Abstract
Context:
participation in regular intensive exercise is associated with a modest increase in left ventricular wall thickness and cavity size. The magnitude of improvement depends on frequency, intensity, type, and duration of exercise program.
Aims:
to determine the effect of sports training on LV morphology and function, lung function, and to know the intensity of the exercise program enough for these changes.
Settings and design:
this was a longitudinal study (20 weeks duration) done on the medical college students.
Material and methods:
three groups, doing exercise at different intensities, high intensity group (HG) [74.9±3.9 %HRmax], low intensity group (LG) [59.46±4.1%HRmax] and no exercise group (NG) were made, and their assessments were done using the echocardiography and pulmonary function test three times, first before start of the exercise program, second at the end of 10th week, and then at the end of the 20th week.
Statistical analysis used:
3 × 3 Anova test and Bonferroni's post-test using Graph pad prism5 software.
Results:
significant improvement was seen in HG in majority of cardio respiratory parameters (VO2max, heart rate, LVIDD, LVIDS, EDV, MVV, PEFR, FVC) as compared to the LG (VO2max, heart rate, MVV, PEFR) and this improvement was specially seen at the end of the twentieth week.
Conclusions:
twenty weeks of training is helpful in improving aerobic power, MVV, and PEFR even the exercise is of moderate (LG) to high intensity (HG) but for overall cardio respiratory development physical training must be associated with very hard intensity if duration of the exercise program is short.
Keywords: left ventricular Internal diameter at end-diastole, left ventricular Internal diameter at end-systole, end diastolic volume, ejection fraction %, maximum voluntary ventilation, peak expiratory flow rate, forced vital capacity.
INTRODUCTION
Regular participation in intensive physical exercise is associated with central and peripheral cardiovascular adaptations that facilitate the generation of a large and sustained cardiac output and enhance the extraction of oxygen from exercising muscle for aerobic glycolysis. An increase in the cardiac size is fundamental to the ability to generate a large stroke volume.
The major factor that affects exercise training improvement includes initial fitness level, frequency, intensity, and duration of exercise and type (mode) of training. Of these, exercise intensity is the most crucial.[1]
The fact that the cardio respiratory system can adapt to endurance training is supported by numerous studies. In foreign countries, lot of work has been done to assess the capability of athletes and marathon runners and to study cardio respiratory adaptive changes resulting due to physical training in them.[2,3] In our country, especially in this central part of India, only few studies have been carried out.[4] Longitudinal studies of exercise-induced changes in normal subjects with exercise program of different intensities are limited. Hence, we undertook a longitudinal study to assess the effect of varying exercise intensity on the cardio respiratory function. In the present exercise program, we had taken 20 weeks duration of the exercise program, as previously author had done a study with 13 weeks duration with moderate intensity and he had found no significant result.[5]
MATERIALS AND METHODS
Design and Setting
This was a longitudinal study where we had made three groups. Three groups were randomly selected from medical college. Two groups were involved in exercise and other group was not involved in. Three groups were as follows:
High-intensity group [mean age ± s.d., 20.12±0.925 years, n=14];
Low-intensity group [ mean age ± s.d., 20.28±1.4 years, n=13];
Group not doing any exercise [mean age± s.d., 19.86±1.01years, n=12].
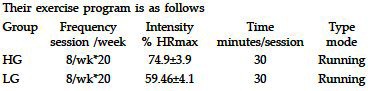
During the initial first week, they were allowed to run on the trade mill at their comfortable speed; they were encouraged to increase their intensity slowly, then two groups were made according to intensity at which they were comfortable. They were free to leave if they were not comfortable. Thus, they were given enough time so that they are not straight away started with the exercise program and this way allow them to adjust for the program. Our idea behind this first week was to make them comfortable with the instrument as well as the environment.
