Abstract
Background:
This study aimed to investigate whether magnesium supplementation might affect serum magnesium, high sensitive C-reactive protein (hs-CRP), plasma fibrinogen, and interleukin 6 (IL-6) levels in healthy middle-aged overweight women. The relationships, if any, between serum magnesium and the inflammatory markers were also examined cross-sectionally in the entire participants at the beginning of the study.
Materials and Methods:
This double-blinded, placebo-controlled, randomized trial included 74 middle-aged overweight women. Participants were randomly assigned to receive either 250 mg magnesium as magnesium oxide or placebo daily for 8 weeks. Serum magnesium, hs-CRP, fibrinogen and IL-6 concentrations were measured before and after the intervention.
Results:
Serum magnesium was found to be inversely correlated with hs-CRP (rs =−0.22, P=0.05) in the entire participants at baseline. Serum hs-CRP declined significantly in both groups as compared with baseline values (median change=0.8 mg/L; PMagnesium= 0.03, PPlacebo < 0.001). Plasma fibrinogen decreased significantly, by 9%, in the magnesium group at the end of week 8 compared to baseline (P=0.001). Mean concentration of IL-6 was significantly increased in the magnesium group comparing the baseline value(P=0.001). However hs-CRP, fibrinogen and IL-6 levels at week 8 or any changes during the study were not statistically different between the two groups. Serum magnesium showed no significant changes in any groups.
Conclusions:
Serum magnesium had a significant inverse correlation with hs-CRP. In the present study, magnesium as magnesium oxide, 250 mg/day, for 8 weeks did not significantly attenuate inflammatory markers in the magnesium group as compared to the placebo.
Keywords: Magnesium, middle-aged women, overweight, IL-6, hs-CRP, systemic inflammation
INTRODUCTION
Magnesium, the second most abundant intracellular cation, plays a central role in various cellular and metabolic reactions including energy, carbohydrate and lipid metabolism, protein and nucleic acids synthesis, ionic pumps, and calcium-channel function.[1] In spite of several dietary sources including whole grains, green leafy vegetables, legumes and nuts, dietary magnesium intakes are inadequate based on current recommendation. Dietary magnesium insufficiency is more common in adolescent girls and women, and worsens with age.[2,3]
Low magnesium intake is related to type 2 diabetes,[4–6] hypertension,[7] cardiovascular disease,[8] metabolic syndrome,[9,10] and colorectal cancer.[11] Systemic inflammation is one of the common mechanisms underlying the development of these disorders, and the associations observed between magnesium intake and the chronic disease may be, in part, due to magnesium's effects on inflammation. Experimentally induced magnesium deficiency in rodent models promoted an inflammatory response, characterized with increased plasma interleukin 6 (IL-6), fibrinogen and a decrease in plasma albumin.[12,13] In humans, low serum magnesium concentrations have been associated with high C-reactive protein (CRP)levels.[14,15] Several cross-sectional studies have reported inverse relationships between magnesium intake and some inflammatory markers, including high sensitive CRP (hs-CRP) and IL-6.[3,16–19] People with magnesium intake at any level below the Recommended Dietary Allowance (RDA) had a higher likelihood of elevated CRP, implicating that the RDA of magnesium may be an important risk threshold. These associations were stronger in adults aged over 40 or those who were overweight.[3,16–19] In a prospective study, magnesium intake was also inversely related to hs-CRP, fibrinogen and IL-6.[6] These findings provide evidence that dietary magnesium has beneficial effects on inflammatory markers. Little is known about the relationship between supplemental magnesium and inflammation. Because of limited data, it is unclear whether magnesium supplementation, as a simple and safe way to assure adequate magnesium intake, has the same effect as dietary magnesium intake on inflammation.
Therefore, we conducted a randomized clinical trial to investigate the effects of magnesium supplementation on serum magnesium, hs-CRP, plasma fibrinogen, and IL-6 in apparently healthy middle-aged women with BMI≥25, a group predisposed to chronic inflammation and adequate magnesium intake may have the most favorable effect on inflammation. We also examined the associations between serum magnesium and inflammatory markers at baseline.
MATERIALS AND METHODS
Subjects
This study was carried out in Tehran, Iran, in 2010. Participants were recruited from staff at the Tehran University of Medical Sciences (TUMS) through advertisements. Participants were recruited consecutively through a convenience sampling. Volunteers could be included in the study if they were female, aged between 40 and 55 years and had a BMI≥25 kg/m2. Exclusion criteria were diabetes, hypertension, cardiovascular disease, renal failure, liver and gastrointestinal disorders, infectious disease, asthma, thyroid and parathyroid disease, smoking and taking drugs including anti-inflammatory drugs, aminoglycoside antibiotics, magnesium-containing medications, laxative or hormonal products. None of participants had been taken any dietary supplements, at least for the two weeks prior to the study.
