Abstract
Background:
Distraction during painful interventions may reduce pain perception, but results in the literature are inconsistent. The aim of the study was to test the effectiveness of a musical mobile as a distraction tool on pain reduction in infants during a vaccine injection.
Materials and Methods:
The study based on a quasi-experimental model involving a test group and a control group was performed on 120 healthy infants, who were presented to the primary healthcare center for their first DaPT-IPV-Hib combined vaccination. The study was conducted in a room furnished with or without a musical mobile fixed to the head of the examination table, suspended at a distance of 20 – 25 cm from the infant's face. A question form was used to determine the infants’ characteristics, and the Face, Legs, Activity, Cry, Consolability (FLACC) Pain Scale was used to assess their levels of pain. Data were collected between January 1 and May 15, 2008.
Results:
The pain scores of the infants in the test group (during the procedure 5.13 ± 2.11 and after the procedure 1.26 ± 2.01) were lower than the scores of the infants in the control group (during the procedure 6.65 ± 2.69 and after the procedure 3.61 ± 2.27). The crying duration was also shorter among infants in the test group than among infants in the control group (23.53 ± 18.38 vs. 30.88 ± 22.78 seconds) during the vaccination injection.
Conclusions:
A lower pain score and shorter crying duration in response to vaccination in a room furnished with a musical mobile indicates that distracting attention via a musical mobile is a practical way to reduce pain during routine medical interventions in infants.
Keywords: Distraction, infant, musical mobiles, pain, vaccination
The routine vaccine injections are some of the most common and most painful procedures during childhood, especially when they are administered without adopting any pain management practice.[1,2] A majority of these injections are administered in the early periods of infancy.[2] Uncontrolled pain experienced in early periods of life has a negative and long-lasting effect such as distress,[3,4] and can negatively affect the development of the central nervous system.[5–7] Moreover, fear and avoidance of medical care during adulthood are partially related to experiences of many painful procedures and fears experienced during childhood. It is therefore important that the number of painful stimuli be kept at a minimum, and that these stimuli are rendered less painful during this period during each procedure.[8]
Health professionals have the responsibility of using various methods to manage painful procedures, in order to prevent long-lasting adverse effects of pain on children and to reduce the emotional and physical effects of the painful procedures.[9] Many pharmacological and nonpharmacological methods are used to control pain in children. Cognitive–behavioral nonpharmacological methods, including distracting attention, are feasible in acute pediatric pain management.[10] Distraction is a method that increases pain tolerance by drawing attention away from the painful stimulus to other directions,[11] and is considered a powerful means of pain management in children.[12–14] This is especially so in the first seven years of life,[15] because it does not require advanced cognitive skills.[12,13]
Numerous studies have focused on the efficacy of various methods of distracting attention to minimize acute pain in a pediatric population, such as movies,[15] party blowers,[16] nonprocedural talk,[17] interactive robots,[18] virtual reality goggles,[19] kaleidoscopes,[20] bubble-blowing,[21] short stories,[22] and music[23] None of these options totally abolishes the pain from injections,[3] but distraction, in general, helps moderately in preschool-age and older children.[13,24]
In some studies on infants, parental distraction,[25,26] movie distraction[15] or nurse-directed distraction[27] have effectively reduced distress. In other studies, skin-to-skin contact[28] and breastfeeding[8,29] have effectively reduced infants’ immunization pain. A limited number of studies on nonpharmacological methods used for distracting attention during vaccination procedures in infants has used similar distraction methods, but their results are inconsistent.[25,27,30] Some studies of parental distraction[25,27,30] and breastfeeding[31] have been shown as ineffective in reducing infants’ immunization pain. A new distraction method can contribute toward a more effective outcome for infants.
The aim of the current study was to examine the effectiveness of a musical mobile as a distraction tool for pain reduction in infants during the vaccination procedure.
MATERIALS AND METHODS
Study design
The study used a quasi-experimental design.
