Abstract
Background:
Athletes use flavonoids as antioxidant to enhance endurance and physical performance. In vitro data indicate flavonoids have antioxidative and antiinflammatory functions but data in human studies are limited. The aim of this study was to determine the effects of a 2-month flavonoid quercetin supplementation on oxidative stress and inflammatory biomarkers in nonprofessional athletes with regular exercise.
Materials and Methods:
The randomized double-blind clinical trial was done among subjects with systematic and regular exercise for 8 weeks in four groups, each containing 15 individuals: 500 mg quercetin + 250 mg vitamin C as pro-oxidant (Q+C), 500 mg of quercetin alone (Q), 250 mg of vitamin C alone (C), and placebo (Control). IL-6, CRP, E-selectin and F2-isoprostane were measured before and after intervention.
Results:
In 60 participants with mean (±SD) age of 21.0 ± 1.6 years, statistically significant within group differences were observed in IL-6 (P<0.1), CRP (P<0.01) and F2-isoprostane for group 1 and pre- and postchanges in E-selectin was marginally significant for all study groups (P<0.1). Group 1 had marginally smaller F2-isoprostane (P<0.1) and interleukin 6 than control group (P<0.05) and there were marginally differences in CRP between respondents in group 1 and 2 with the control group (P<0.1).
Conclusions:
Eight-week supplementation with quercein-vitamin C was effective in reducing oxidative stress and reducing inflammatory biomarkers including CRP and IL-6 with little effect on E-selectin in healthy subjects.
Keywords: CRP, E-selectin, F2-isoprostane, interleukin 6, Quercetin, ROS production, vitamin C
INTRODUCTION
Nowadays, athletes use supplements to enhance muscle strength and endurance. A group of sport supplements that are good candidates for antioxidant therapy are flavonoids. Flavonoids are a class of polyphenolic compounds available in the fruits and vegetables. Epidemiological studies showed that the risk of cardiovascular disease in subjects, who had a high intake of flavonoids, has been reduced.[1] The most prominent flavonoids in fruits and vegetables are flavonols, and, of these, quercetin is the most commonly consumed in the human diet.[2] Quercetin is claimed to exert many positive effects on health, including protection against various diseases such as osteoporosis, lung cancer, and cardiovascular disease. Especially the ability of quercetin, to reduce reactive species such as peroxynitrite and hydroxyl radical, is proposed to be involved in these sorts of positive effects on health. Therefore, numerous studies have been conducted on the possible pharmacological aspects of this flavonoid.[2] Several in vitro researches confirmed the antioxidant effects of quercetin.[2–4] Contradictory, the studies with human subjects showed little or no effect on inflammatory and oxidative stress biomarkers.[5–7] Particularly, in many human studies on athletes, a significant effect of quercetin supplementation was not observed in the measurement parameters such as F2-isoprostanes, antioxidant capacity, and CRP.[3,8] But there are some other evidences that quercetin effects may be potentiated in the presence of certain pro-oxidants through mediating electron transfer from flavonoid quercetin and by preventing its oxidative degradation.[2] For example in animal studies quercetin co-ingestion with omega-3 long-chain fatty acids, vitamin C, or vitamin E resulted in anti-inflammatory and antioxidative effects.[9-10] Therefore, due to the failure of quercetin alone to reduce inflammation, and reactive oxygen species in many human reports, especially those on athletes with aim of increasing their physical performance,[11,12] and confirming data on co-operative activities of quercetin with ascorbate in enhancing quercetin biological activities such as its antiviral effects or reducing the increased cutaneous vascular permeability occurring in conditions of experimentally induced inflammation,[13,14] this research was conducted to explore whether the efficacy of the co-ingestion of quercetin with vitamin C, could improves its anti-inflammatory and anti-oxidative stress activity in athletes with regular exercise training.