Study design
This study was a randomized, double-blind, placebo-controlled trial. Participants were randomly assigned into two groups using a random number table. The intervention group (n=37) received a tablet containing 250 mg magnesium in the form of magnesium oxide daily, while the controls (n=37) received a placebo for 8 weeks; the placebo had an identical appearance to the magnesium supplement, and contained corn starch, lactose, and stearic acid. The participants and all investigators were blinded to the treatment allocation, except an independent statistician who provided the randomization list. They were instructed to consume their pills once a day with lunch in order to reduce the risk of diarrhea and stomach upset. Subjects were contacted at 2 week intervals to assess safety and compliance, and had been asked not to change their usual diet, physical activity and to avoid taking any other dietary supplements during the study. Dietary intakes were assessed by a 24 h food recall completed for 2 days (1 week day and 1 weekend day) at entry and after 8 weeks of supplementation. The daily nutrient intakes, including energy, total fat, Saturated Fatty Acid (SFA), Monounsaturated Fatty Acid (MUFA), Polyunsaturated Fatty Acid (PUFA), protein, carbohydrate, fiber, magnesium, selenium, zinc, Vitamins A, C, E, and B2 were determined by Nutritionist 4 software (N-squared Computing, San Bruno, CA). Physical activity levels were assessed at entry and end of the study using the short form of the International Physical Activity Questionnaire (IPAQ) and expressed as Met-Min/Week.[20] This study was approved by the ethics committee of the School of Public Health, Tehran University of Medical Sciences, and written informed consent was obtained from each subject.
Anthropometric and biochemical measurements
Height and weight were measured using standard protocols, with light clothes and without shoes, to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as body weight (kg) divided by height squared (m2). Body fat mass and fat percent were measured by the Bioelectrical Impedance Analyzer (Quad scan 4000; Bodystat-England).[21]
Blood samples were collected twice, in the morning after a 10-12 h overnight fast, both before and after the intervention. Blood was divided into 3 tubes: (1)The Clot tube (for serum Mg and hs-CRP measurements) (2)The Sodium citrate containing tube (for plasma fibrinogen measurement) and (3)The EDTA containing tube (for Plasma IL-6 measurement). All tubes were centrifuged at 3000-4000 RPM for 10 min to separate serum and plasma. Serum magnesium was measured using the spectrophotometric method by an autoanalyzer (Hitachi 912; Roche Diagnostics). Serum hs-CRP values were measured using the immunoturbidimetric method (Pars Azmun, Iran) on the Hitachi 912. Plasma fibrinogen was determined using the Clauss method and IL-6 was measured by an enzyme-linked immunosorbent assay (Bender Medsystems, Vienna, Austria). The sensitivities of the assays for hs-CRP and IL-6 were 0.1 mg/L and 0.92 pg/mL, respectively.
Statistical analysis
The number of participants estimated for each group was 31 at 80% power and α of 0.05 to detect a difference of 2 mg/L in hs-CRP concentrations between groups with an SD of 2.8 mg/L.[22] To allow for dropouts, it was decided to recruit 37 participants for each group.
The relationships between serum magnesium and each of hs-CRP, fibrinogen and IL-6 levels were examined using Spearman or Pearson correlation analysis in all the participants at the beginning of the study(n=74). The Kolmogorov-Smirnov test was applied to assess normality of data. Normally distributed data within groups were compared using paired-samples t-test and between groups by independent-samples t-test. Comparison of non-normally distributed data was conducted using Wilcoxon signed ranks and the Mann-Whitney U-test. Physical activity and hs-CRP concentrations were non-normally distributed at all time points. IL-6 in placebo group and its change in magnesium group were also non-normally distributed. Values are presented as mean±SD at any given time point when the data were normally distributed and as median (IQR; interquartile range) when the data had a non-normal distribution. Statistical analyses were performed by SPSS version 15 (SPSS Inc., Chicago, IL). A two-tailed P value≤0.05 was considered significant statistically.
RESULTS
Relationship between serum magnesium and inflammatory markers
The mean age and BMI of 74 healthy women who participated in the study were 46.3±4.2 years and BMI 28±3 kg/m2, respectively. The median hs-CRP for all participants was 2.3 mg/L. Fourteen out of 74 participants (18.9%) demonstrated high hs-CRP levels (≥3 mg/L) and 58 participants (78.4%) had a CRP level between 1 and 3 mg/L. All of participants had normomagnesemia except for one with a serum magnesium level of 1.7 mg/dl. There was a negative correlation between serum magnesium and hs-CRP (rs =−0.22, P=0.05); while neither IL-6 nor fibrinogen concentrations were correlated with serum magnesium level.
