDL levels using t Student's t and ANOVA tests [Table 2].
Table 2.
Association of SHBG and testosterone levels with of some variable categories in hypogonadal type 2 diabetic men
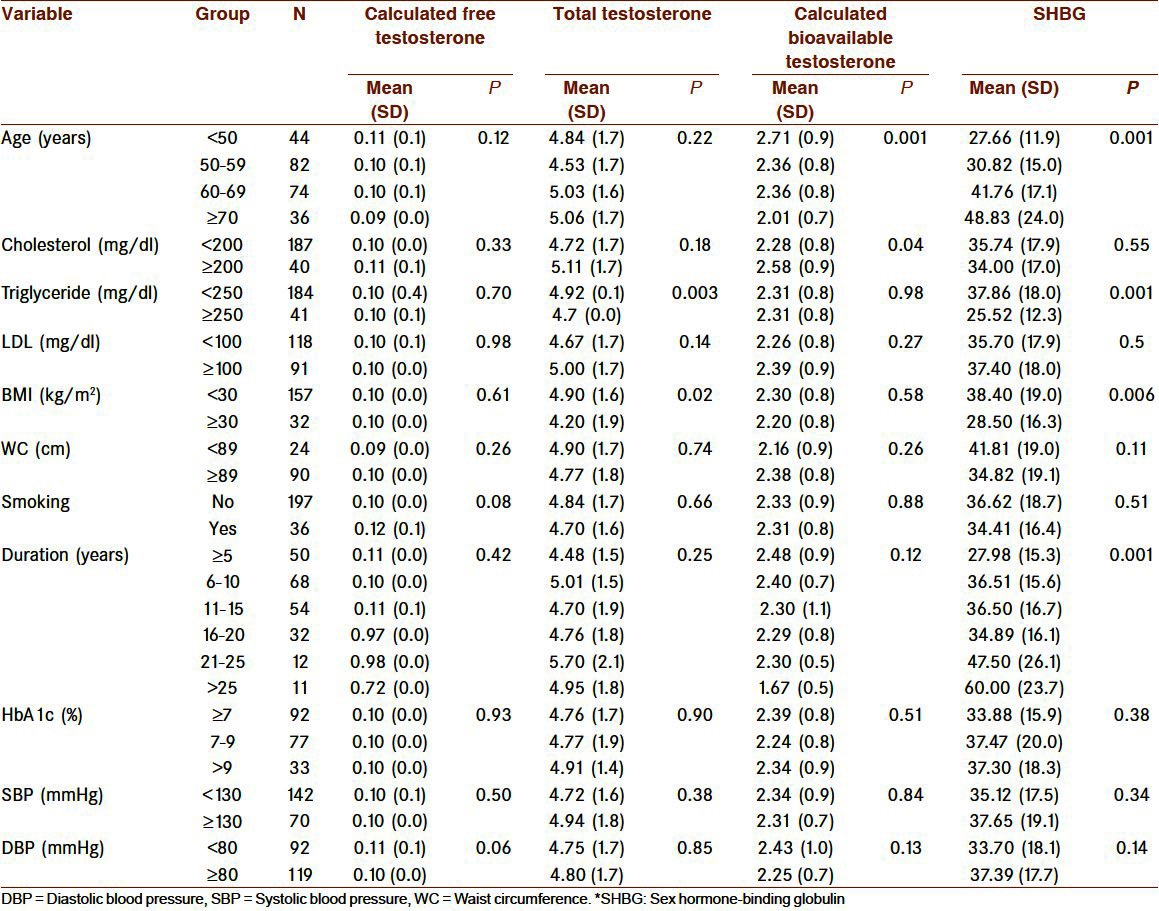
The correlation between TT, cFT, and cBT levels with age, SHBG, total cholesterol, triglyceride, LDL, HbA1c, systolic, and diastolic blood pressure, duration of diabetes, BMI and waist circumference was determined by Pearson correlation coefficient. The relationship between hypogonadism and obesity and smoking status was done. Both overt and borderline hypogonadism were considered as hypogonadism in this model. Results were considered statistically significant at P values less than 0.05.