MATERIALS AND METHODS
Subjects
This randomized, double-blind placebo-controlled clinical trial was conducted among 60 men physical education students volunteered for this investigation. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences. This study was registered in the Iranian Registry of Clinical Trials (www.irct.ir) on December 23, 2011 with IRCT registration number: 01112055062N4. Healthy subjects who were fit and physically active, performing a continuous graded exercise test (GXT) on a HP cosmos treadmill (Mercury, Germany) prior to and following the intervention, with no disease and medication use were included in the study. After laboratory tests, subjects with primary abnormal tests (CRP> 3) and any drugs reactions during the study were excluded from the study. Participants were supplemented orally for 8 weeks with quercetin (Solaray, USA), vitamin C (Razi, Iran), or placebo (Pharmacy Faculty, Isfahan University of Medical Sciences, Iran). Sixty subjects were randomized using permuted block randomization to one of four groups, each containing 15 individuals: 500 mg quercetin + 250 mg vitamin C as pro-oxidant (Q+C), 500 mg of quercetin alone (Q), 250 mg of vitamin C alone (C), or placebo (Control). Written informed consent was obtained from each subject. During the study, subjects agreed to avoid the use of vitamin/mineral supplements, nutritional supplements, herbs, and drugs known to affect immune function for 3 weeks before and during the 8 weeks of study. Before and after the supplementation period, participants performed their normal exercise. The following information was also reported by each subject: diet, physical activity, power status, and gastrointestinal side effects, skin or other.
Collection and handling of blood samples
A total of 15 mL of blood was drawn fasting period before and after supplementation, and serum samples were stored at -70° C until analysis. C-reactive protein was measured by enzyme immunoturbidometric assay (Randox laboratory Ltd, Belfast, United Kingdom) and IL-6, E-selectin F2-isoprostane was determined with the solid-phase enzyme-linked immunosorbent assay kits (Bender MedSystems GmbH, Vienna, Austria).
Statistical analysis
The results are presented as mean±standard error. One-way multivariate analysis of covariance (MANCOVA) controlling for the pretest differences, followed by Dunnett's posthoc comparison was used for multiple between group comparisons. Within group comparisons were done using paired samples t-test. Due to non-normality of the studied variables (positive skewed distribution) logarithmic transformation was done and homogeneity of covariance matrix has been tested via Box’M statistics. Analyses were performed with the SPSS version 16 (SPSS Inc, Chicago, IL) statistical package.
RESULTS
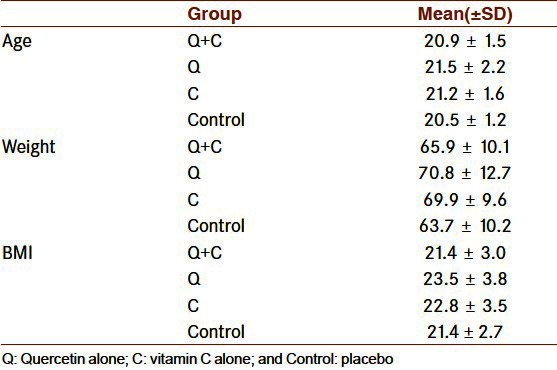
General mean ± SD for all study sample for age (years), weight (kg), and body mass index (BMI, kg/m2) was (21.0 ± 1.6), (67.5 ± 10.8) and (22.3 ± 3.3), respectively. Table 1 presents these variables for each studied groups. There are no statistically significant differences between groups in terms of basic characteristics.
Table 1.
Basic Characteristics of the participants in studied groups
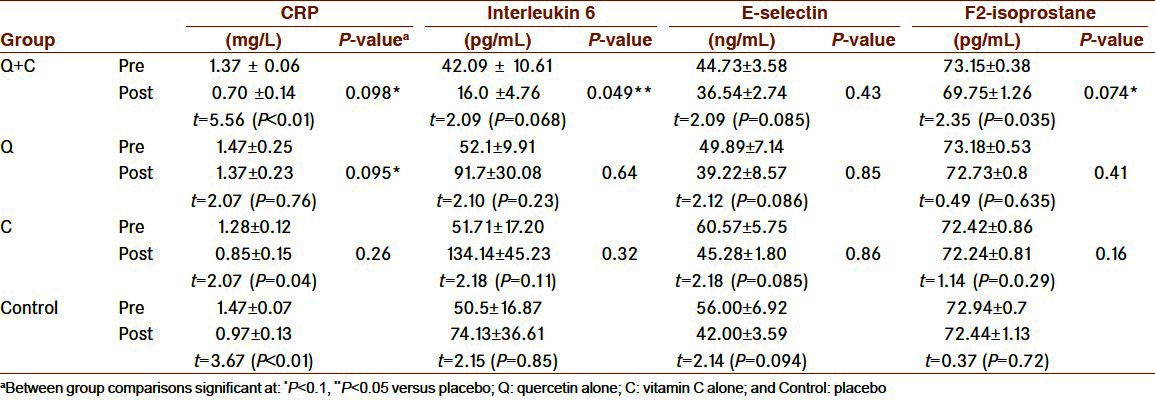
Pre- and postchanges in CRP for group 1 and control was statistically significant at P<0.01 while for group 3 at P<0.1. Within group differences in interleukin 6 and F2-isoprostane only for group 1 was marginally significant (P<0.1). Also, pre- and postchanges in E-selectin was marginally significant for all study groups (P<0.1).