Setting and samples
The research was conducted at a primary healthcare center in the east of Turkey. According to records, this healthcare center had provided service for 14,040 people during 2007, and 334 infants had been administered with their first Diphtheria, acellular Pertussis, Tetanus (DaPT) vaccination. Power analysis was performed to calculate the appropriate sample size for this study of infants. The power calculation indicated that a total sample of 84 infants would achieve a power of 80% with the alpha set at 0.05 (two-tailed).[32] The research population thus comprised of 120, two-month-old, healthy infants, who were presented to the healthcare center for their first Diphtheria, acellular Pertussis, Tetanus, Inactivated Polio, Haemophilus Influenzae type b (DaPT-IPV-Hib) combined vaccination between 1 January and 15 May 2008. The infants were divided into the control and test groups, and were to receive vaccination in a room furnished with or without a musical mobile. In the healthcare center, vaccination procedures are normally performed on two consecutive days every week. Infants who came in first place to the healthcare center for vaccination were assigned to the test group and infants who came in second place to the center were assigned to the control group. It was lasted in this way. For both groups, the injections were administered by the same staff nurse from the primary healthcare center.
Inclusion criteria:
Infants who did not meet certain criteria were excluded from the study. The inclusion criteria were, (1) absence of any neurological or chronic disorders, (2) older than 38 weeks of age, (3) no treatment of any kind received at a healthcare institution before the study, (4) no analgesic medicine taken in the last three hours before the vaccination procedure, (5) attending for vaccination against Kvit and Hepatitis B (twice) and being screened for phenylketonuria according to health policy in Turkey, (6) being accompanied by a parent, and (7) not crying before vaccination procedure. No measurements were made on 20 infants because they were excluded from the study (11 infants were crying, two were younger than 38 weeks of age, and seven were brought to the center by other relatives).
Data Collecting Tools
The data were collected between January 1 and May 15, 2008. The question form and FLACC Pain Scale were used in data collection and the infants’ responses to the procedure were video recorded.
Question Form:
This form, prepared by the researchers, based on relevant literature, comprised questions to collect participants’ demographic data, such as, gender, age, and weight.[14,25,33] The form was filled out during face-to-face interviews held with the parents of the infants, who had volunteered to participate in the study.
Face, Legs, Activity, Cry, Consolability Pain Scale:
The FLACC is used to assess the behavioral reactions to pain by infants and children (two months to seven years), who cannot express their own pain and with whom oral communication cannot be established.[34] The FLACC pain scale assesses five behavioral areas (facial expression of the child, the position of the legs, activity, crying, and consolability) with scores ranging from 0 to 2 for each item. The Cronbach's alpha values for FLACC were reported to be 0.95 – 0.99 during the procedure and 0.92 – 0.99 after the procedure.[35] This scale was adapted to the Turkish community.[36] In our research, the Cronbach's alpha values for FLACC were 0.84 and 0.76 during and after the procedure, respectively.
Using the FLACC Pain Scale, the infants’ behavioral reactions to pain during and after the vaccination procedure were determined in the control and test groups. These responses were coded separately by the researcher and a registered nurse, who was blind to the subject group. The mobile was not visible on the video recording, and the music was muted before the nurse coded the response.
Interrater reliability coefficients greater than 0.41, demonstrate an acceptable agreement between users.[34] In our participants, the interrater reliability for this scale was acceptable, as demonstrated by the kappa values for each of the five categories, ranging from 0.43 to 0.75.
Crying Duration
Crying was described as a loud, high-pitched sound made by infants in response to painful stimuli. The crying duration is an indicator of pain level.[34] Its duration was documented for the period from the onset of crying (when the needle was inserted) till the crying was not audible, for five seconds. The crying duration was recorded and scored in seconds.
Procedure
For the vaccination procedure, a quiet, well-lit room, at a warm temperature was selected. All participants received Bacillus Calmette-Guerin (BCG) vaccination and DaPT-IPV-Hib combined vaccination, at two months of age, according to the vaccination program accepted by The Health Ministry. The DaPT-IPV-Hib combined vaccine was first performed, while the participants were in supine position on the examination table, by the same staff nurse. The nurse was instructed to behave in the same way toward all infants in both groups, to avoid confounded outcome and bias. All infants were awake and had clean diapers at the time of injection, and their parents were in the procedure room. During the vaccination procedure, the parents of infants in both groups were allowed to calm their babies by touching and talking to them, but not to feed and do anything that would distract the infant's attention (giving toys, showing a dummy, clapping, etc.).
The preparation and actual procedure were consistent for all the infants. The injection site was cleaned with alcohol and allowed to air dry before the needle was inserted. The vaccination was administered when infants were in the quiet, alert state. A dose of 0.5 ml DaPT-IPV-Hib was given with a 23 mm gauge needle into the vastus lateralis muscle, at a 90° angle. The duration of the DaPT-IPV-Hib injection was approximately 20 seconds (for the standard technique, the needle was inserted at 90 degrees with steady pressure and aspiration was performed for 5–10 seconds. The vaccine was slowly injected over 5–10 seconds and the needle was then slowly withdrawn).[37] A light pressure was applied to the site after injection.
