Abstract
Background:
The aim of this study was to test the potential properties of metformin (MF) to protect the kidney from gentamicin (GM)-induced renal toxicity.
Materials and Methods:
In this preclinical study, 50 male Wistar rats were randomly divided into five groups of 10 rats in each. In the first group (group I), they were kept in the same condition as others without receiving drugs for 10 days. In group II, the rats were injected intraperitoneally with 100 mg/kg/day of GM for 10 consecutive days. Group III rats received 100 mg/kg/day MF orally for 10 days. In group IV, the rats received GM (100 mg/kg; intraperitoneally) for 10 days and 100 mg/kg/day MF orally for the next 10 days. In the last group (group V), the rats received a combination of GM 100 mg/kg/day intraperitoneally and MF 100 mg/kg/day orally for 10 days simultaneously. Serum blood urea nitrogen (BUN) and creatinine (Cr) values were measured and renal tissues of the animals were processed for light microscope examination.
Results:
The levels of BUN in groups II, IV, and V, and also the serum level of Cr in groups II and V were increased significantly after the experiment. Furthermore, post-treatment with MF or co-treatment with MF could prevent the elevation of serum BUN and Cr induced by GM and also attenuates the damage score (P < 0.05).
Conclusions:
MF may prevent or ameliorate GM-induced acute renal failure, and therefore it might be beneficial in patients under treatment with this medicine.
Keywords: Gentamicin, metformin, nephroprotection, tubular toxicity
INTRODUCTION
Metformin (MF) is used for the treatment of diabetes mellitus. It is a biguanide developed from galegine, a guanidine derivative. Chemically, it is a hydrophilic base which exists at physiological pH as the cationic species.[1,2] This drug was observed to reduce hypergly-cemia, improve glucose utilization, reduce free fatty acid utilization, gluconeogenesis, serum lipids, insulin, insulin-like growth factor 1 (IGF-1), reduce body weight, and decrease metabolic immunodepression both in humans and rodents.[1–4] Various studies revealed that MF is more than a simple antidiabetic agent. Indeed, for an equivalent effect on glycemic control after a long time of treatment, MF was found to be obviously superior to other therapeutic measures for reducing vessel diseases and all-cause related mortality.[4] It was suggested that insulin and MF treatments, y improving glycoxidative, inflammatory, and fibrotic renal damage markers, play a key role in the prevention of diabetic nephropathy.[5,6] The role of mitochondria in programmed cell death is associated with the release of apoptotic signaling molecules. The production of reactive oxygen species (ROS) by mitochondria also contributes to cell degradation process.[6] Previous investigators pointed to mitochondrial effects of MF.[7] Mitochondria are believed to have a role in a various renal diseases, and mitochondrial dysfunction could have a major role in nephrotoxicity.[8] Gentamicin (GM) is probably the most the commonly used and studied of all the aminoglycosides.[9,10] The limitation to the use of this antibiotic is its tubular toxicity.[8,9] GM inhibits oxidative phosphorylation and reduces ATP levels in renal tubular cells.[11] Hence, GM-enhanced ROS formation in isolated cortical mitochondria[11–17] and ROS-induced cell death were found to have a role in GM-mediated acute renal failure.[8] Apart from the superiority of MF to other antidiabetic drugs,[15,16] various investigations strongly suggest that this antidiabetic agent prevented oxidative stress-induced death in several cell types through a mechanism dependent on the mitochondrial permeability transition pore (PTP) opening.[6,8,13,17–19] Thus, MF may afford protection against GM-induced tubular injury by affecting the mitochondria through a mechanism dependent on the mitochondrial PTP opening.[6,20] However, less data are present regarding the renoprotective effects of MF, and previous studies on attenuation of GM tubular toxicity by MF need to be re-tested. Therefore, we aimed to test the potential properties of MF to protect the kidney from GM-induced acute renal failure. Also, finding whether delayed treatment with MF exerts similar benefits on GM-induced renal toxicity in rats was the second aim of this study.