Effects of magnesium supplementation on serum magnesium and inflammatory markers
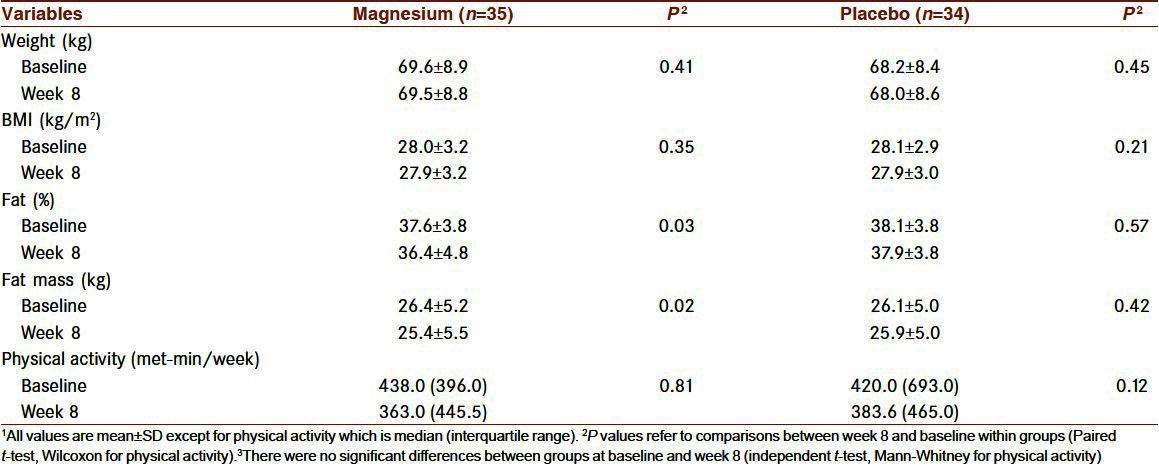
Sixty-nine participants completed the study. Five persons (3 in the placebo and 2 in the magnesium group) withdrew from the study because of personal reasons. Forty-one of 69 women (59.4%) were premenopausal. The χ2 test showed that the distributions of women based on their menstrual cycles were the same between groups. Anthropometric measurements and physical activity levels of the participants who completed the study are shown in Table 1. There were no significant differences between the two groups at baseline and at week 8. Body weight, BMI, and physical activity did not change significantly within the groups throughout the study. Body fat percent and fat mass decreased significantly only in the magnesium group as compared to baseline values (mean change of fat mass percent:−1.2±3.2%, P=0.03; mean change of fat mass:−0.9±2.4 kg, P=0.02).
Table 1.
Anthropometric and physical activity characteristics of participants before and after 8 weeks of treatment1
Dietary intakes are shown in Table 2. Dietary intakes were not different between the 2 groups before and after intervention, except for SFA intake, which was higher in the magnesium group at week 8 (P=0.04). Dietary magnesium intakes (non-supplemental) did not change during the study within each group.