For the test group, a musical mobile (Exor Baby Musical Mobile) was fixed to the head of the same examination table, leaving a 20–25 cm distance between the infant's face and the mobile.[38] There were six stuffed toys with various colors attached to the rotating mobile accompanied by the tune of ‘Twinkle, Twinkle, Little Star’. The mobile was operated when the infant was laid on the examination table and it was not turned off until the infant ceased crying.
The infant's whole body and the crying sounds, which were indicative of pain,[39,40] were video recorded (Canon Ixus 75 7.1 MP digital video camera).
Data analysis
In the evaluation of the data, the crying durations of the infants were recorded and scored in seconds, and the pain reactions during and after the procedure were scored between 0 and 10, according to the FLACC Pain Scale. For the data analysis, Statistical Package for the Social Sciences (SPSS 11.0) computer program was used. Statistical significance was considered at a P-value less than 0.05.
In the data analysis, the following calculations and tests were used: Power analysis was used for sample size calculation; Cronbach's alpha coefficient for determining the consistency of the scale items; Kappa analysis for interrater reliability; percentages and chi-square test to understand whether there was a homogenous distribution between the experimental and control groups; mean and percentage distributions for determining the infants’ characteristics; means for evaluating the scale scores; t-test for determining intra- and intergroup differences in the FLACC scores of the experimental and control groups; and the t-test for determining the intergroup differences in the crying durations of the experimental and control groups.
Ethical considerations
The study was approved by the local institution based on regulation No. 2007 3.1 / 22, dated October 24, 2007, by the Ethical Board of Ataturk University, Health Sciences Institute. As responses should be given voluntarily in all researches, whereby information is obtained, it was ensured that the parents of the infants to be included in the study were volunteers. Moreover, the parents of the infants were informed of the aim and protocol of the research (why the babies were recorded, and for what purpose these recordings were going to be used), and both their written and oral consents (informed consent principle) were received.[41] All infants’ parents agreed to fill out the question form and were aware that the video recording was going to be short and that their babies were not going to be imposed with any extra burden.
RESULTS
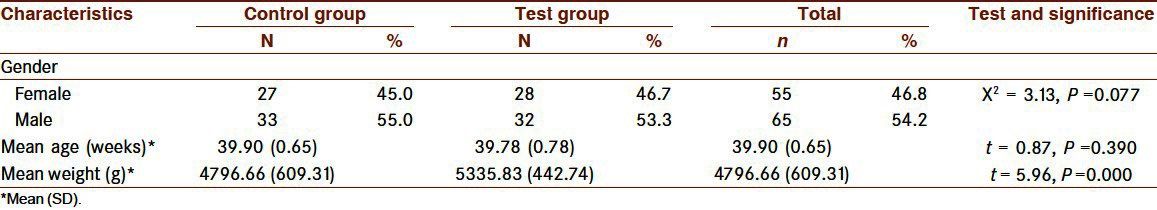
There were 55 female infants (46.8%) and 65 male infants (54.2%), and gender distribution by the groups was homogenous (X2 : 3.13, P > 0.05). The weight of the infants was within normal range (3. – 97. percentile ranges for two-month-old Turkish girls and boys). Body weight and not the age of the infants was different between the groups (t = 5.96, P < 0.001). The mean weight and age were 5,335.83 (442.74) g and 39.78 (0.78) weeks, respectively, for the control group and 4,796.66 (609.31) g and 39.90 (0.65) weeks for the test group [Table 1]. Within the groups, however, there were no differences in weight and age by gender.
Table 1.
Demographic characteristics
The pain scores for the test group were lower than those for the control group during the vaccination procedure (5.13 (2.11) vs. 6.65 (2.69), P < 0.01) and after the vaccination procedure (from after the needle was removed until the infants ceased crying) (1.26 (2.01) versus 3.61 (2.27), P < 0.001) [Table 2 and Figure 1]. The pain score during, and after the vaccination procedure, was however, independent from the body weight [Table 3]. The infants in the test group had a shorter crying duration than those in the control group [23.53 (18.38) vs. 30.88 (22.78) seconds, P < 0.05, Table 2]
Table 2.