MANCOVA result showed that there was marginally statistical between group differences (Wilk's λ= 0.261, F= 2.22; P<0.1). Group 1 had marginally smaller F2-isoprostane (P<0.1) and interleukin 6, than control group (P<0.05), and there were marginally differences in CRP between respondents in group 1, and 2 with the control group (P<0.1). There were no differences in E-selectin between active treatment groups and control group [Table 2].
Table 2.
Plasma concentrations of inflammatory and oxidative stress markers before and after ingestion of Quercetin + vitamin C (Q+C), quercetin alone (Q), Vitamin C alone (C), and control group
DISCUSSION
In this research in the supplemented athletes, the CRP and IL-6 levels in Q+C group was lower than placebo, quercetin alone and vitamin C [Figures 1a and 1b]. In a similar study in a two-week supplementation with quercetin + omega-3 fatty acids on trained cyclists after three day heavy exercise, quercetin-omega-3 supplement reduced inflammatory biomarkers (in particular CRP and IL-6) versus placebo,[15] while quercetin alone compared with placebo did not alter exercise-induced inflammatory markers in other studies.[11,12]
Figure 1.
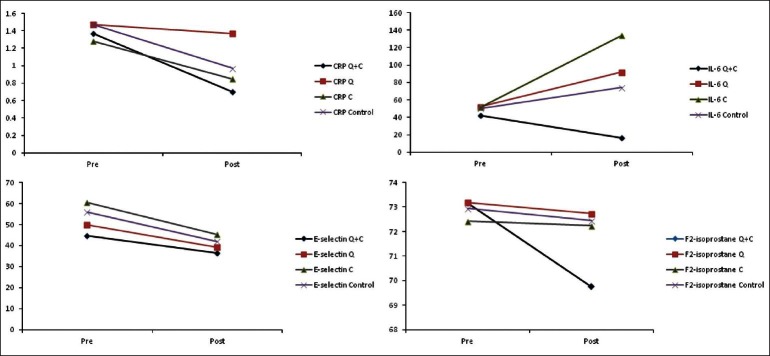
Estimated marginal means of concentrations of inflammatory and oxidative stress markers before and after ingestion of quercetin + vitamin C (Q+C), quercetin (Q), vitamin C (C), and control group
There is some evidence that quercetin metabolites in the endothelial tissue accumulate and act as an antioxidant supplement.[16] The intervention studies have also confirmed quercetin to improve endothelial function,[1] but according to our findings in this research, there was no relationship between oral intake of quercetin and E-selectin as one of the endothelial markers during regular training [Table 2]. In comparisons with similar studies, a clinical trial on flavonoid hesperidin in individuals with metabolic syndrome, reduced concentrations of inflammatory markers including CRP and E-selectin,[17] while in another study in 3 month on isoflavonoids in postmenopausal women, no difference was found for CRP and E-selectin biomarkers between groups.[18] So to have a better judge about the effect of quercetin supplementation on vascular dysfunction further studies are required.
Heavy exercise can increase oxygen consumption, mitochondrial oxidative phosphorylation and increased oxidative stress leading to free radical generation and lipid peroxidation.[19] In spite of many in vitro studies on quercetin as an antioxidant, free radical scavenger and metal chelator,[19–21] recent studies in humans suggest that supplementation with quercetin exerts very low or limited antioxidant effects.[22–24] But, however, our finding showed significant differences in F2-isoprostanes – a reliable index of in vivo free radical generation and oxidative damage of lipids – between pre- and postsupplementation in the group taking quercetin + vitamin C. Consequently, the data about CRP, IL-6, and F2-isoprostane clear that quercetin is without effect except in the presence of vitamin C. The probable mechanism of co-operative activity of quercetin + vitamin C, might be the second-order reaction of vitamin C (ascorbate), to regenerate quercetin oxidized forms.[13] During the protection against oxidative stress by scavenging free radicals, quercetin is oxidized into reactive products referred to as o-quinone/quinonmethide (QQ). QQ can be recycled with other antioxidants such as ascorbate to the parent compound quercetin and it becomes available again for the antioxidant network.[2] Moreover, there are some evidences that in the absence of enough plasma levels of ascorbate or glutathione (GSH), quercetin oxidation products, like semiquinone radicals will exert toxic effects.[25,26] Therefore, adequate glutathion plasma level or ascorbate suggested to be maintained when quercetin in high doses are supplemented [Figure 2].