Table 2.
Dietary intakes of participants before and after 8 weeks of treatment1
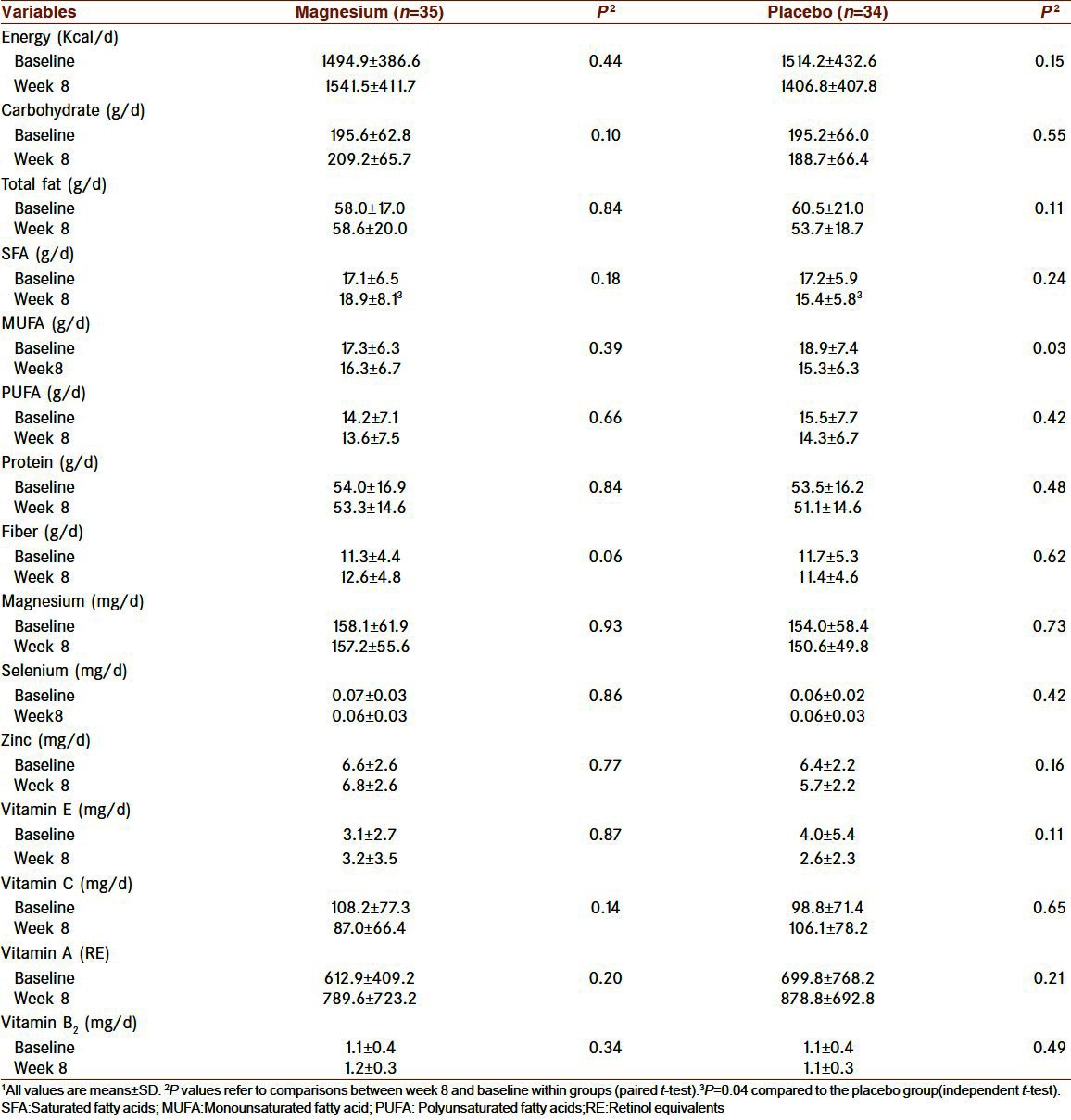
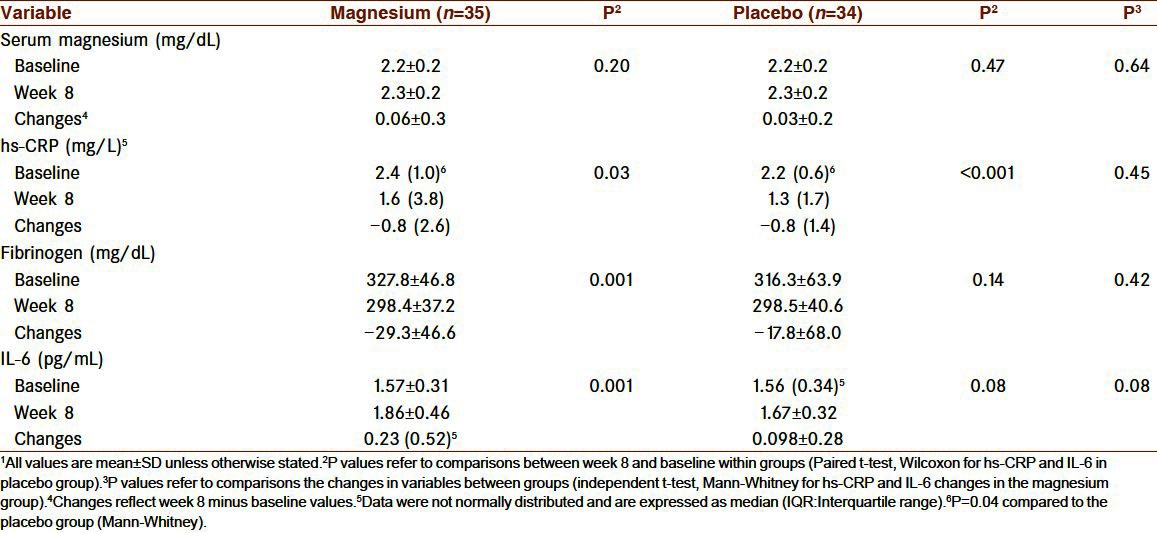
Table 3 shows effects of magnesium supplementation on serum magnesium and inflammatory markers. There were no statistically significant differences between the groups at the beginning of the study, apart from hs-CRP concentration, which was higher in the magnesium group (P=0.04). Serum magnesium did not change significantly during the study in either group, being the same at the end of the study between groups. At the end of week 8, hs-CRP decreased significantly in the magnesium (P=0.03) and placebo (P<0.001) groups compared to baseline but hs-CRP reduction was not significantly different between the groups. Plasma fibrinogen declined significantly in the magnesium group at the end of the supplementation period compared to baseline, whereas no significant change was observed in the placebo group. The reduction in fibrinogen concentration was not significant compared to placebo group. IL-6 concentrations increased in both groups slightly and significantly in magnesium group (P=0.001), however either the concentrations at the end of the study or the changes during the study were not significantly different between the 2 groups.
Table 3.