RESULTS
Thirty seven out of 325 selected type 2 diabetic male patients, were not enrolled the study because of already mentioned exclusion criteria. All eligible patients (n = 288) were screened by ADAM's questionnaire. Twenty three (8%) out of 288 patients had no symptoms of hypogonadism. Therefore, they were considered clinically eugonadal and were not evaluated further. Of those who had positive ADAM's questionnaire (n = 265, 92%), 18 noncompliant patients did not follow the study. Total testosterone and SHBG were measured in 247 patients. Four men were excluded, again, because of the elevated baseline total testosterone, due to unreported exogenous androgen usage. However, the data of 243 hypogonadal diabetic men have been reported.
The baseline data of patients are presented in Table 1.
Table 1.
Baseline characteristics in diabetic males (n = 243), clinical and laboratory data
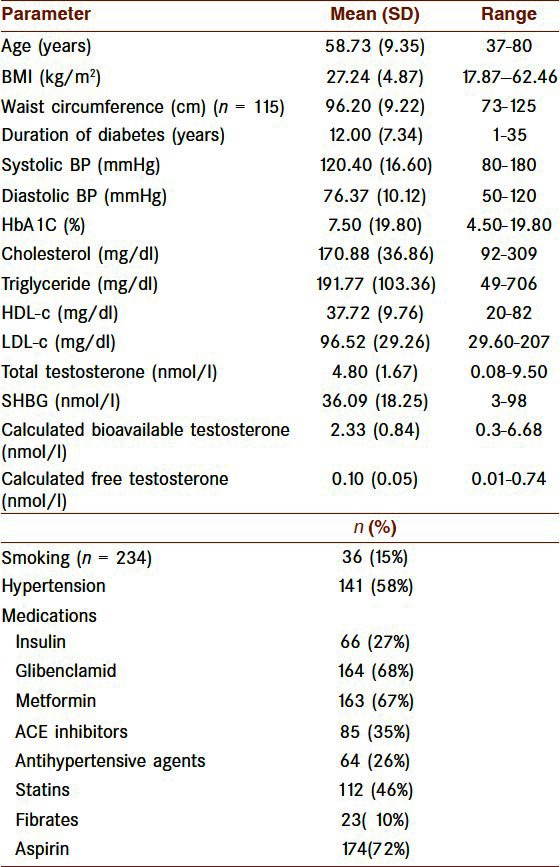
The most common symptoms of the diabetic men with low testosterone levels were erectile dysfunction, deterioration of work performance and loss of energy occurring in 211 (86.8 %), 204 (84%), and 199 (81.9%), respectively.
Calculated free testosterone correlates with cBT and TT (r = 0.6; P = 0.001 and r = 0.5; P = 0.001, respectively). Calculated bioavailable testosterone correlates directly with cFT, TT and LDL (r = 0.6; P = 0.001, r = 0.7; P = 001, r = 0.1; P = 0.05, respectively). It was inversely correlated with SHBG (r = -0.3; P = 0.001).
SHBG correlates with TT, systolic and diastolic blood pressure (r = 0.5; P = 0.001, r = 0.2; P = 0.02 and r = 0.2; P = 0.02, respectively) and also with cBT, BMI and triglyceride (r = 0.3; P = 0.001, r = 0.2; P = 0.003 and r = 0.3; P = 0.001).
Eighteen men (7.4%) had overt hypogonadism (total testosterone ≤8 nmol/l) and 24 men (9.9%) had borderline hypogonadism (total testosterone between 8 nmol/l and 12 nmol/l). Overt hypogonadism based on calculated bioavailable testosterone which defined as level ≤2.5 nmol/l was seen in 149 men (61.6%) and calculated bioavailable testosterone between 2.5 nmol/l and 4 nmol/l found in 89 men (36%), as borderline hypogonadism. Two hundred twenty seven men (96%) had calculated free testosterone≤0.16 nmol/l.
The age distribution by decades and the mean of total, calculated free and bioavailable testosterone and SHBG levels were shown in Table 2. Those patients less than 50 years were 18.6%, between 50 and 59 years were 34.7%, between 60 and 69 years were 31.4% and more than 69 years were 15.3% of all hypogonadal men [Table 2].