From second week onward, their intensity was controlled with the help of pulse oximeter. Maximal heart rate (HRmax) was calculated with help of formula (220 – age in years). Initial 5 min were their warm up period and they encouraged to exercise at above respective heart rate for the rest 25 to 30 min and also their exercise was not abruptly stopped. Such schedule was followed for the total of 20 weeks. Many dropouts were there. Numbers shown are for those who were able to complete the exercise for the total of 20 weeks. During these sessions, they were also closely monitored.
Inclusion criteria were mainly healthy volunteer, age group between 17–22 years, and nonsmokers. Smokers, subjects with history cardiac, respiratory, and diabetics and subjects whose clinical finding were abnormal were excluded from the study.
Approval for the above study was taken from institutional ethics committee.
Their cardio respiratory assessment was done three times, first before start of the exercise program, second at the end of 10th week and then at the end of 20th week.
For pulmonary function test:-MIR Spirolab (Via Del Maggiolino, 125, 00153, Rome, Italy). The pulmonary function test was recorded in morning session. On the previous day, students were told to avoid any physical exertion till the test is done.
All the subjects were made familiar with the instrument and the procedure for performing the test. The data of the subject with regard to name, age, height, weight, sex, date of performing the test, atmospheric temperature were fed to the computerized Medspiror.
The tests were performed in the sitting position. The subject was given demonstration of every procedure. Three consecutive reading were taken and the best reading among three was selected.
For echocardiography:-PHILIPS, i.e., 33, S5-1 – sector array transducer. Appointments were given to four subjects daily. They were told to take lunch, avoid any physical exertion for 4–5 h prior to the test and assemble in the Department of Cardiology, where their echocardiography was done by the cardiologist.
Subjects were examined and conventional two-dimensionally (2D) guided M-mode recording were recorded. A variable frequency phased array transducer was used. All the conventional acoustic window were used, and usual plane of view (parasternal long and short axes; apical four-chamber view) were registered. The gain, depth compensation, and darkest gray scale were individually adjusted according to thoracic wall thickness, depth of cardiac chamber, and positional relationship between cardiac chambers and the ultrasonic beam.
The transducer was placed in the fourth intercostal space at the left sternal border and was adjusted. The transducer was directed posteriorly, slightly laterally, and inferiorly so as to record the clear and simultaneous echoes from the left ventricular wall and the interventricular septum both. Cardiac dimensions were measured in M-mode echocardiogram combined B-mode from 2D images.
At rest, left ventricular end diastolic and end systolic diameters, interventricular septum, and left posterior wall thicknesses were measured from the parasternal long and short-axis view, just below the mitral valve level according to the recommendations of the American Society of Echocardiography.[6,7] To assess the objectivity of echocardiographic readings, all recording were evaluated by two independent experts.
Calculation
Ejection fraction = end diastolic volume – end systolic volume/end diastolic volume* 100
The Devereux modified American Society of echocardiography cube formula was used to calculate LV mass.[7]
LV mass = 0.8 (1.04 ([LVIDD + PWTD + IVSTD]3− [LVIDD]3)) + 0.6 g.
For maximal oxygen consumption
Subjects underwent measurement of maximal oxygen consumption (VO2max) performed by open circuit spirometry using a progressive treadmill walking protocol to volitional fatigue using a Medical Graphics CPX-D. Requirements to assure subjects reached their maximal oxygen consumption by this protocol included at least two of the following three criteria: 1) maximal heart rate>200 beats per minute; 2) respiratory exchange ratio (VCO2/VO2) >1.0; and 3) a plateau in oxygen consumption. All subjects reached their maximal oxygen consumption according to the above criteria.
For fat mass and fat %:- OMRON BF 300 (Test Medical Symptom @, Inc, 6633, Ashman road, Maria Stein OH.45860) was used. It works according to Bioelectrical impedance analysis method (BIA). Any metallic object such as watch, rings, necklace, and coins were removed. Data such as height, weight, age, and sex were entered into the machine and subjects were demonstrated how to hold the machine. Digital recording of fat mass was recorded and then lean body mass was calculated by deducting fat mass from total mass (weight).