Inflammatory markers before and after 8 weeks of treatment1
Safety and compliance
Magnesium supplement was generally well tolerated; there were no serious symptoms due to magnesium oxide or placebo. Three participants reported loose stool (1 subject in the magnesium group and 2 subjects in the placebo group) that resolved after a few days without medication and did not lead to discontinuation of the study. Compliance with treatment, assessed by pill counts, was approximately 93% for both the magnesium and the placebo groups.
DISCUSSION
In the present study, serum magnesium was inversely correlated with only hs-CRP, among other inflammatory markers (rs =−0.22, P=0.05) at the beginning of the study. The association between serum magnesium and CRP has been investigated in few studies. Guerrero-Romero and Rodríguez-Morán investigated 371 non-diabetic, non-hypertensive Mexican adults and reported a negative correlation between serum magnesium and CRP(r=−0.39, P=0.002); after controlling for ages, sex, BMI, and glucose tolerance, elevated CRP was 2.11 times more likely in subjects with the lowest quintile of serum magnesium.[14] They also found a negative association between serum magnesium and hs-CRP in 488 healthy Mexican children, aged 10-13 years (r=−0.708, P<0.001) and the likelihood of elevated hs-CRP was 4.1 times higher in children with lowest serum magnesium.[15] In 4497 Americans, aged 18-30 years, Kim et al. evaluated the correlations between serum magnesium and other inflammatory markers, including fibrinogen and IL-6 in addition to hs-CRP, and they also found an inverse correlation between serum magnesium and only hs-CRP, a finding in agreement with ours.[6]
Several cross-sectional studies have reported an inverse association between dietary magnesium intake and inflammation. Song et al., for the first time, investigating 11686 women≥45 years of age, showed that CRP concentration is 12% lower in the highest dietary magnesium intake quintile.[16] In an analysis of 5773 adults, aged≥17 years, King et al. found their participants, who consumed less than the RDA recommendations for magnesium intake, were 1.48-1.75 times more likely to have elevated CRP (≥3 mg/L).[3] Chacko et al., in a 3713 multiethnic cohort of postmenopausal women, showed an inverse association between magnesium intake and IL-6, in addition to hs-CRP.[19] Recently, in a longitudinal study of 4497 Americans, aged 18-30 years, Kim et al. found negative long-term associations between magnesium intake, hs-CRP, fibrinogen, and IL-6.[6] The contributions of total magnesium intake from supplements were small in those studies, so their findings support the beneficial effects from magnesium intake through its food sources. While dietary magnesium intake is highly correlated with other dietary nutrients including fiber, potassium, and folate, it is not clear that the associations observed are due to magnesium alone or from some other nutrient or combination of nutrients. It has also not determined whether increasing magnesium intake can reduce CRP and other inflammatory markers.
Inadequate magnesium intake commonly occurs in various populations. This nutritional problem was also highly prevalent among our participants, the dietary magnesium intake of 55.4% were lower than 50% of the RDA and about 93.2% consumed less than the American Estimated Average Requirement (EAR) of 265 mg/day. King et al., in a cross-sectional study of individuals with dietary magnesium intakes ≤50 % RDA recommendations, and took magnesium supplements, found that the likelihood of elevated CRP levels were 22% lower than those not taking supplements.[23] In the present study, we have examined whether 250 mg magnesium/day, in the form of magnesium oxide taken for 8 weeks, alter inflammatory markers in apparently healthy middle-aged overweight women.
After an 8-week supplementation, mean plasma fibrinogen attenuated significantly, by 9%, in magnesium group but this reduction did not reach significant compared to placebo group. It was indicated that the risk of myocardial infraction (MI) has been increased as much as 45% by a 1 g/L rise in plasma fibrinogen. Based on this estimation, the 29.3 mg/dL decrease in fibrinogen levels observed in our study can reduce the risk of MI by 12%.[24] Serum hs-CRP reduced in both group significantly; hs-CRP concentrations at end of the study and their changes were not different between 2 groups. In contrast to hs-CRP and fibrinogen, IL-6 concentrations increased slightly in both group however no significant differences were observed between two groups in regard of IL-6 levels or their changes during the study.
In a clinical trial, Sarafian et al., found that 300 mg/day magnesium as magnesium citrate, given to heart failure patients for 5 weeks, significantly attenuated serum log CRP from 1.4±0.4 to 0.8±0.3 (P<0.001).[25] Those patients demonstrated high serum CRP and low serum magnesium levels; however it was not tested whether the reduction in log CRP was significant compared to the magnesium untreated controls. In adults older than 51 years with poor quality sleep, 320 mg magnesium/day as magnesium citrate for 7 weeks decreased plasma CRP in participants with baseline values above 3 mg/dL.[26] Individuals with high inflammatory stress or low magnesium status might respond better and faster to magnesium supplementation. In our study, the dose administered and duration of magnesium supplementation may not be adequate enough to achieve the desired outcome; because of unavailability of related information on the direct anti-inflammatory effect of magnesium supplement, we cannot explain precisely why we saw no favorable effects for magnesium compared to the placebo.