With advancing age, the concentration of SHBG increased (r = 0.4; P = 0.001) and cBT decreased (r = -0.3; P = 0.001).
Total testosterone and SHBG decreased by obesity (BMI≥30kg/m2) (P = 0.02 and P = 0.006, respectively), However, cFT and cBT were not be affected by obesity [Table 2].
Both cBT and cFT, inversely, correlated with age (r = -0.2; P = 0.04 and r = -0.2; P = 0.04, respectively).
Hypogonadism (TT ≤12 nmol/l) was not correlated with obesity, smoking status, age, duration of diabetes, systolic and diastolic blood pressure, waist circumference, BMI, HbA1c, SHBG, and lipid profile. It was correlated with bioavailable testosterone and CFT (P < 0.0001).
DISCUSSION
Some, but not all[24] cross-sectional studies have reported the association between low total testosterone and type 2 diabetes.[5–11,13] Our study has shown that the prevalence of hypogonadism in men with type 2 diabetes is high, especially, if it was defined based on calculated free and bioavailable testosterone.
The design of Kapoor et al, study was very similar to ours.[19] They measured total testosterone level in symptomatic patients and calculated bioavailable testosterone. The prevalence of overt hypogonadism, derived from TT, in our study was 7.4% versus 17% in Kapoor et al, study and the borderline hypogonadism was 9.9% versus 25%, respectively. According to cBT, the prevalence of overt hypogonadism in our research was 61.6% versus 14% in their study and the borderline hypogonadism was 36% versus 29%, respectively.[19] The cut off point for low cFT was 0.16 nmol/l in our study, while it was 0.25 nmol/l in Kapoor et al, study. As a result, the prevalence of hypogonadism was 96% in our patients and 42% of Kaoor et al, study. The lower prevalence of hypogonadism in Kapoor study based on cFT, would not be due to different cut off point, as it was higher than ours.
There are just a few studies,[12,14,25] which have reported the prevalence of hypogonadism according to cBT and cFT. Measurement of free testosterone by equilibrium dialysis technique is difficult and not available everywhere, but cFT values are reliable by Vermeulen equation, and can also be used instead of free testosterone, in a clinical setting.[26] We did not measure the free testosterone directly. It was calculated by electronic calculator using TT and SHBG. The calculator had been designed based on Vermeulen et al, method of calculation.[21]
In our study, TT had strong correlation with cFT and cBT (r = 0.5; P = 0.001 and r = 0.7; P = 0.001, respectively). However, the big difference between the prevalence of hypogonadism according to TT and cFT (or cBT) could not be explained.
We observed no correlation between obesity (BMI≥30 kg/ m2), age, duration of diabetes, smoking status and HbA1c and the prevalence of hypogonadism based on low total testosterone. Hypogonadism did not have correlation with the degree of glycemic control. Similar to ours, other studies have found that low testosterone concentrations are not related to glycosylated hemoglobin or duration of diabetes, but had association with obesity.[27] According to a literature review, hypogonadism in diabetes is more related to insulin resistance than glycemic control itself.[27]
In a study, recently done in USA, obesity and diabetes were found to be independent risk factors for low free testosterone in men.[28]
Trials of long duration are required to establish the benefits and risks of testosterone replacement in patients with type 2 diabetes and low testosterone.[27]
CONCLUSION
Hypogonadism is highly prevalent in type 2 diabetes patients in Isfahan population. There is no correlation between obesity, smoking status, age, duration of diabetes, systolic, between hypogonadism of diabetic men and diastolic blood pressure, waist circumference, BMI, HbA1c, SHBG, and lipid profile.