For pulse oximetry: pulse oximeter manufactured by Nidek Medical India
Then the data of the observation for all parameters were statistically analyzed by calculating mean and standard deviation. The data were analyzed using Graph pad prism5 software. Statistical difference between the data obtained in various groups was evaluated by 3 × 3 Anova test and Bonferroni's post test and P value <0.05 was considered as statistically significant.
RESULT
The physical characteristics of the subject in the three groups (before the start of exercise program) are shown in the Graph 1. There was no significant difference in the body composition in the three groups. No significant difference was seen in aerobic power in three groups. HG were doing exercise at intensity 74.9±3.9 %HRmax, and LG were doing exercise at intensity 59.46±4.1%HRmax and this was significant (P<0.01).
Graph 1.
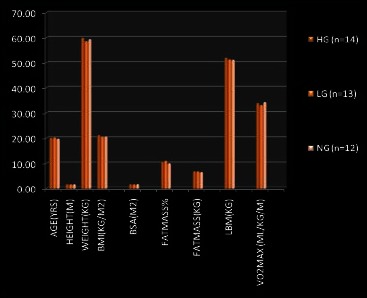
Physical characteristics of the subject in three groups
Cardio respiratory changes that had occurred in the HG group are shown in the Tables 1 and 2. There was very significant rise in aerobic power at the end of 20 weeks. The heart rate significantly decreased but 10 weeks were not enough for this change. If we see other cardiac morphological and functional changes, there was no significant changes at the end of 10th week but significant increase was seen left ventricular Internal diameter at end-diastole (LVIDD), left ventricular Internal diameter at end-systole (LVIDS), and end diastolic volume (EDV) at the end of 20 weeks but this duration was not enough for other parameters. LV mass and left ventricular posterior wall thickness at end systole, end diastole (LVPWS, LVPWD) and stroke volume (SV), ejection fraction(EF%) also increased but change was nonsignificant.
Table 1.
Cardiac changes with 10 and 20 weeks of exercise in HG
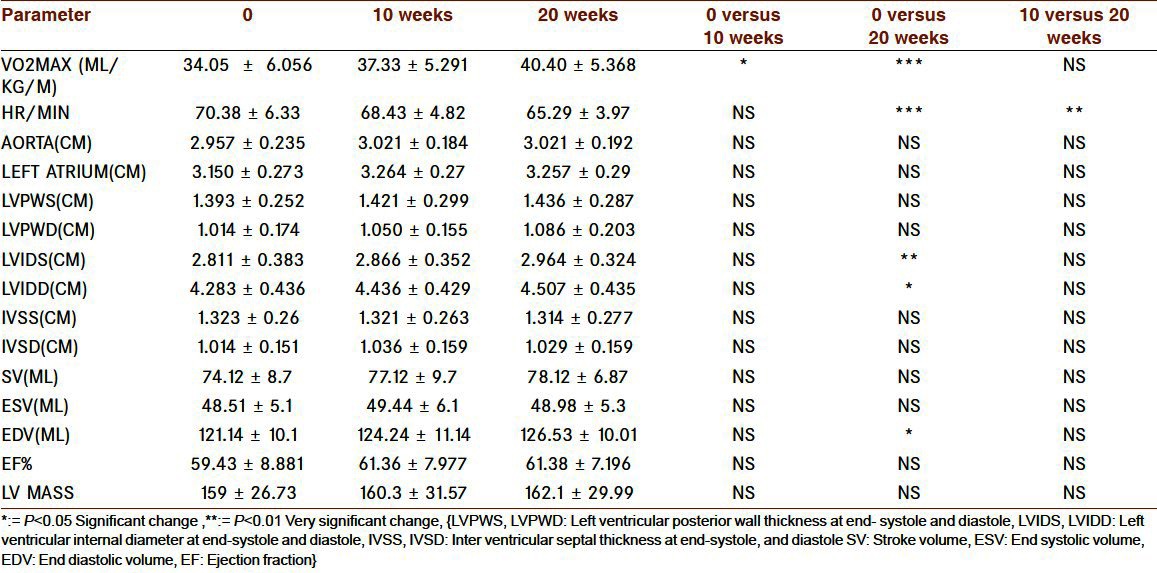
Table 2.