MATERIALS AND METHODS
Drugs and chemicals
MF (Metformin Hexal; Germany) was supplied as a white powder, soluble in distilled water, and freshly prepared as an aqueous solution to be given as a single daily oral dose of 100 mg/kg/day.[8] GM treatment protocols used in the present study have been reported earlier.[21]
Animals
In a preclinical study, 50 male Wistar rats with a weight range of 200–250 g, purchased from Jundishapur University of Medical Sciences, Ahvaz, Iran, were transferred to the animal house of Shahrekord University of Medical Sciences, Shahrekord, Iran. Animals were housed at an environment of controlled temperature (25 ± 3°C) and humidity (50–60%) with a 12-hour dark–light cycle (lights on at 7 AM) and free access to pelleted diet and tap water for 2 weeks to acclimatize to the new environment. The animal experimentation was conducted in accordance with the National Institute of Health guide for the careful use of laboratory animals.[22] Their general health state and activity were monitored closely during the experiment.
Experimental design
The animals were divided into five groups (10 rats each) as follows:
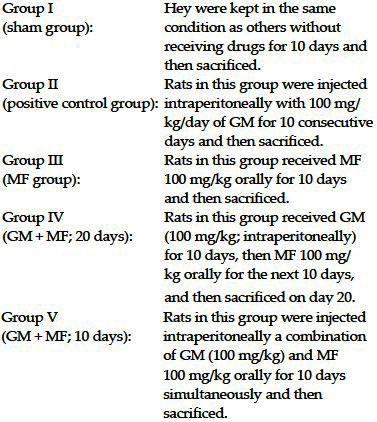
On the first day (before experiment) and the final day (day of sacrificing), serum samples were obtained to measure blood urea nitrogen (BUN) and serum creatinine (Cr) for all the rats. Rats were sacrificed (i.p.) under general anesthesia with ketamine. The kidneys were removed for histologic examinations.
Determination of serum BUN and Cr level
BUN and Cr levels were measured by a colorimetric method using commercial kits on an autoanalyzer.
Histopathologic evaluations
The kidneys of each animal were dissected out, then fixed in buffered formalin for 12 h and processed for histopathologic examination. Three-micrometer-thick paraffin sections were stained with hematoxylin and eosin (H and E) for light microscope examination using conventional protocol. Histopathologic studies were performed under a light microscope. Slides were coded and examined by a histopathologist who was blinded to the treatment groups. All specimens were examined for six morphologic parameters including epithelial cell vacuolization, degeneration, tubular cell flattening, hyaline cast, tubular dilatation, and debris materials in tubular lumen on a semi-quantitative score from 1 to 5, while the score of zero was assigned to the normal tissue without damage.[9,23,24]
Statistical analyses
Data were expressed as mean ± SEM. The t-paired test was used to compare the serum BUN and Cr levels before and after the experiments. One-way analysis of variance (ANOVA) was applied to compare the serum BUN and Cr levels between the groups. To compare the pathology damage score between the groups, Kruskal–Wallis and Mann–Whitney U-test were applied. P values <0.05 were considered statistically significant.
RESULTS
The effects of MF on BUN and Cr levels
The data for serum levels of BUN and Cr are shown in Figure 1. No significant differences were observed before the experiment. The levels of BUN in groups II, IV, and V, and the serum levels of Cr in groups II and V were increased significantly after the experiment (P < 0.05).
Figure 1.
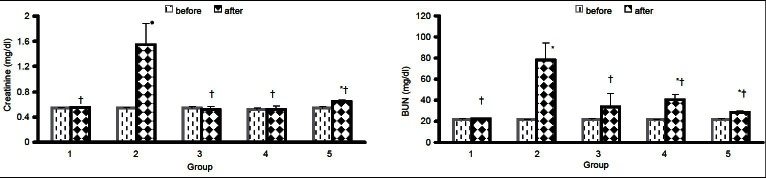
The serum levels of BUN and Cr before and after the experiment in five groups of animals. Group I, sham group; group II, positive control group treated with gentamicin; group III, treated with metformin; group IV, treated with gentamicin for 10 days and post-treatment with metformin for the next 10 days; and group V, co-administration of metformin and gentamicin for 10 days. The symbols (*) and (†) stand for significant difference before the experiment (P < 0.05) and significant difference from positive control group (P < 0.05), respectively
The increase in serum BUN and Cr levels was highly significant in group 2 versus groups 4 and 5, therefore MF has a protective effect.
