Abstract
Background:
To determine whether the phosphodiesterase type 5 inhibitor, Sildenafil citrate, affects uteroplacental perfusion.
Materials and Methods:
Based on a randomized double-blinded and placebo-controlled trial, forty one pregnant women with documented intrauterine growth retardation at 24-37 weeks of gestation were evaluated for the effect of a single dose of Sildenafil citrate on uteroplacental circulation as determined by Doppler ultrasound study of the umbilical and middle cerebral arteries. Statistical analysis included χ2-test to compare proportions, and independent-samples t-test and paired student's t-test to compare continuous variables.
Results:
Sildenafil group fetuses demonstrated a significant decrease in systolic/diastolic ratios (0.60 [SD 0.40] [95% Cl 0.37-0.84], P=0.000), and pulsatility index (0.12 [SD 0.15] [95% Cl 0.02-0.22], P=0.019) for the umbilical artery and a significant increase in middle cerebral artery pulsatility index (MCA PI) (0.51 [SD 0.60] [95% Cl 0.16-0.85], P=0.008).
Conclusion:
Doppler velocimetry index values reflect decreased placental bed vascular resistance after Sildenafil. Sildenafil citrate can improve fetoplacental perfusion in pregnancies complicated by intrauterine growth restriction. It could be a potential therapeutic strategy to improve uteroplacental blood flow in pregnancies with fetal growth restriction (FGR).
Keywords: Cerebroplacental ratio, Doppler flow velocimetry, fetal growth restriction, pulsatility index, resistance index, systolic/diastolic ratio
INTRODUCTION
Excluding fetal anomalies, after prematurity, fetal growth restriction (FGR) is the second common cause of perinatal morbidity.[1] It has been long recognized that in preeclampsia, a severe maternal endothelial dysfunction must be identical placental pathology to that underlying fetal growth restriction.[2] Vascular endothelial activation is also present in pregnancies with fetal growth restriction without preeclampsia.[3] Pregnancies with fetal growth restriction are associated with elevated peripheral resistance in the maternal arterial system as seen in pregnancies with preeclampsia.[4] A poor perinatal outcome is expected in pregnancies with high vascular resistance in uterine circulation, but the pregnancies in which the resistance values are normalized in the later trimesters have a significantly better outcome.[5]
In a normal pregnancy, the trophoblast produces nitric oxide (NO) which plays an important role in vasodilatation in the fetoplacental circulation to improve oxygen and nutritional supply to the fetus.[6,7] Nitric oxide relaxes arterial and venous smooth muscle potently and might inhibit platelets aggregation and adhesion. Nitric oxide donors, as vasodilating agents, must be the possible therapeutic approach for embryo development and fetus growth. The umbilical vein endothelial cells in FGR do not respond to chronic hypoxia, which may lead to fetoplacental vasoconstriction.[8] As a locally potent vasodilator, nitric oxide helps regulate perfusion by counter balancing the effects of other vasoactive agents.[9]
Moreover, increased circulating phosphodiesterase (PDE) activity is suspected in women with preeclampsia.[10] In pregnancies with fetal growth restriction and without preeclampsia, a reversible increased myometrial arterial tone by phosphodiesterase inhibition has been reported in vitro.[11]
Sildenafil citrate is a selective inhibitor of cyclic guanosine mono phosphate (cGMP)–specific phosphodiesterase (PDE)-5 and enhances the relaxation and cyclic guanosine mono phosphate (cGMP) accumulation elicited by exogenous and neural-released nitric oxide in corpus cavernosum.[12] Sildenafil citrate increases uterine blood flow and potentiates estrogen-induced vasodilation.[13] Intravaginal administration of Sildenafil in the success of in vitro fertilization describes no deleterious effects on mother and fetus.[14] The Natural Killer Cells activity and endometrial thickness were significantly changed after vaginal Sildenafil therapy so it might be an interesting therapeutic option before conception in women with recurrent reproductive failure.[15] A study showed that intravenous Sildenafil in newborn piglets reversed increased pulmonary vascular resistance induced by meconium aspiration syndrome.[16]
In an animal study on guinea pigs, low doses of Sildenafil favored fetal tolerability to induced intrapartum asphyxia and high doses of Sildenafil increased the fetal weight by 1.5 times.[17] Improved growth of a fetus with growth restriction whose mother is treated with Sildenafil for pulmonary hypertension is noted.[18] Incubation with Sildenafil citrate limits the effects of vasoconstrictors on myometrial small arteries of normal pregnancy women and pregnant women with fetal growth restriction.[19] So that phosphodiesterase inhibitors seem to improve uterine perfusion safely in pregnancies with fetal growth restriction. We intended to administer Sildenafil in pregnancies with fetal growth restriction in an attempt to induce vasodilatation and improve uteroplacental perfusion resulting in improved Doppler indices.