Cardiac changes with 10 and 20 weeks of exercise in HG
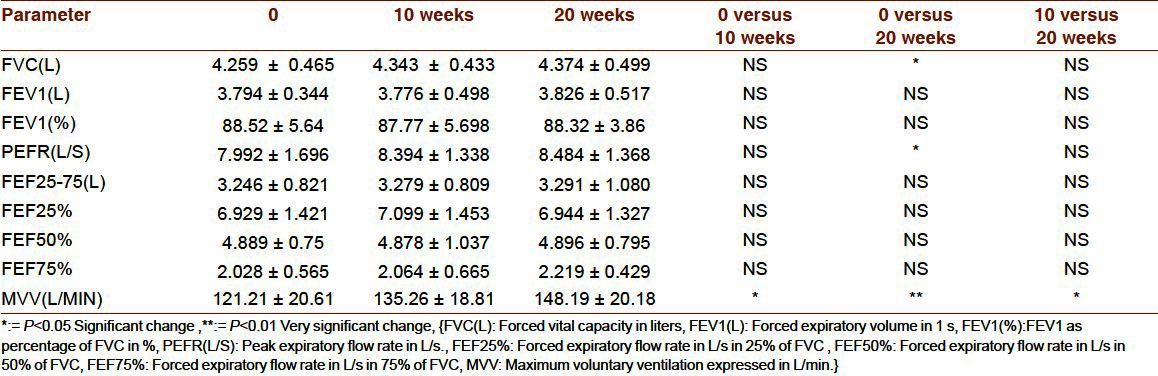
In respiratory function assessment, 10-week duration was enough for MVV (maximum voluntary ventilation), but not for other variable. With 20 weeks of exercise, significant increase is seen in PEFR (peak expiratory flow rate), FVC (forced vital capacity) and MVV.
Thus in HG group, aerobic power and MVV were the initial changes observed with 10 weeks while other cardio respiratory parameters took 20 weeks. Twenty weeks duration was not enough for all cardio respiratory parameters.
In LG group [Tables 3 and 4], ten-week exercise was not enough for any of the variable but with 20 weeks we got the significant change in aerobic power, heart rate (decrease), PEFR, and MVV while other parameters showed nonsignificant change. Changes in NG group are displayed Graph 2 and Graph 3, here no significant changes was seen in any of the parameters.
Table 3.
Cardiac changes with 10 and 20 weeks of exercise in LG
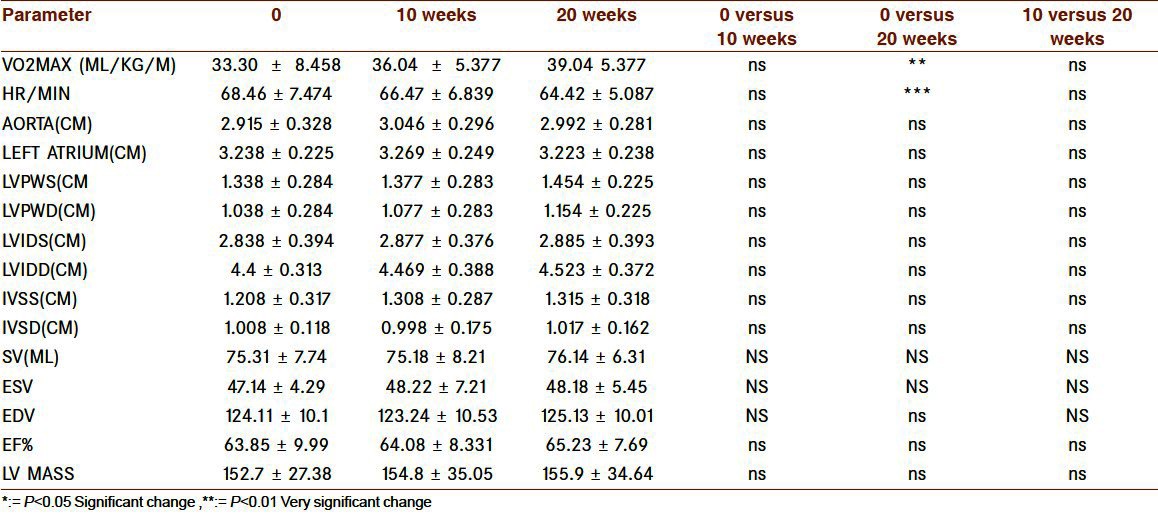
Table 4.