The effects of MF on damage score
The pathology damage score indicated a higher score for all GM treated groups, which was significantly different from non-GM treated groups (P < 0.05). However, post-administration of MF after 10 days of GM treatment (group IV) and co-administration of MF and GM for 10 days attenuated the damage score significantly (P < 0.05), when compared with group II. As it is demonstrated in Figure 2, co-administration of GM and MF for 10 days or post-treatment with MF after 10 days of treatment with GM could reduce the damage induced by GM.
Figure 2.
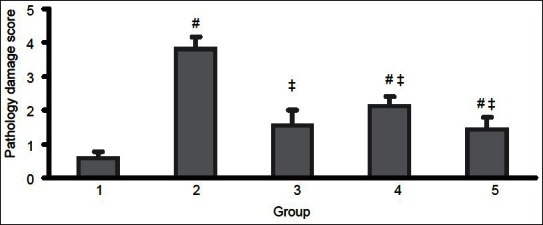
The pathology damage score in five groups of animals. Group I, sham group; group II, positive control group treated with gentamicin; group III, treated with metformin; group IV, treated with gentamicin for 10 days and post-treatment with metformin for the next 10 days; and group V, co-administration of metformin; and gentamicin for 10 days. The symbols (#) and (‡) stand for significant difference from group I (P < 0.05) and group II (P < 0.05), respectively
DISCUSSION
In this study, we found that MF could ameliorate GM-induced kidney tissue toxicity. We also found that MF was still effective when the drug was administered after progression of tubular damage by GM. MF is used for the treatment of diabetes as a sugar-lowering agent.[15,16] In addition, MF is recommended as the drug of first choice in type 2 diabetes.[16–19] There are potential survival benefits associated with the use of MF, in addition to the benefits in respect to cardiovascular outcomes and metabolic parameters as suggested by recent studies.[2–5] MF exerts its metabolic activity through the induction of the adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway which acts as a sensor detecting variations of intracellular energy levels.[19]
It was suggested that hypoxia-induced hypoxia-inducible factor (HIF)-1α accumulation in diabetic nephropathy could be suppressed by GM through the repression of oxygen consumption.[9,23–27] HIF-1α plays an important role in chronic hypoxia and tubulointerstitial fibrosis, which are presently considered to be the common pathways for various progressive kidney diseases, including diabetic nephropathy.[9,23–27] Alterations in epithelial cell polarity and in the subcellular distributions of epithelial ion transport proteins are key molecular consequences of acute kidney injury and intracellular energy depletion.[28] AMPK, a cellular energy sensor, is rapidly activated in response to renal ischemia, and AMPK activity may influence the maintenance or recovery of epithelial cell organization in mammalian renal epithelial cells subjected to energy depletion.[28,29] At a molecular level, energy deprivation causes key energy-dependent membrane proteins to become displaced and dysfunctional.[13,29,30] Specially, in the proximal tubule, the Na–K-ATPase is internalized from the basolateral membrane, disrupting the cell's capacity to maintain normal transepithelial sodium transport.[13,28,29] Preservation of a polarized plasma membrane distribution of Na–K-ATPase in renal epithelia is essential for the maintenance of both solute reabsorption and volume homeostasis. It was shown that Na–K-ATPase becomes mislocalized after energy deprivation.[7,8,15] ATP depletion also perturbs the distribution of tight junction proteins, further disrupting epithelial cell polarity and organization[7] and leading to back leak of extracellular fluid into the urinary space. Such molecular insults result in accumulation of potentially harmful toxins.[8] MF activates AMPK in rat kidney lysates.[25,26] MF treatment increases detectable p-AMPK in a dose-dependent manner, and MF-induced AMPK activation occurs in proximal tubules as well as in distal segments.