Both the amounts and type of dietary fat can modulate pro-inflammatory cytokines and markers of inflammation.[27] Dietary intakes showed that all types of dietary fats decreased in placebo group during the study in parallel with the reduction in the total amount of fat intake. At end of the study, SFA intake was significantly lower in placebo group compared to magnesium group. SFAs, especially palmitic acid, have been shown to promote inflammation indirectly by impairing the glucose and lipid metabolism.[28] An increase in IL-6 and fibrinogen followed by consuming a diet rich with stearic acid, a SFA with neutral effect on cholesterol levels, indicating that other mechanism may also be involved.[29] These findings suggest that the changes in quantity and quality of dietary fat intakes, especially SFA, may cause for decline in hs-CRP and fibrinogen concentrations in placebo group.
The mechanisms through which magnesium affects inflammation are largely unknown; increased intracellular calcium due to magnesium deficiency is one of the proposed underlying mechanisms.[30] In our study, percentage of fat declined significantly from 37.6±3.8 to 36.4±4.8% and fat mass from 26.4±5.2 to 25.4±5.5 kg in the magnesium group. No significant changes in physical activity levels and dietary intake during the study, raising the hypothesis that could magnesium supplementation has affected body composition? These modest changes in body composition may be important because of the relationship between fat mass and inflammatory markers which have been observed in both obese and non-obese individuals.[31,32] It is now recognized that adipose tissue secrete many inflammatory cytokines and chemokines,[30] and blood inflammatory markers are correlated with the degree of adiposity.[33,34] Thus, it is possible that magnesium can reduce inflammatory markers by changing body fat mass along with the other proposed mechanisms. Limited cross-sectional studies have reported an inverse or no association between magnesium and adiposity as measured by BMI, waist circumference or percentage of body fat.[35–38] Our finding provides direct evidence supporting the link between magnesium intake and body composition. Further research is needed to investigate the associations between magnesium and body composition.
In our study, 250 mg/day magnesium, administered as magnesium oxide for 8 weeks had no effect on serum magnesium in the magnesium group. However all previous studies do not report magnesium supplementation to cause consistent increases in serum magnesium levels; in some studies, it did increase, following magnesium supplementation,[25,39,40] while in others not,[41,42] or even showed a decrease.[43] Vormann suggested that in adaptations to a wide variety of magnesium intakes, the total intake and excretion of magnesium remains in balance.[44] Lukaski and Nielsen have indicated that serum magnesium concentration has slight responses to changes in magnesium intake.[45] In addition, Song et al.[46] and Dimai et al.[41] have shown that serum magnesium fluctuated in a cyclic manner in response to magnesium supplementation, indicating that there is a time course of response to magnesium supplementation and that serum magnesium and supplemental magnesium intake do not have a linear trend. Therefore, it is clear that serum magnesium cannot be a sensitive indicator for magnesium intake despite frequent usage. In response to magnesium supplementation, the magnesium status of the participants at entry of the study may be also important. In certain cases, in whom magnesium supplementation increased serum magnesium, the participants were hypomagnesemic.[39,40] In our study, serum magnesium levels of participants were within normal range (1.8-2.6 mg/dL) at baseline.
To our knowledge, this is the first study that has evaluated the effects of magnesium supplementation on several markers of inflammation. By excluding subjects with known factors affecting inflammation, we tried to examine the effect of magnesium on chronic subclinical inflammation, an important risk factor for age-related diseases including type 2 diabetes, hypertension, and cardiovascular disease. We also investigated the effects of magnesium in middle-aged overweight women who are prone to chronic inflammation and age related diseases, a group in whom the associations between magnesium intake and inflammatory markers have been stronger and more pronounced.[3,16,19]
We had some limitations in this study. First, the duration of the study was short and the dose of magnesium administered was rather modest. Second, we did not determine the effect of magnesium supplementation on serum ionized magnesium or intracellular magnesium. Third, we determined treatment compliance by performing pill count, whereas urinary magnesium concentration was not measured to confirm it. Fourth, the kit applied to measure plasma IL-6 was not sensitive enough to indicate possible changes in its concentration in healthy subjects.
In summary, 250 mg/day magnesium administered for 8 weeks did not influence serum total magnesium, hs-CRP, fibrinogen, and IL-6, compared to placebo. Further clinical trials are required to examine the effectiveness of magnesium supplementation on inflammation. Some questions that remain to be answered are can magnesium supplementation consider as another way to reduce inflammation, which doses and for what durations would magnesium supplementation be effective and can magnesium intake affect body composition? Meanwhile, promoting intakes of magnesium near the RDA, especially through food sources, is prudent in order to reduce chronic diseases risk and improve health.