Respiratory changes with 10 and 20 weeks of exercise in LG
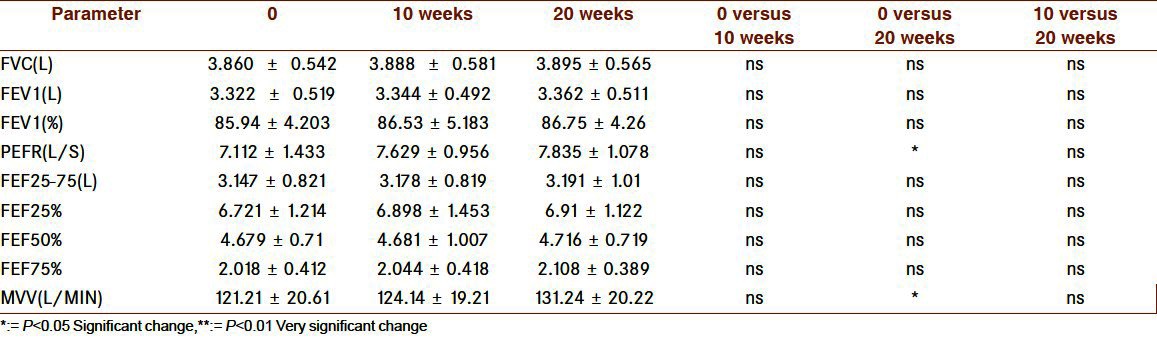
Graph 2.
Cardic changes with 10 and 20 weeks of exercise in NG
Graph 3.
Respiratory changes with 10 and 20 weeks of exercise in NG
DISCUSSION
In this study, response of the exercise to different exercise program with different intensity is studied. With higher intensity, changes were more as compared to the changes with lower intensity. Now we will discuss these finding under two separate heading, that is, cardiac changes and respiratory changes.
Cardiac changes
In this study, volunteers were involved in 20-week endurance exercise programs with different intensity. Endurance training is associated with an increased cardiac output and volume load on the left and right ventricles, causing the endurance-trained heart to generate a mild to moderate dilatation of the left ventricle combined with a mild to moderate increase in left ventricular wall thickness that is called eccentric left ventricular hypertrophy.[8,9] We expected such eccentric LVH; what we got was the some form of eccentric hypertrophy with 20 weeks of exercise in the subjects doing exercise with high intensity but these 20 weeks were not enough when the subjects were doing exercise with low intensity.