Intra- and intergroup comparisons of the mean FLACC pain scores during and after vaccination and crying duration
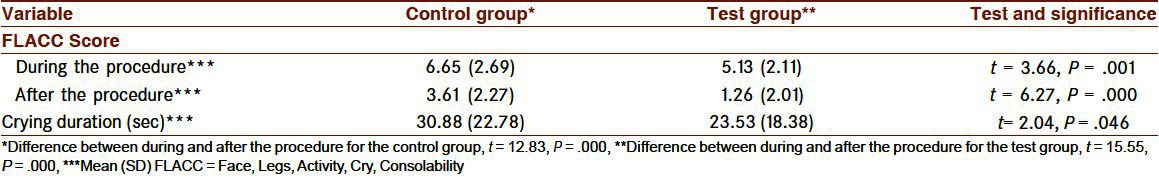
Figure 1.
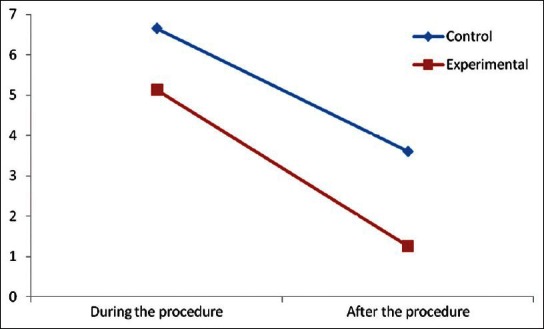
Changes in pain scores in infants subjected to vaccination under different conditions
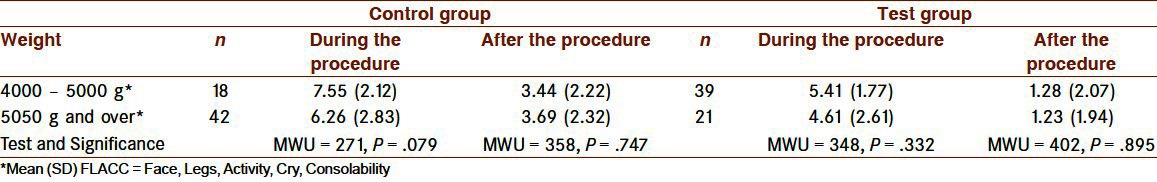
Table 3.
Group comparison of the mean FLACC pain scores during and after the procedure when the infants were categorized by weight
DISCUSSION
Despite achieving less pain and a shorter crying duration with distraction via a musical mobile, the current study had some limitations. First, variables such as pain score and crying durations were subjective and based on observational measures. Physiological indices (e.g., heart rate, blood pressure, and oxygen saturation) could have been assessed to more accurately determine this factor. Second, there was some difficulty balancing the behavior of parents during the procedure, especially as the parents of infants in the test group had to be be more comfortable, which could contribute to the infant's anxiety and pain.[42,43] Thus in the current study, both groups were similar in terms of gender distribution, but not in terms of weight. However, weight was not a contributing factor to pain score.
Previous studies addressing nonpharmacological methods, such as parental holding, sucrose, and breastfeeding, confirmed pain reduction in infants when they were subjected to painful procedures.[3,8,44] However, studies on the effectiveness of distraction to reduce anxiety and pain in infants are sparse and have mixed results.[14,25,27] In this study, the efficacy of distracting Turkish infants via a musical mobile, a multi-sensorial and new practical intervention, while being injected with their first DaPT-IPV-Hib combined vaccine was evaluated. The results shown in Table 2 reveal a significant reduction in pain when infants were distracted during vaccination. Pain reduction was also reported in previous studies when using various distraction methods in American children during vaccination[45,46] and during other injection procedures[23,40] as well as in American infants during vaccination[25,27] The device used in the present study is clinically significant, because it is multi-sensorial and practical, but results are based on observational measures. It has been reported that distraction strategies that use two senses (visual with audio) appear to be more effective at reducing pain than the use of either one alone; and content, intensity, and combinations of multisensory stimuli are important elements of distraction interventions.[47] The Human Response Model (HRM) has been focused on individual adaptation to health conditions. According to the HRM, the domains of person and environment are modifiable and non-modifiable factors,[48] and these may contribute negatively (vulnerability or risk) or positively (resilience or resource). Modifiable person or environment factors can be considered as potential targets for interventions.[49] In the HRM, sounds and sights have been shown as environment factors that are modifiable, among the factors affecting an individual's adaptation to acute pain.[48]
Crying is known to be a behavioral reaction to pain.[39,40] Infants vaccinated in a room equipped with a musical mobile cried for a shorter time than those not distracted by any device [Table 2]. Previous studies have also shown that various distraction techniques were effective in reducing the crying duration during vaccination[8] and blood sampling from the food.[33,39]
When infants were categorized by weight [Table 3], there were no differences in pain scores during and after the vaccination procedure in both groups. Effects on the FLACC pain score among those infants with low weight were also shown among infants given sucrose and pacifiers as analgesia during venipuncture.[50]
CONCLUSION
In conclusion, a musical mobile with both visual and auditory elements is a validated and reliable observational measure and a practical way of distracting infants from vaccination pain. It can be regularly used to reduce pain during the vaccination procedure. However, it would be useful to assess and compare the pain responses of infants from different cultures and communities, and children at different ages, to the use of the musical mobile. It can be used during different painful procedures and its effectiveness can also be compared with other distraction methods.