Reduced flow / increased resistance in uterine and umbilical arteries, indicative of reduced uteroplacental flow in pregnancies with fetal growth restriction, has been documented by noninvasive Doppler ultrasound velocimetry.[20] Fetal brain perfusion in pregnancies with fetal growth restriction shows clear regional variations, which changes with progression of hemodynamic deterioration. The presence of neurological damage in frontal lobe networking, limbic system and hippocampus can be associated with an impaired blood supply.[21] The blood flow centralization process preserves brain oxygen supply in the presence of chronic hypoxia, identified clinically by a reduced Doppler pulsatility index (PI) in the middle cerebral artery (MCA).[22] In pregnancies with fetal growth restriction, we tried to investigate Sildenafil effect on both brain sparing phenomenon and placental circulation by Doppler flow velocimetry in middle cerebral arteries and umbilical artery, respectively.
The main aim of this study was to decrease the poor perinatal outcome expected in pregnancies with high vascular resistance in the uterine circulation, because we believe that normalizing the resistance values in later trimesters results in a significantly better outcome.
MATERIALS AND METHODS
This double-blind, placebo-controlled study was conducted in Iran. Based on routine examination in the prenatal care clinic in Arash University Hospital, 76 women 24-37 weeks gestation were identified as pregnancies with Intra Uterine Growth Retardation (IUGR) between June 2008 and February 2010. The percentage of sonographic estimate was within 3% of actual birth weight. Pregnancies with fetal anomalies or chromosomal abnormalities, maternal cardiovascular morbidity, users of any vasodilator agents, diastolic blood pressure more than 110 mmHg and maternal obesity (body mass index (BMI)>34) were excluded. The patients had undergone ultrasound examination in the first trimester for accurate evaluation of the gestational age and after the first trimester to identify structural malformations. They all underwent ultrasound biometry at the second and third trimesters. In a prospective study, 59 patients without exclusion criteria agreed to randomization to either a single dose of Sildenafil citrate or placebo. Pregnancy category for this drug is B and pharmacokinetic data suggest 25-50 mg of Sildenafil administered orally as the initial dose for most patients. A large increase in circulating plasma volume during pregnancy may alter Sildenafil pharmacokinetics. Each participant signed an informed written consent form prior to randomly receiving a 50 mg tablet of Sildenafil citrate or placebo orally in a double blind manner. Maximum observed plasma concentration must reach within 30-120 minutes of oral dosing. A local pharmacy prepared the oral tablets for our study from a domestic manufacturer (Zahravi). The study was approved by the ethics committee of Tehran University of Medical Sciences, Iran.
Pulsed-wave Doppler velocimetry measurements were performed with color flow Doppler guidance on the umbilical and middle cerebral arteries with an angle of insonation of <30°, before and after 2 hours of tablet ingestion. Patients were asked about drug complications and fetal movement changes. Vascular flow velocity investigation was carried out by means of color Doppler imaging system Medisone, Accuivix v20 equipped with a 3.5-5 MHz convex transducer and a 100 Hz high pass filter, between 11 am and 2 pm by one experienced well-known operator in our center. Pregnant women were examined in a semi-recumbent position and after identification of the umbilical and middle cerebral arteries, the recording was performed at the apparent entrance point into the placenta for the umbilical artery and into the Willis Circle for middle cerebral arteries. The systolic/diastolic ratio (S/D), resistance index (RI) and pulsatility index (PI) for the middle cerebral and umbilical arteries were obtained by averaging the value of three consecutive waveforms and then the total average systolic/diastolic ratio (S/D), resistance index (RI) and pulsatility index (PI) from the left and right middle cerebral artery and total average systolic/diastolic ratio (S/D), resistance index (RI) and pulsatility index (PI) from the both umbilical arteries were calculated. The outcome was defined as the improvement of uteroplacental perfusion measured by Doppler indices. Statistical analysis included χ2-test to compare proportions, and independent–samples independent-samples t-test and paired student's t-test to compare continuous variables. The significance level was set at <0.05, power=95%.
CONCLUSION
Fifty-nine pregnant women with fetal growth restriction (FGR) were divided into two groups, 30 in the placebo and 29 in the Sildenafil group. Unfortunately, 3 patients in the placebo and 15 in the Sildenafil group refused to undergo Doppler velocimetry for the second time.