[28,29] Mitochondria represents one of the major cellular sources of ROS generation[7,15] and mitochondrial toxicity can also be mediated by ROS. ROS are normally produced at low levels by mitochondria themselves. But under pathological conditions, the intracellular and intramitochondrial ROS content may be amplified.[7,15] GM as a mitochondrial toxin can exert various morphological damages to the kidney. Indeed, after GM treatment, intracellular ROS content can reach a toxic level, thus causing cell death and malfunctioning of the organ.[11,30–33] Literature review shows that the renal protective effects of MF in diabetic nephropathy have not been fully evaluated. Meanwhile, in accordance with our results, prevention of histologic changes due to GM toxicity by MF was shown by Morales et al.[8] They found that control and MF-treated rats showed no structural alterations in renal tissues, while massive and diffuse cell necrosis was observed in the proximal tubules of kidneys from rats injected with GM.[8] They could show that tubular lumen was frequently filled with hyaline casts or heterogeneous cellular debris, while in rats treated with GM and MF, most of the proximal tubules were saved and manifestations of necrosis were observed in less than 10% of cells.[8] Similar results were obtained in our study also.
CONCLUSION
Based on the above in vivo results, it was concluded that MF ameliorates GM-induced acute renal failure. Also, the present study shows that delayed treatment of MF for GM-induced acute renal failure was still effective as when MF was given in combination with the GM. Hence, MF is a nephroprotective drug to prevent or attenuate the tubular damage caused by GM or other nephrotoxic agents which act through the same mechanisms as this aminoglycoside. However, these studies are promising and warrant a more comprehensive trial.
ACKNOWLEDGMENT
This study was supported by a grant from Research Deputy and Medical Plants Research Center of Shahrekord University of Medical Sciences (Grant #994).
Footnotes
Source of Support: None
Conflict of Interest: None declared
REFERENCES
- 1.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Assadi F. The epidemic of pediatric chronic kidney disease the danger of skepticism. J Nephropathology. 2012;1:61–4. doi: 10.5812/nephropathol.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nye HJ, Herrington WG. Metformin: The safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract. 2011;118:c380–3. doi: 10.1159/000323739. [DOI] [PubMed] [Google Scholar]
- 4.Gheissari A, Mehrasa P, Merrikhi A, Madihi Y. Acute kidney injury: A pediatric experience over 10 years at a tertiary care center. J Nephropathol. 2012;1:101–8. doi: 10.5812/nephropathol.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 6.Louro TM, Matafome PN, Nunes EC, da Cunha FX, Seiça RM. Insulin and metformin may prevent renal injury in young type 2 diabetic Goto-Kakizaki rats. Eur J Pharmacol. 2011;653:89–94. doi: 10.1016/j.ejphar.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Zorov DB. Amelioration of aminoglycoside nephrotoxicity requires protection of renal mitochondria. Kidney Int. 2010;77:841–3. doi: 10.1038/ki.2010.20. [DOI] [PubMed] [Google Scholar]
- 8.Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arévalo M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–9. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 9.Rafieian-Kopaei M, Nasri H, Nematbakhsh M, Baradaran A, Gheissari A, Rouhi H, et al. Erythropoietin ameliorates genetamycin-induced renal toxicity: A biochemical and histopathological study. J Nephropathol. 2012;1:109–16. doi: 10.5812/nephropathol.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 11.Kadkhodaee M. Erythropoietin; bright future and new hopes for an old drug. J Nephropathol. 2012;1:81–2. doi: 10.5812/nephropathol.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons CF, Jr, Bogusky RT, Humes HD. Inhibitory effects of gentamicin on renal mitochondrial oxidative phosphorylation. J Pharmacol Exp Ther. 1980;214:709–15. [PubMed] [Google Scholar]