ACKNOWLEDGMENT
We thank the parents of the infants who participated in the study and the physicians, nurses, midwives, and healthcare workers employed at Veyisefendi Healthcare Center, Erzurum Health Directory of The Ministry of Health of Turkey.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Felt BT, Mollen E, Diaz S, Renaud E, Zeglis M, Wheatcroft G, et al. Behavioral interventions reduce infant distress at immunization. Arch Pediatr Adolesc Med. 2000;154:719–24. doi: 10.1001/archpedi.154.7.719. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases. Recommended childhood and adolescent immunization schedule-United States. Pediatrics. 2003;111:212–6. doi: 10.1542/peds.111.1.212. [DOI] [PubMed] [Google Scholar]
- 3.Reis EC, Roth EK, Syphan JL, Tarbell SE, Holubkov R. Effective pain reduction for multiple immunization injections in young infants. Arch Pediatr Adolesc Med. 2003;157:1115–20. doi: 10.1001/archpedi.157.11.1115. [DOI] [PubMed] [Google Scholar]
- 4.Young KD. Pediatric procedural pain. Ann Emerg Med. 2005;45:160–71. doi: 10.1016/j.annemergmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Taddio A, Goldbach M, Ipp M, Stevens B, Koren G. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet. 1995;345:291–2. doi: 10.1016/s0140-6736(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 6.Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603. doi: 10.1016/S0140-6736(96)10316-0. [DOI] [PubMed] [Google Scholar]
- 7.Gradin M, Eriksson M, Holmqvist G, Holstein A, Schollin J. Pain reduction at venipuncture in newborns: Oral glucose compared with local anesthetic cream. Pediatrics. 2002;110:1053–7. doi: 10.1542/peds.110.6.1053. [DOI] [PubMed] [Google Scholar]
- 8.Efe E, Özer ZC. The use of breast-feeding for pain relief during neonatal immunization injections. Appl Nurs Res. 2007;20:10–6. doi: 10.1016/j.apnr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Rogers TL, Ostrow C. The use of EMLA cream to decrease venipuncture pain in children. J Pediatr Nurs. 2004;19:33–9. doi: 10.1016/j.pedn.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Chambless DL, Ollendick TH. Empirically supported psychological interventions: Controversies and evidence. Annu Rev Psychol. 2001;52:685–716. doi: 10.1146/annurev.psych.52.1.685. [DOI] [PubMed] [Google Scholar]
- 11.McCaffery M. Nursing approaches to nonpharmacological pain control. Int J Nurs Stud. 1990;27:1–5. doi: 10.1016/0020-7489(90)90018-e. [DOI] [PubMed] [Google Scholar]
- 12.Reyes S. Nursing assessment of infant pain. J Perinat Neonatal Nurs. 2003;17:291–303. doi: 10.1097/00005237-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 13.DeMore M, Cohen LL. Distraction for pediatric immunization. J Clin Psychol Med Settings. 2005;12:281–92. [Google Scholar]
- 14.Cohen LL, MacLaren JE, Fortson BL, Friedman A, DeMore M, Lim CS, et al. Randomized clinical trial of distraction for infant immunization pain. Pain. 2006;125:165–71. doi: 10.1016/j.pain.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Cohen LL, Blount RL, Cohen RJ, Schaen ER, Zaff JF. Comparative study of distraction versus topical anesthesia for pediatric pain management during immunizations. Health Psychol. 1999;18:591–8. doi: 10.1037//0278-6133.18.6.591. [DOI] [PubMed] [Google Scholar]
- 16.Manimala M, Blount RL, Cohen LL. The influence of parental reassurance and distraction on children's reactions to an aversive medical procedure. Child Health Care. 2000;29:161–77. [Google Scholar]