Figure 2.
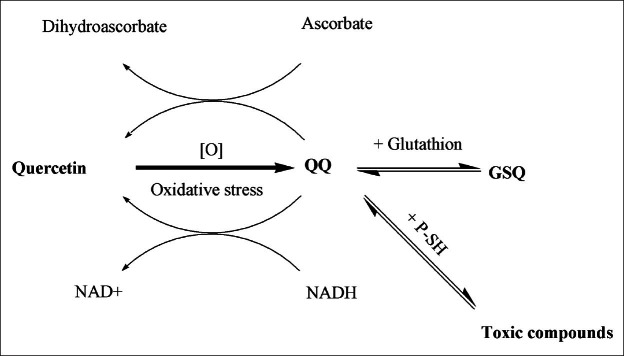
During the protection against oxidative stress by scavenging free radicals, quercetin is oxidized into reactive products referred to as o-quinone/quinonmethide (QQ). QQ can be recycled with other antioxidants such as ascorbate and NADH to the parent compound quercetin and it becomes available again for the antioxidant network. QQ is toxic in the absence of glutathion (GSH) or ascorbate. Therefore, an adequate glutathion plasma level or ascorbate suggested to be maintained when quercetin in high doses are supplemented. NAD+= nicotinamide adenine dinucleotide; NADH= reduced NAD+; P-SH= protein-thiols; GSQ: glutathione quercetin
In summary, ingestion of quercetin with pro-oxidants like vitamin C, may improve its bioavailability probably though regeneration of oxidized form of quercetin to parent compound and resupplying the flavonoid. Additional researches are requested to optimize the ascorbate or other pro-oxidants needed as co-factor in flavonoid-antioxidant network.
The limitation of this study is not measuring other inflammatory factors especially IL-8 and IL-10. One another limitation of this study was the sample size. Although the data had normal distribution, but larger number of participants, increase the statistical precision and reduce the standard errors.
ACKNOWLEDGMENTS
The authors are grateful for the students of Isfahan Faculty of Nursing and Midwifery, who participated in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–8. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- 2.Boots WA, Haenen GR, Bast A. Health effects of quercetin. From antioxidant to nutraceutical. Euro J Pharmacol. 2008;585:325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Loke WM, Proudfoot JM, McKinley AJ, Needs PW, Kroon PA, Hodgson JM, et al. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J Agric Food Chem. 2008;56:3609–15. doi: 10.1021/jf8003042. [DOI] [PubMed] [Google Scholar]
- 4.Najafzadeh M, Reynolds PD, Baumgartner A, Anderson D. Flavonoids inhibit the genotoxicity of hydrogen peroxide (H2O2) and of the food mutagen 2-amino-3-methylimadazo[4,5-f]-quinoline (IQ) in lymphocytes from patients with inflammatory bowel disease (IBD) Mutagenesis. 2009;24:405–11. doi: 10.1093/mutage/gep016. [DOI] [PubMed] [Google Scholar]
- 5.Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a highcardiovascular disease risk phenotype: A double-blinded, placebocontrolled cross-over study. Br J Nutr. 2009;102:1065–74. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 6.Knab AM, Shanely RA, Henson DA, Jin F, Heinz SA, Austin MD, et al. Influence of quercetin supplementation on disease risk factors in community-dwelling adults. J Am Diet Assoc. 2011;111:542–9. doi: 10.1016/j.jada.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Shanely RA, Knab AM, Nieman DC, Jin F, McAnulty SR, Landram MJ. Quercetin supplementation does not alter antioxidant status in humans. Free Radic Res. 2010;44:224–31. doi: 10.3109/10715760903407293. [DOI] [PubMed] [Google Scholar]
- 8.McAnulthy SR, McAnulthy LS, Nieman DC, Quindry JC, Hosick PA, Hundson MH, et al. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl Physiol Nutr Metab. 2008;33:254–62. doi: 10.1139/H07-177. [DOI] [PubMed] [Google Scholar]
- 9.Camuesco D, Comalada M, Concha A, Nieto A, Sierra S, Xaus J, et al. Intestinal anti-inflammatory activity of combined quercitrin and dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, in rats with DSS-induced colitis. Clin Nutr. 2006;25:466–76. doi: 10.1016/j.clnu.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Mostafavi-Pour Z, Zal F, Monabati A, Vessal M. Protective effects of a combination of quercetin and vitamin E against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Hepatol Res. 2008;38:385–92. doi: 10.1111/j.1872-034X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 11.Nieman DC, Henson DA, Davis JM, Murphy EA, Jenkins DP, Gross SJ, et al. Quercetin's influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007;103:1728–35. doi: 10.1152/japplphysiol.00707.2007. [DOI] [PubMed] [Google Scholar]