No change was found in left ventricular posterior wall thickness in systole and diastole by some (LVPWS and LVPWD)[2] whereas some studies noted significant increase.[3,10] Left ventricular Internal diameter at end-diastole(LVIDS) and at end-systole (LVIDD) does not change significantly in several studies[2,3] while in some it changed significantly.[11] Inter ventricular septal thickness at end-diastole (IVSD) and at end-systole (IVSS) remains unchanged[2] but increased in some studies.[3]
Ejection fraction indicates the contractile status of heart. Many author found no improvement in ejection fraction.[10–12] Significant change in end diastolic volume was also shown by some[11] while some got no improvement.[3,10] Bjornstad et al. concluded that the systolic function at rest is similar in age matched top athletes, athletic students, and control.[12] Many authors found significant improvement in LV mass.[3,10,11] LA dimension increase was seen in highly trained athletes,[13,14] while aortic dimension does not changed even in highly trained athletes.[14]
Contribution of Frank–Starling law Inverse correlation between the left ventricular size and heart rate was shown,[15] so bradycardia may be triggering factor for hypertrophy. Most of author had got the bradycardia[2,3,10,11] even Goodman JM et al,[15] with 6 days of endurance training, found nonsignificant decrease in the heart rate. Exercise training besides causing vagal dominance, decreases the intrinsic firing rate of sinoatrial pacemaker tissue.[16] Due to the lower heart rate, more time is allowed for ventricular filling for accommodating more volume of blood in the left ventricle, which causes stretching of myocardial fibers, this increases the end-diastolic diameter of the ventricles. Increase in the size of left ventricular chamber increases the reserve capacity of chambers as well as increase force of contraction of left ventricle to pump out more volume of blood during each stroke.[15] Thus, in our study, volume load that has lead to the enlargement of left ventricular internal diameter was mainly seen in the HG group where intensity of exercise program was high, and in LG group where intensity was low as compared HG, though decrease in the heart rate was also seen, significant increase in the LV diameter was not seen, may be more duration is required for this.
We had expected eccentric left ventricular hypertrophy but in our case left ventricular posterior wall thickness does not change significantly even in HG group; thus, left ventricular diameter may be the first morphological change seen in dynamic exercise and for other morphological change, high intensity, and longer duration may be important.
Respiratory changes
Author expected improvement in respiratory function in HG group but the possibility of deterioration in the lung function was also their as exercise-induced bronchoconstriction can be triggered by intense exercise, cold dry environments, chronic asthma, or by a variety of air pollutants.[17] We got improvement in the lung function not only in the HG group, but also in LG group improvement was seen in few parameters as compared to HG group. Deterioration was not seen in any group.
FVC:-Our finding in athletes is in agreement with several studies[4,18–20] but contrast with some.[21] FEV1 and FEV1%:-Our findings are matching with[18–20,22] but several studies have found contradictory results.[19,21] PEFR and mid-forced expiratory flow (FEF25%–75%), found in our study is in harmony with some studies[4,19] while significant improvement was found in some studies.[21,22] Significant improvement in MVV was also seen by some author.[23]
Improvement in the lung function may be because regular forceful inspiration and expiration for prolonged period during playing lead to strengthening of the respiratory muscles, both voluntary and involuntary. This helps the lung to inflate and deflate maximally. This maximum inflation and deflation is an important physiological stimulus for release of lung surfactant[24] into alveolar space thereby increasing the lung compliance and decreasing the bronchial smooth muscle tone.[21]
Since there is no improvement in any of the PFT parameter studied besides PEFR and MVV in LG group, it denotes that, adaptive changes are less, it is clearly due to less intensity of the exercise program. Besides less intensity, longer duration might be other factor. Besides duration, swimming should be the exercise for respiratory development. Swimming training attenuates the dysanaptic development of respiratory system and improved airway conductance[25] and may lead to alveolar hyperplasia as a result of intermittent hypoxia;[21,22] thus, all athletes should be involved in some form of swimming for overall development because continuing exercise with same tempo may sometimes be difficult.
Histamine is one inflammatory mediator that has been shown to increase during heavy exercise, which could cause bronchoconstriction at the level of the small airways and/or increase microvascular permeability. This is believed to result from the repetitive dehydration of the small airways when large volume of cold air is inhaled.[26,27] Such decline was not shown in our study and also by Kipplen et al. who did not find significant evidence of the lung function impairment in healthy Mediterranean athletes even after 1 year of endurance training.[28] The reason for this not significant change may be beneficial effect of exercise that is overcoming the effect of different mediator responsible for bronchoconstriction.
Thus overall cardiorespiratory development was more with the exercise program of higher intensity (74.9±3.9 %HRmax).