ACKNOWLEDGMENTS
We would like to express our appreciation to the staff of Tehran University of Medical Sciences for their cooperation and participation in this study. This work was financially supported by Tehran University of Medical sciences. This trial was registered at the Iranian Registry of Clinical Trials (IRCT) (ID No.IRCT138805312365N1).
Footnotes
Source of Support: Tehran University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Gallagher M. The nutrients and their metabolism. In: Mahan LK, Escott-Stump S, editors. Krause's food & nutrition therapy. 12th ed. Philadelphia: Saunders; 2008. pp. 110–3. [Google Scholar]
- 2.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–82. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 3.King DE, Mainous AG, Geesey ME, Woolson RF. Dietary magnesium and C-reactive protein levels. J Am Coll Nutr. 2005;24:166–71. doi: 10.1080/07315724.2005.10719461. [DOI] [PubMed] [Google Scholar]
- 4.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med. 2007;167:956–65. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 5.Villegas R, Gao YT, Dai Q, Yang G, Cai H, Li H, et al. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89:1059–67. doi: 10.3945/ajcn.2008.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DJ, Xun P, Liu K, Loria C, Yokota K, Jacobs DR, Jr, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33:2604–10. doi: 10.2337/dc10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Y, Sesso HD, Manson JE, Cook NR, Buring JE, Liu S. Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am J Cardiol. 2006;98:1616–21. doi: 10.1016/j.amjcard.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Al-Delaimy WK, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Magnesium intake and risk of coronary heart disease among men. J Am Coll Nutr. 2004;23:63–70. doi: 10.1080/07315724.2004.10719344. [DOI] [PubMed] [Google Scholar]
- 9.He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113:1675–82. doi: 10.1161/CIRCULATIONAHA.105.588327. [DOI] [PubMed] [Google Scholar]
- 10.Puchau B, Zulet MA, de Echávarri AG, Hermsdorff HH, Martinez JA. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults. Nutrition. 2010;26:534–41. doi: 10.1016/j.nut.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Bergkvist L, Wolk A. Magnesium intake in relation to risk of colorectal cancer in women. JAMA. 2005;293:86–9. doi: 10.1001/jama.293.1.86. [DOI] [PubMed] [Google Scholar]
- 12.Malpuech-Brugère C, Nowacki W, Daveau M, Gueux E, Linard C, Rock E, et al. Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta. 2000;1501:91–8. doi: 10.1016/s0925-4439(00)00018-1. [DOI] [PubMed] [Google Scholar]
- 13.Blache D, Devaux S, Joubert O, Loreau N, Schneider M, Durand P, et al. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic Biol Med. 2006;41:277–84. doi: 10.1016/j.freeradbiomed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero-Romero F, Rodríguez-Morán M. Relationship between serum magnesium levels and C-reactive protein concentration, in non-diabetic, non-hypertensive obese subjects. Int J Obes Relat Metab Disord. 2002;26:469–74. doi: 10.1038/sj.ijo.0801954. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Morán M, Guerrero-Romero F. Serum magnesium and C-reactive protein levels. Arch Dis Child. 2008;93:676–80. doi: 10.1136/adc.2006.109371. [DOI] [PubMed] [Google Scholar]
- 16.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:1438–44. doi: 10.2337/diacare.28.6.1438. [DOI] [PubMed] [Google Scholar]
- 17.Bo S, Durazzo M, Guidi S, Carello M, Sacerdote C, Silli B, et al. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clin Nutr. 2006;84:1062–9. doi: 10.1093/ajcn/84.5.1062. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr. 2007;85:1068–74. doi: 10.1093/ajcn/85.4.1068. [DOI] [PubMed] [Google Scholar]
- 19.Chacko SA, Song Y, Nathan L, Tinker L, de Boer IH, Tylavsky F, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care. 2010;33(2):304–10. doi: 10.2337/dc09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 21.Shalileh M, Shidfar F, Haghani H, Eghtesadi S, Heydari I. The influence of calcium supplement on body composition, weight loss and insulin resistance in obese adults receiving low calorie diet. J Res Med Sci. 2010;15:191–201. [PMC free article] [PubMed] [Google Scholar]
- 22.Brighenti F, Valtueña S, Pellegrini N, Ardigò D, Del Rio D, Salvatore S, et al. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr. 2005;93:619–25. doi: 10.1079/bjn20051400. [DOI] [PubMed] [Google Scholar]
- 23.King DE, Mainous AG, Geesey ME, Egan BM, Rehman S. Magnesium supplement intake and C-reactive protein levels in adults. Nutrition Research. 2006;26:193–6. [Google Scholar]
- 24.van der Bom JG, de Maat MP, Bots ML, Haverkate F, de Jong PT, Hofman A, et al. Elevated plasma fibrinogen: cause or consequence of cardiovascular disease? Arterioscler Thromb Vasc Biol. 1998;18:621–5. doi: 10.1161/01.atv.18.4.621. [DOI] [PubMed] [Google Scholar]