- 17.Gonzalez JC, Routh DK, Armstrong FD. Effects of maternal distraction versus reassurance on children's reactions to injections. J Pediatr Psychol. 1993;18:593–604. doi: 10.1093/jpepsy/18.5.593. [DOI] [PubMed] [Google Scholar]
- 18.Pringle B, Hilley L, Gelfand K, Dahlquist LM, Switkin M, Diver T, et al. Decreasing child distress during needle sticks and maintaining treatment gains overtime. J Clin Psychol Med Settings. 2001;8:119–30. [Google Scholar]
- 19.Hoffman HG, Patterson DR, Magula J, Carrougher G, Zeltzer K, Dagadakis S, et al. Water-friendly virtual reality pain control during wound care. J Clin Psychol. 2004;60:189–95. doi: 10.1002/jclp.10244. [DOI] [PubMed] [Google Scholar]
- 20.Güdücü Tüfekci F, Çelebioğlu A, Küçükoğlu S. Turkish children loved distraction: Using kaleidoscope to reduce perceived pain during venipuncture. J Clin Nurs. 2009;18:2180–6. doi: 10.1111/j.1365-2702.2008.02775.x. [DOI] [PubMed] [Google Scholar]
- 21.Sparks L. Taking the “ouch” out of injections for children: Using distraction to decrease pain. MCN Am J Matern Child Nurs. 2001;26:72–8. doi: 10.1097/00005721-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Mason S, Johnson MH, Woolley C. A comparison of distractors for controlling distress in young children during medical procedures. J Clin Psychol Med Settings. 1999;6:239–48. [Google Scholar]
- 23.MacLaren JE, Cohen LL. A comparison of distraction strategies for venipuncture distress in children. J Pediatr Psychol. 2005;30:387–96. doi: 10.1093/jpepsy/jsi062. [DOI] [PubMed] [Google Scholar]
- 24.Uman LS, Chambers CT, McGrath PJ, Kisely S. A systematic review of randomized controlled trials examining psychological interventions for needle-related procedural pain and distress in children and adolescents: An abbreviated cochrane review. J Pediatr Psychol. 2008;33:824–54. doi: 10.1093/jpepsy/jsn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cramer-Berness LJ, Friedman AG. Behavioral interventions for infant immunizations. Child Health Care. 2005;34:95–111. [Google Scholar]
- 26.Bustos T, Jaaniste T, Salmon K, Champion GD. Evaluation of a brief parent intervention teaching coping-promoting behavior for the infant immunization context: A randomized controlled trial. Behav Modif. 2008;32:450–67. doi: 10.1177/0145445507309031. [DOI] [PubMed] [Google Scholar]
- 27.Cohen LL. Reducing infant immunization distress through distraction. Health Psychol. 2002;22:207–11. [PubMed] [Google Scholar]
- 28.Chermont AG, Falcão LF, de Souza Silva EH, de Cássia Xavier Balda R, Guinsburg R. Skin-to-skin contact and / or oral 25% dextrose for procedural pain relief for term newborn infants. Pediatrics. 2009;124:1101–7. doi: 10.1542/peds.2009-0993. [DOI] [PubMed] [Google Scholar]
- 29.Dilli D, Küçük IG, Dallar Y. Interventions to reduce pain during vaccination in infancy. J Pediatr. 2009;154:85–90. doi: 10.1016/j.jpeds.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Cohen LL, Bernard RS, McClellan CB, Piazza-Waggoner C, Taylor BK, MacLaren JE. Topical anesthesia versus distraction for infants’ immunization distress: Evaluation with 6-month follow-up. Child Health Care. 2006;35:103–21. [Google Scholar]
- 31.Abdel RA, Az El-Dein N. Effect of breast-feeding on pain relief during infant immunization injections. Int J Nurs Pract. 2009;15:99–104. doi: 10.1111/j.1440-172X.2009.01728.x. [DOI] [PubMed] [Google Scholar]
- 32.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 33.Mathai S, Natrajan N, Rajalakshmi NR. A comparative study of non-pharmacological methods to reduce pain in neonates. Indian Pediatr. 2006;43:1070–5. [PubMed] [Google Scholar]