These all cardio respiratory changes are what physicians are concerned about but what athlete is concerned, is about his performance on the field; it mainly depends on aerobic power, that is, VO2max that is the internationally accepted parameter to evaluate the cardio respiratory fitness.[1] In our study, the magnitude of improvement was more in the HG group. Recent studies such as ours are also in favor of high-intensity aerobic endurance training[29,30] for improvement in the aerobic power. Thus to summarize
Twenty weeks of training is helpful in improving the aerobic power, MVV, and PEFR even the exercise is of moderate to high intensity. For overall cardiorespiratory development physical training must be associated with very hard intensity if duration of the exercise program is short. With moderate intensity of exercise, the exercise program should be of long duration. Thus, while assessing the athletes in the clinic, stress has to be given on intensity of exercise, it is important to ask history of not only duration but also of intensity especially when duration of the training program is short, so that proper evaluation of athlete is possible. Also the athletes coming to us for advice from professional training point of view must be told about the importance of intensity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Saltin B. Training for Anaerobic and aerobic power. In: McArdle WD, editor. Exercise physiology, Energy, Nutrition and Human Performance. V edition. Philadelphia, Baltimore, Newyork, London, Hongkong, Sydney, Tokyo: Lippincort William and Wilkins Publisher; 2001. pp. 459–99. [Google Scholar]
- 2.McDonald MP, Sanfilippo AJ, Savard GK. Baroreflex function and cardiac structure with moderate endurance training in normotensive men. J Appl Physiol. 1993;74:2469–77. doi: 10.1152/jappl.1993.74.5.2469. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues AC, de Melo Costa J, Alves GB, Ferreira da Silva D, Picard MH, Andrade JL, et al. Left ventricular function after exercise training in young men. Am J Cardiol. 2006;97:1089–92. doi: 10.1016/j.amjcard.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 4.Prakash S, Meshram S, Ramtekkar U. Athletes, yogis and individuals with sedentary lifestyles; do their lung functions differ? Indian J Physiol Pharmacol. 2007;51:76–80. [PubMed] [Google Scholar]
- 5.Hulke SM, Phatak MS. Effect of Short Term Training on Cardio respiratory Parmeters during Physical Education Training Course: A longitudinal Study. Research and Reviews: A Journal of Medicine. 2011;1:34–44. [Google Scholar]
- 6.Sahn J, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M- mode echocardiographic measurement. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Liebson PR, Horan MJ. Recommendations concerning the use of echocardiography in Hypertension and general population research. Hypertension. 1987;9:97–104. doi: 10.1161/01.hyp.9.2_pt_2.ii97. [DOI] [PubMed] [Google Scholar]
- 8.Fogard R. Athlete's Heart. Heart. 2003;89:1455–61. doi: 10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogard RH. Impact of different sports and training on cardiac structure and function. Cardiol Clin. 1997;15:397–412. doi: 10.1016/s0733-8651(05)70348-9. [DOI] [PubMed] [Google Scholar]
- 10.Sipola P, Heikkinen J, Laaksonen DE, Kettunen R. Influence of 12 weeks of jogging on magnetic resonance-determined left ventricular characteristics in previously sedentary subjects free of cardiovascular disease. Am J Cardiol. 2009;103:567–71. doi: 10.1016/j.amjcard.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Baggish AL, Yared K, Wang F, Weiner RB, Hutter AM, Jr, Picard MH. The impact of endurance exercise training on left ventricular systolic mechanics. Am J Physiol Heart Circ Physiol. 2008;295:H1109–16. doi: 10.1152/ajpheart.00395.2008. [DOI] [PubMed] [Google Scholar]