Maternal characteristics were significantly similar between the two groups [Table 1]. Among the 41 pregnancies, there were 6 with oligohydramnios (14/6%, 4 in the case and 2 in the control group), 3 with hypertension (7/3%, all in the control group) and 1 with previous stillbirth (in the placebo group). This study included 38 asymmetric (92/7%) and 3 symmetric (7/3%) fetuses with growth restriction. All symmetric ones were in the control group. Two hours after tablet ingestion, 1 in the Sildenafil and 2 in the placebo group expressed headache and 1 in the placebo group reported flushing but there was no report of nausea, myalgia and arthralgia. However, nine in the placebo and 3 in the Sildenafil group reported better fetal movement. The means (95% CI) of the umbilical artery (UA) pulsatility index (PI) and Systolic/Diastolic ratio(S/D) significantly decreased 2 hours after Sildenafil ingestion as compared to the placebo group [Table 2]. Mean umbilical artery systolic/diastolic ratio (UA S/D) significantly decreased in the Sildenafil group as compared to the the placebo group (0.60 [SD 0.40] [95% Cl 0.37-0.84], P=0.000). Mean umbilical artery pulsatility index (UA PI) significantly decreased in the Sildenafil group in comparison with the placebo group (0.12 [SD 0.15] [95% Cl 0.02-0.22], P=0.019. In middle cerebral arteries, a significant increase (95% CI) was noted in mean Pulsatility Index (PI), resistance index (RI) and Systolic/Diastolic ratio (S/D) after Sildenafil administration [Table 3]. Mean Middle Cerebral Artery Pulsatility Index (MCA PI) significantly increased in the Sildenafil group (0.51 [SD 0.60] [95% Cl 0.16-0.85], P=0.008).
Table 1.
Maternal characteristics (data are presented as mean±SD)
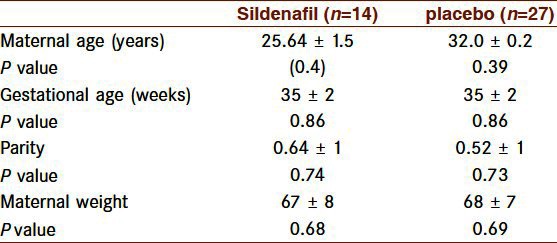
Table 2.
Umbilical artery Doppler indices in the two groups (data are presented as mean±SD)
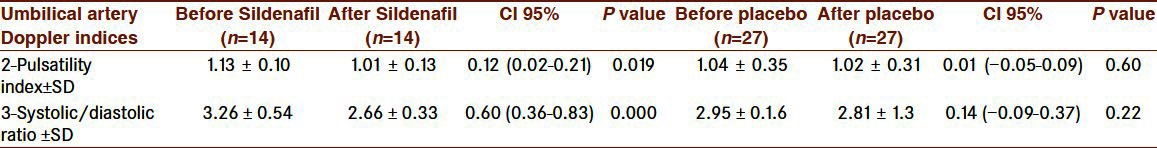
Table 3.
Middle cerebral arteries Doppler indices in the two groups (data are presented as mean±SD)
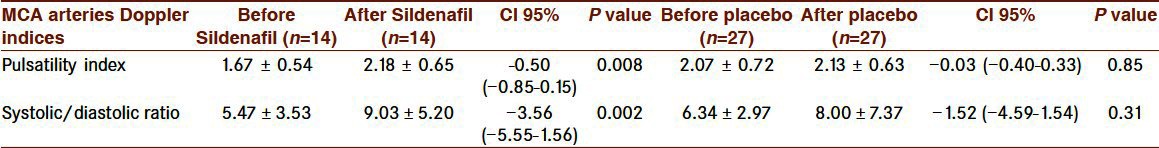
No significant improvement was detected in the perfusion of umbilical and middle cerebral arteries in the control group [Tables 2 and 3]. Mean (95% CI) umbilical and middle cerebral arteries pulsatility index and systolic/diastolic ratio were similar before and after placebo.
DISCUSSION
This study investigated the effect of Sildenafil citrate on uteroplacental perfusion in FGR pregnancies and concluded that Sildenafil 50 mg was associated with significant changes in the fetoplacental Doppler flow velocimetry waveforms, as compared to controls. This hypothesis stems from the similarities between the pathophysiology associated with preeclampsia and FGR due to a relative placental hypoperfusion. The study showed that FGR pregnancies treated with Sildenafil showed significant improvements in umbilical and middle cerebral arteries Doppler velocimetry.