- 25.Almoznino-Sarafian D, Berman S, Mor A, Shteinshnaider M, Gorelik O, Tzur I, et al. Magnesium and C-reactive protein in heart failure: an anti-inflammatory effect of magnesium administration? Eur J Nutr. 2007;46:230–7. doi: 10.1007/s00394-007-0655-x. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen FH, Johnson LK, Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res. 2010;23:158–68. doi: 10.1684/mrh.2010.0220. [DOI] [PubMed] [Google Scholar]
- 27.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009;55:123–39. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- 28.Aeberli I, Molinari L, Spinas G, Lehmann R, l’Allemand D, Zimmermann MB. Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am J Clin Nutr. 2006;84:748–55. doi: 10.1093/ajcn/84.4.748. [DOI] [PubMed] [Google Scholar]
- 29.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–73. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen FH. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev. 2010;68:333–40. doi: 10.1111/j.1753-4887.2010.00293.x. [DOI] [PubMed] [Google Scholar]
- 31.Bo M, Raspo S, Morra F, Cassader M, Isaia G, Poli L. Body fat is the main predictor of fibrinogen levels in healthy non-obese men. Metabolism. 2004;53:984–8. doi: 10.1016/j.metabol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Neyestani TR, Salekzamani S, Kalayi A, Alavi-Majd H, Houshiarrad A, Nikooyeh B, et al. Predictors of serum levels of high sensitivity C-reactive protein and systolic blood pressure in overweight and obese nondiabetic women in Tehran: a cross-sectional study. Metab Syndr Relat Disord. 2011;9:41–7. doi: 10.1089/met.2010.0075. [DOI] [PubMed] [Google Scholar]
- 33.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–77. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 34.Garanty-Bogacka B, Syrenicz M, Goral J, Krupa B, Syrenicz J, Walczak M, et al. Changes in inflammatory biomarkers after successful lifestyle intervention in obese children. Endokrynol Pol. 2011;62:499–505. [PubMed] [Google Scholar]
- 35.Lima Mde L, Cruz T, Rodrigues LE, Bomfim O, Melo J, Correia R, et al. Serum and intracellular magnesium deficiency in patients with metabolic syndrome--evidences for its relation to insulin resistance. Diabetes Res Clin Pract. 2009;83:257–62. doi: 10.1016/j.diabres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Song CH, Choi WS, Oh HJ, Kim KS. Associations of serum minerals with body mass index in adult women. Eur J Clin Nutr. 2007;61:682–5. doi: 10.1038/sj.ejcn.1602568. [DOI] [PubMed] [Google Scholar]
- 37.Evangelopoulos AA, Vallianou NG, Panagiotakos DB, Georgiou A, Zacharias GA, Alevra AN, et al. An inverse relationship between cumulating components of the metabolic syndrome and serum magnesium levels. Nutr Res. 2008;28:659–63. doi: 10.1016/j.nutres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Abbasalizad Farhanghi M, Mahbob S, Ghaem Magami S, Safayian A, Vahed Jabbari M, Ostadrahimi A. Serum magnesium concentration and its relationship with body composition in obese and non obese reproductive age women. Iranian J Endocrinology and Metabolism. 2008;10:169–75. [Google Scholar]
- 39.Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care. 2003;26:1147–52. doi: 10.2337/diacare.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 40.Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-Martínez AM, Montes-Villarreal J, Treviño-Ortiz JH, et al. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab. 2004;30:253–8. doi: 10.1016/s1262-3636(07)70116-7. [DOI] [PubMed] [Google Scholar]
- 41.Dimai HP, Porta S, Wirnsberger G, Lindschinger M, Pamperl I, Dobnig H, et al. Daily oral magnesium supplementation suppresses bone turnover in young adult males. J Clin Endocrinol Metab. 1998;83:2742–8. doi: 10.1210/jcem.83.8.5015. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter TO, DeLucia MC, Zhang JH, Bejnerowicz G, Tartamella L, Dziura J, et al. A randomized controlled study of effects of dietary magnesium oxide supplementation on bone mineral content in healthy girls. J Clin Endocrinol Metab. 2006;91:4866–72. doi: 10.1210/jc.2006-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S, Park HK, Son SP, Lee CW, Kim IJ, Kim HJ. Effects of oral magnesium supplementation on insulin sensitivity and blood pressure in normo-magnesemic nondiabetic overweight Korean adults. Nutr Metab Cardiovasc Dis. 2009;19:781–8. doi: 10.1016/j.numecd.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Vormann J. Magnesium: nutrition and metabolism. Mol Aspects Med. 2003;24:27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 45.Lukaski HC, Nielsen FH. Dietary magnesium depletion affects metabolic responses during submaximal exercise in postmenopausal women. J Nutr. 2002;132:930–5. doi: 10.1093/jn/132.5.930. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050–6. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]