- 12.Björnstad H, Smith G, Storstein L, Meen HD, Hals O. Electrocardiographic and echocardiographic findings in top athletes, athletic students and sedentary controls. Cardiology. 1993;82:66–74. doi: 10.1159/000175856. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea A, Riegler L, Cocchia R. Left atrial volume index in highly trained athletes. Am Heart J. 2010;159:1155–61. doi: 10.1016/j.ahj.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Kasikcioglu E, Oflaz H, Akhan H. Left atrial geometric and functional remodeling in athletes. Int J Sports Med. 2006;27:267–71. doi: 10.1055/s-2005-865660. [DOI] [PubMed] [Google Scholar]
- 15.Goodman JM, Liu PP, Green HJ. Left ventricular adaptations following short-term endurance training. J Appl Physiol. 2005;98:454–60. doi: 10.1152/japplphysiol.00258.2004. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer ME, Allert JA, Adams HR, Laughlin MH. Adrenergic responsiveness and intrinsic sinoatrial automaticity of exercise-trained rats. Med Sci Sports Exerc. 1992;24:887–94. [PubMed] [Google Scholar]
- 17.Wagner IM, Wagner PD. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J Appl Physiol. 1998;85:1523–32. doi: 10.1152/jappl.1998.85.4.1523. [DOI] [PubMed] [Google Scholar]
- 18.Hagberg JM, Yerg JE, 2nd, Seals DR. Pulmonary function in young and older athletes and untrained men. J Appl Physiol. 1988;65:101–5. doi: 10.1152/jappl.1988.65.1.101. [DOI] [PubMed] [Google Scholar]
- 19.Armour J, Donnely PM, Bye PT. The large lung of elite swimmers: An increased alveolar number? Eur Respir J. 1993;6:237–47. [PubMed] [Google Scholar]
- 20.Adegoke OA, Arogundade O. The effect of chronic exercise on lung function and basal metabolic rate in some nigerian athletes. Afr J Biomed Res. 2002;1:9–11. [Google Scholar]
- 21.Mehrota PK, Verma N, Tiwari S, Kumar P. Pulmonary functions in Indian sportsmen playing different sports. Indian J Physiol Pharmacol. 1998;42:412–16. [PubMed] [Google Scholar]
- 22.Doherty M, Dimitriou L. Comparison of lung volume in Greek swimmers, land based athletes, and sedentary controls using allometric scaling. Br J Sports Med. 1997;31:337–41. doi: 10.1136/bjsm.31.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh AK, Ahuja A, Khanna GL. Pulmonary capacities of different groups of sportsmen in India. Br J Sports Med. 1985;19:232–4. doi: 10.1136/bjsm.19.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrain JN, Georke J, Clement JA. Surfactant release in exercised rat stimulated by air inflation. J Appl Physiol. 1981;51:905–10. doi: 10.1152/jappl.1981.51.4.905. [DOI] [PubMed] [Google Scholar]
- 25.Courteix D, Obert P, Lecoq AM, Guenon P, Koch G. Effect of intensive swimming training on lung volume, airway resistance and on the maximal expiratory flow-volume relationship in prepubescent girls. Eur J Appl Physiol. 1997;76:264–9. doi: 10.1007/s004210050246. [DOI] [PubMed] [Google Scholar]
- 26.Rundell KW, Jenkinson DM. Exercise induced bronchospasm in the elite athlete. Sports Med. 2002;32:583–600. doi: 10.2165/00007256-200232090-00004. [DOI] [PubMed] [Google Scholar]
- 27.Anselme F, Caillaud C, Couret I, Rossi M, Prefaut C. Histamine and exercise-induced hypoxemia in highly trained athletes. J Appl Physiol. 1994;76:127–32. doi: 10.1152/jappl.1994.76.1.127. [DOI] [PubMed] [Google Scholar]
- 28.Kippelen P, Caillaud C, Robert E, Connes P, Godard P, Prefaut C. Effect of endurance training on lung function: A one year study. Br J Sports Med. 2005;39:617–21. doi: 10.1136/bjsm.2004.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665–71. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 30.Gormley SE, Swain DP, High R, Spina RJ, Dowling EA, Kotipalli US, et al. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc. 2008;40:1336–43. doi: 10.1249/MSS.0b013e31816c4839. [DOI] [PubMed] [Google Scholar]