One study showed that birth weight was significantly lower in patients with persistently abnormal velocimetric profile versus those with normal velocimetry at 24 weeks or those with later normalization.[5] Efforts for later normalization of fetoplacental perfusion in FGR pregnancies with placental abnormalities might increase birth weight. Growth restriction caused by placental abnormalities is usually the consequence of inadequate substrates for fetal metabolism and decreased oxygen availability.[23] Availability of nutrients to the fetus plays a crucial role in fetal growth, and fetal overgrowth observed in diabetic pregnancies results from increased substrate availability that stimulates fetal insulin secretion and fetal growth. To achieve optimal fetal growth, adequate blood flow in uteroplacental vascular function is essential. Abnormal vasculature adaptation, resulting in aberrant blood flow, has been implicated as a possible cause of fetal growth restriction (FGR). Sildenafil, as a vasodilator, should be an alternative in the treatment of Intra Uterine Growth Retardation (IUGR) and preeclampsia by later normalization in velocimetric profile. As a therapeutic agent in FGR gestations by promoting myometrial small artery vasodilatation, reducing in maternity peripheral resistance and increasing flow within the uteroplacental bed, can improve uteroplacental perfusion. PDE-5 inhibitors can reduce vasoconstriction and improve relaxation of FGR myometrial small arteries.
Not only an extensive report of the preclinical evaluation could not demonstrate any evidence of teratogenicity by Sildenafil, even at doses much higher than that evaluated in the present study but also for preeclampsia treatment, Sildenafil in the escalating dose regimen 20-80 mg tid was well tolerated, without increasing in maternal or fetal morbidity or mortality.[24] It might safely reduce perinatal morbidity and mortality increasing uteroplacental impaired perfusion.
Although there is no study on its placental transfer, Sildenafil has a relatively low molecular weight and might cross the placenta and exert a direct effect on fetoplacental circulation as a mild NO donor in the peripheral vasculature. No significant difference was observed in drug complications attributed to Sildenafil in single 50 mg therapy. Common adverse reactions of the administration of Sildenafil citrate in erectile dysfunction including headache, flushing, dyspepsia, nasal congestion, urinary tract infection, abnormal vision and diarrhea, which have been reported in clinical trials, may also be observed in its prospective use in pregnancy.[25]
The better fetal movement which was observed in the control group could be due to the placebo effect.
It would have been useful to compare the changes of Doppler indices of the uterine arteries possibly induced by Sildenafil. Although Samangaya et al. ruled out prolonged pregnancy in women with preeclampsia using Sildenafil.[24] To our knowledge, there is no report to determine the changes in uteroplacental perfusion induced by PDE-5 inhibitors. Although Sildenafil might actually improve fetal well being and mitigate growth retardation associated with placental insufficiency, it may not be an appropriate therapeutic strategy in all FGR pregnancies apparently. In cases with no genetic abnormalities, especially with impaired fetal Doppler velocimetry, the findings of our study could justify a clinical trial investigating whether Sildenafil citrate administration may lead to improved fetal well being. Our findings would have been much more reliable if we had enough FGR pregnancies with impaired Doppler indices to assess the hypothesis more accurately.
The increased risk of hypertension, diabetes mellitus and coronary artery diseases in subjects with a history of long-term deficient growth in the uterus is well documented. The implications of Sildenafil for increasing fetal weight in FGR pregnancies have to be considered for future studies.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Akbar Fotouhi for his contribution in this study.
Financial support
This study was funded by Tehran University of Medical Sciences project number is 283/1387.