- 12.Nieman DC, Henson DA, Davis JM, Dumke CL, Gross SJ, Jenkins DP, et al. Quercetin ingestion does not alter cytokine changes in athletes competing in the Western States Endurance Run. J Interferon Cytokine Res. 2007;27:1003–12. doi: 10.1089/jir.2007.0050. [DOI] [PubMed] [Google Scholar]
- 13.Skaper SD, Fabris M, Ferrari V, Dalle Carbonare M, Leon A. Quercetin protects cutaneous tissue-associated cell types including sensory neurons from oxidative stress induced by glutathione depletion: Cooperative effects of ascorbic acid. J Free Radic Biol Med. 1997;22:669–78. doi: 10.1016/s0891-5849(96)00383-8. [DOI] [PubMed] [Google Scholar]
- 14.Vrijsen R, Everaert L, Boeye A. Antiviral activity of flavones and potentiation by ascorbate. J Gen Virol. 1988;69:1749–51. doi: 10.1099/0022-1317-69-7-1749. [DOI] [PubMed] [Google Scholar]
- 15.Nieman DC, Henson DA, Maxwell K, Williams A, McAnulty SR, Jin F, et al. Influence of supplemental quercetin and epigallocatechin 3-gallate on exercise performance, mitochondrial biogenesis, immunity, inflammation, and oxidative stress. Med Sci Sports Exerc. 2009;41:1467–75. doi: 10.1249/MSS.0b013e318199491f. [DOI] [PubMed] [Google Scholar]
- 16.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–34. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 17.Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E782–92. doi: 10.1210/jc.2010-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikander E, Metsä-Heikkilä M, Tiitinen A, Ylikorkala O. Evidence of a lack of effect of a phytoestrogen regimen on the levels of C-reactive protein, E-selectin, and nitrate in postmenopausal women. J Clin Endocrinol Metab. 2003;88:5180–5. doi: 10.1210/jc.2003-030362. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson PM. Antioxidants and physical performance. Crit Rev Food Sci Nutr. 1995;35:131–41. doi: 10.1080/10408399509527692. [DOI] [PubMed] [Google Scholar]
- 20.Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, González-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005;135:2299–304. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- 21.Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, et al. The Protective Effect of Quercetin against Oxidative Stress in the Human RPE In Vitro. Invest Ophthalmol Vis Sci. 2008;49:1712–20. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 22.Abbey EL, Rankin JW. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int J Sport Nutr Exerc Metab. 2011;21:91–6. doi: 10.1123/ijsnem.21.2.91. [DOI] [PubMed] [Google Scholar]
- 23.Zohrab’ian RO. Effect of antioxidants lipin and quercetin on indices of lipid peroxidation and antioxidant system in recipients of renal allotransplants. Klin Khir. 2010;7:41–4. [PubMed] [Google Scholar]
- 24.Heinz SA, Henson DA, Nieman DC, Austin MD, Jin F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br J Nutr. 2010;104:849–57. doi: 10.1017/S000711451000156X. [DOI] [PubMed] [Google Scholar]
- 25.Boots WA, Kubben N, Haenen GR, Bast A. Oxidized quercetin reacts with thiols rather than with ascorbate: Implication for quercetin supplementation. Biochem Biophys Res Commun. 2003;308:560–5. doi: 10.1016/s0006-291x(03)01438-4. [DOI] [PubMed] [Google Scholar]
- 26.Bors W, Michel C, Schikora S. Interaction of flavonoids with ascorbate and determination of their univalent redox potentials: A pulse radiolysis study. Free Radic Biol Med. 1995;19:45–52. doi: 10.1016/0891-5849(95)00011-l. [DOI] [PubMed] [Google Scholar]