ACKNOWLEDGMENTS
We would like to thank Dr Sharareh Navvab, Ms Atsa Norouzy, Ms Fahimeh Akbari, our research laboratory personnels, and all of the patients who accepted to take part in this study. We also thank Mr Majid Abyar for his technical help in computer affairs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 yr. Diabetes. 1987;36:523–34. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- 2.Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci. 2001;56:M5–13. doi: 10.1093/gerona/56.1.m5. [DOI] [PubMed] [Google Scholar]
- 3.Tong PC, Ho CS, Yeung VT, Ng MC, So WY, Ozaki R, et al. Association of testosterone, insulin-like growth factor-I, and C-reactive protein with metabolic syndrome in Chinese middle-aged men with a family history of type 2 diabetes. J Clin Endocrinol Metab. 2005;90:6418–23. doi: 10.1210/jc.2005-0228. [DOI] [PubMed] [Google Scholar]
- 4.Amini M, Afshin-Nia F, Bashardoost N, Aminorroaya A, Shahparian M, Kazemi M. Prevalence and risk factors of diabetes mellitus in the Isfahan city population (aged 40 or over) in 1993. Diabetes Res Clin Pract. 1997;38:185–90. doi: 10.1016/s0168-8227(97)00099-5. [DOI] [PubMed] [Google Scholar]
- 5.Andersson B, Marin P, Lissner L, Vermeulen A, Bjorntorp P. Testosterone concentrations in women and men with NIDDM. Diabetes Care. 1994;17:405–11. doi: 10.2337/diacare.17.5.405. [DOI] [PubMed] [Google Scholar]
- 6.Ando S, Rubens R, Rottiers R. Androgen plasma levels in male diabetics. J Endocrinol Invest. 1984;7:21–4. doi: 10.1007/BF03348370. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Khaw KT, Yen SS. Endogenous sex hormone levels in older adult men with diabetes mellitus. Am J Epidemiol. 1990;132:895–901. doi: 10.1093/oxfordjournals.aje.a115732. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117:807–11. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- 9.Betancourt-Albrecht M, Cunningham GR. Hypogonadism and diabetes. Int J Impot Res. 2003;15(Suppl 4):S14–20. doi: 10.1038/sj.ijir.3901031. [DOI] [PubMed] [Google Scholar]
- 10.Chang TC, Tung CC, Hsiao YL. Hormonal changes in elderly men with non-insulin-dependent diabetes mellitus and the hormonal relationships to abdominal adiposity. Gerontology. 1994;40:260–7. doi: 10.1159/000213594. [DOI] [PubMed] [Google Scholar]
- 11.Defay R, Papoz L, Barny S, Bonnot-Lours S, Caces E, Simon D The CALedonia DIAbetes Mellitus (CALDIA) Study Group. Hormonal status and NIDDM in the European and melanesian populations of new caledonia: a case-control study. Int J Obes Relat Metab Disord. 1998;22:927–34. doi: 10.1038/sj.ijo.0800697. [DOI] [PubMed] [Google Scholar]
- 12.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–8. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- 13.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–8. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–40. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 15.Nieschlag E, Behre HM, Bouchard P, Corrales JJ, Jones TH, Stalla GK, et al. Testosterone replacement therapy: current trends and future directions. Hum Reprod Update. 2004;10:409–19. doi: 10.1093/humupd/dmh035. [DOI] [PubMed] [Google Scholar]
- 16.Braunstein GD. Chapter 13. In: Greenspan F, editor. Basic and Clinical Endocrinology. New York: Lange/McGraw-Hill; 2007. pp. 470–501. [Google Scholar]
- 17.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 18.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–55. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–7. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 20.Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–42. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 22.Morales A, Lunenfeld B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. International Society for the Study of the Aging Male. Aging Male. 2002;5:74–86. [PubMed] [Google Scholar]
- 23.Talaei A, Amini M, Alikhani S, Delavari A, Mahdavi A. Waist circumference cut off in relation to hypertension in Iran. Iran J Endocrinol Metab. 2008;4:375–82. [Google Scholar]
- 24.Matsumoto AM, Bremner WJ. Serum testosterone assays-accuracy matters. J Clin Endocrinol Metab. 2004;89:520–4. doi: 10.1210/jc.2003-032175. [DOI] [PubMed] [Google Scholar]
- 25.Corrales JJ, Burgo RM, Garca-Berrocal B, Almeida M, Alberca I, González-Buitrago JM, et al. Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism. 2004;53:666–72. doi: 10.1016/j.metabol.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 27.Dandona P, Dhindsa S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96:2643–51. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–92. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]