Footnotes
Source of Support: Tehran University of Medical Sciences project number is 283/1387
Conflict of Interest: None declared
REFERENCES
- 1.Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110:S99–107. doi: 10.1016/s0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension. 2010;55:380–5. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson MR, Anim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? BJOG. 2002;109:836–9. doi: 10.1111/j.1471-0528.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 4.Schiessl B, Kainer F, Oberhoffer R, Jundt K, Friese K. Doppler sonography of the uterine and the cubital arteries in normal pregnancies, preeclampsia and intrauterine growth restriction: evidence for a systemic vessel involvement. J Perinat Med. 2006;34:139–44. doi: 10.1515/JPM.2006.025. [DOI] [PubMed] [Google Scholar]
- 5.Soregaroli M, Valcamonico A, Scalvi L, Danti L, Frusca T. Late normalisation of uterine artery velocimetry in high risk pregnancy. Euro J Obstet Gynecol Reprod Biol. 2001;95:42–5. doi: 10.1016/s0301-2115(00)00358-4. [DOI] [PubMed] [Google Scholar]
- 6.Rosselli M, Keller RJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4:3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay B, Sooranna SR, Johnson MR. Nitric oxide synthase activities in human myometrium and villous trophoblast throughout pregnancy. Obstet Gynecol. 1996;87:249–53. doi: 10.1016/0029-7844(95)00391-6. [DOI] [PubMed] [Google Scholar]
- 8.Bewley S, Chard T, Grudzinskas G, Campbell S. The relationship of uterine and umbilical Doppler resistance to fetal and placental protein synthesis in the second trimester. Placenta. 1993;14:663–70. doi: 10.1016/s0143-4004(05)80383-2. [DOI] [PubMed] [Google Scholar]
- 9.Nanetti L, Giannubilo SR, Raffaelli F, Curzi CM, Vignini A, Moroni C, et al. Nitric oxide and peroxynitrite platelet levels in women with small-for-gestational-age fetuses. BJOG. 2008;115:14–21. doi: 10.1111/j.1471-0528.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller SL, Loose JM, Jenkin G, Wallace EM. The effects of sildenafil citrate (Viagra) on uterine blood flow and well being in the intrauterine growth-restricted fetus. Am J Obstet Gynecology. 2009;200(102):e1–7. doi: 10.1016/j.ajog.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Wareing M, Myers JE, O’Hara M, Kenny LC, Taggart MJ, Skillern L, et al. Phosphodiesterase-5 inhibitors and omental and placental small artery function in normal pregnancy and pre-eclampsia. Eur J Obstet Gynecology Reprod Biol. 2006;127:41–9. doi: 10.1016/j.ejogrb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–71. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- 13.Zoma WD, Baker RS, Friedman A, Clark KE. Sildenafil citrate (Viagra) increases uterine blood flow and potentiates estrogen-induced vasodilation. Am J Obstet Gynecol. 2004;190:1291–7. doi: 10.1016/j.ajog.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Jerzak M, Kniotek M, Mrozek J, Górski A. Sildenafil increases successful pregnancies after recurrent miscarriage. Fertil Steril. 2008;90:1848–53. doi: 10.1016/j.fertnstert.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Jerzak M, Kniotek M, Mrozek J, Górski A, Baranowski W. Sildenafil citrate decreased natural killer cell activity and enhanced chance of successful pregnancy in women with a history of recurrent miscarriage. Fertil Steril. 2008;90:1848–53. doi: 10.1016/j.fertnstert.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Shekerdemian LS, Ravn HB, Penny DJ. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2002;165:1098–102. doi: 10.1164/ajrccm.165.8.2107097. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Aparicio P, Mota-Rojas D, Nava-Ocampo AA, Trujillo-Ortega ME, Alfaro-Rodríguez A, Arch E, et al. Effects of sildenafil on the fetal growth of guinea pigs and their ability to survive induced intrapartum asphyxia. Am J Obstet Gynecol. 2008;198(127):e1–6. doi: 10.1016/j.ajog.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 18.Lacassie HJ, Germain AM, Valdés G, Fernández MS, Allamand F, López H. Management of Eisenmenger syndrome in pregnancy with sildenafil and L-arginine. Obstet Gynecol. 2004;103:1118–20. doi: 10.1097/01.AOG.0000125148.82698.65. [DOI] [PubMed] [Google Scholar]
- 19.Wareing M, Myers JE, O’Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol Metab. 2005;90:2550–5. doi: 10.1210/jc.2004-1831. [DOI] [PubMed] [Google Scholar]
- 20.Bower S, Kingdom J, Campbell S. Objective and subjective assessment of abnormal uterine artery Doppler flow velocity waveforms. Ultrasound Obstet Gynecol. 1998;12:260–4. doi: 10.1046/j.1469-0705.1998.12040260.x. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Andrade E, Figueroa-Diesel H, Jansson T, Rangel-Nava H, Gratacos E. Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstet Gynecol. 2008;32:71–6. doi: 10.1002/uog.5377. [DOI] [PubMed] [Google Scholar]
- 22.Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol. 2000;16:407–13. doi: 10.1046/j.1469-0705.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 23.Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–6. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- 24.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28:369–82. doi: 10.3109/10641950802601278. [DOI] [PubMed] [Google Scholar]
- 25.Boyce EG, Umland EM. Sildenafil citrate: A therapeutic update. Clin Ther. 2001;23:2–23. doi: 10.1016/s0149-2918(01)80027-8. [DOI] [PubMed] [Google Scholar]