- 13.Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, et al. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003;52:2570–7. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 14.Tayebi Khosroshahi H. Short history about renal transplantation program in Iran and the world: Special focus on world kidney day 2012. J Nephropathol. 2012;1:5–10. doi: 10.5812/jnp.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, et al. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta. 2011;1812:77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 17.El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Curr Diabetes Rev. 2011;7:392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 18.Ghamari Z. Nephro and neurotoxicity, mechanisms of rejection: A review on Tacrolimus and Cyclosporin in organ transplantation. J Nephropathol. 2012;1:23–30. doi: 10.5812/jnp.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 20.Tavafi M. Inhibition of gentamicin – induced renal tubular cell necrosis. J Nephropathol. 2012;1:83–6. doi: 10.5812/nephropathol.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derakhshanfar A, Bidadkosh A, Hashempour Sadeghian M. L-methionine attenuates gentamicin nephrotoxicity in male Wistar rat: Pathological and biochemical findings. Iran J Vet Res. 2009;10:323–8. [Google Scholar]
- 22.Jabbari M, Rostami Z, Jenabi A, Bahrami A, Mooraki A. Simvastatin ameliorates gentamicin-induced renal injury in rats. Saudi J Kidney Dis Transpl. 2011;22:1181–6. [PubMed] [Google Scholar]
- 23.Nematbakhsh M, Ashrafi F, Safari T, Talebi A, Nasri H, Mortazavi M, et al. Administration of vitamin E and losartan as prophylaxes in cisplatin-induced nephrotoxicity model in rats. J Nephrol. 2012;25:410–7. doi: 10.5301/jn.5000018. [DOI] [PubMed] [Google Scholar]
- 24.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–96. [PMC free article] [PubMed] [Google Scholar]
- 25.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011;60:981–92. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyauchi K, Takiyama Y, Honjyo J, Tateno M, Haneda M. Upregulated IL-18 expression in type 2 diabetic subjects with nephropathy: TGF-beta1 enhanced IL-18 expression in human renal proximal tubular epithelial cells. Diabetes Res Clin Pract. 2009;83:190–9. doi: 10.1016/j.diabres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Khajehdehi P. Turmeric: Reemerging of a neglected Asian traditional remedy. J Nephrol Pathol. 2012;1:17–22. doi: 10.5812/jnp.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, Kashgarian M, Caplan MJ. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol. 2011;301:F1346–57. doi: 10.1152/ajprenal.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takiar V, Nishio S, Seo-Mayer P, King JD, Jr, Li H, Zhang L, et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA. 2011;108:2462–7. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hariprasad G, Kumar M, Rani K, Kaur P, Srinivasan A. Aminoglycoside induced nephrotoxicity: molecular modeling studies of calreticulin-gentamicin complex. J Mol Model. 2012;18:2645–52. doi: 10.1007/s00894-011-1289-8. [DOI] [PubMed] [Google Scholar]
- 31.Morales AI, Rodríguez-Barbero A, Vicente-Sánchez C, Mayoral P, López-Novoa JM, Pérez-Barriocanal F. Resveratrol inhibits gentamicin-induced mesangial cell contraction. Life Sci. 2006;78:2373–7. doi: 10.1016/j.lfs.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 32.Kopple JD, Ding H, Letoha A, Ivanyi B, Qing DP, Dux L, et al. L-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol Dial Transplant. 2002;17:2122–31. doi: 10.1093/ndt/17.12.2122. [DOI] [PubMed] [Google Scholar]
- 33.Juan SH, Chen CH, Hsu YH, Hou CC, Chen TH, Lin H, et al. Tetramethylpyrazine protects rat renal tubular cell apoptosis induced by gentamicin. Nephrol Dial Transplant. 2007;22:732–9. doi: 10.1093/ndt/gfl699. [DOI] [PubMed] [Google Scholar]