- 34.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 35.Vaughan M, Paton EA, Bush A, Pershad J. Does lidocaine gel alleviate the pain of bladder catheterization in young children? A randomized, controlled trial. Pediatrics. 2005;116:917–20. doi: 10.1542/peds.2005-0103. [DOI] [PubMed] [Google Scholar]
- 36.Şenaylı Y, Özkan F, Şenaylı A, Bıçakçı Ü. Çocuklarda postoperatif ağrının FLACC (YBAAT) ağrı skalasıya değerlendirilmesi (Evaluation of postoperative pain in children with FLACC Pain Scale) Turkiye Klinikleri J Anest Reanim. 2006;4:1–4. [Google Scholar]
- 37.Ipp M, Taddio A, Sam J, Gladbach M, Parkin PC. Vaccine-related pain: Randomised controlled trial of two injection techniques. Arch Dis Child. 2007;92:1105–8. doi: 10.1136/adc.2007.118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avcı N. İstanbul: Morpa Kültür Yayınları Ltd.Ş; 2004. Gelişimde 0-3 yaş (0-3 years in development) pp. 119–21. [Google Scholar]
- 39.Yılmaz G, Gürakan B, Saatçi Ü. Topuk kanı alınma sonrası bebeklerin ağlama sürelerine etki eden faktörler (Factors influencing the duration of crying of infants after heel lance) Çocuk Sağ ve Hast Dergisi. 2002;45:233–6. [Google Scholar]
- 40.Özyazıcıoğlu N, Çelebioğlu A. Hemşirelik yüksekokulu öğrencilerinin yenidoğanda ağrıya ilişkin bilgi ve görüşleri (Information and opinions of nursing college students related pain in infant) Atatürk Üniversitesi HYO Dergisi. 2007;11:9–16. [Google Scholar]
- 41.Karataş N. Hemşirelik araştırmalarında etik (Ethic in nursing researches) HEMAR-G Dergisi. 2000;1:5–8. [Google Scholar]
- 42.Güdücü Tüfekci F, Erci B. Ağrılı işlemler sırasında ebeveynlerin bulunması konusunda çocukların, ebeveynlerin, sağlık çalışanlarının görüşleri (The opinion of children, parents and health staff about parental presence during procedures) Atatürk Üniversitesi HYO Dergisi. 2007;10:52–62. [Google Scholar]
- 43.O’Keefe N. Pain and children. World Ir Nurs. 2001;9:34–6. [Google Scholar]
- 44.Thyr M, Sundholm A, Teeland L, Rahm VA. Oral glucose as an analgesic to reduce infant distress following immunization at the age of 3, 5 and 12 months. Acta Paediatr. 2007;96:233–6. doi: 10.1111/j.1651-2227.2007.00021.x. [DOI] [PubMed] [Google Scholar]
- 45.French GM, Painter EC, Coury DL. Blowing away shot pain: A technique for pain management during immunization. Pediatrics. 1994;93:384–8. [PubMed] [Google Scholar]
- 46.Megel ME, Houser CW, Gleaves LS. Children's responses to immunizations: Lullabies as a distraction. Issues Compr Pediatr Nurs. 1998;21:129–45. doi: 10.1080/014608698265456. [DOI] [PubMed] [Google Scholar]
- 47.Kline GA. Does a view of nature promote relief from acute pain? J Holist Nurs. 2009;27:159–66. doi: 10.1177/0898010109336138. [DOI] [PubMed] [Google Scholar]
- 48.Heitkemper M, Bond E. State of nursing science: On the edge. Biol Res Nurs. 2003;4:151–62. doi: 10.1177/1099800402239725. [DOI] [PubMed] [Google Scholar]
- 49.Heitkemper M, Levy R, Jarrett M, Bond E. Interventions for irritable bowel syndrome: A nursing model. Gastroenterol Nurs. 1995;18:224–30. doi: 10.1097/00001610-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Curtis SJ, Jou H, Ali S, Vandermeer B, Klassen T. A randomized controlled trial of sucrose and / or pacifier as analgesia for infants receiving venipuncture in a pediatric emergency department. BMC Pediatr. 2007;18:27. doi: 10.1186/1471-2431-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]


