Abstract
Background:
Human growth hormone (hGH) is a single-chain polypeptide that participates in a wide range of biological functions such as metabolism of proteins, carbohydrates and lipids as well as in growth, development and immunity. Growth hormone deficiency in human occurs both in children and adults. The routine treatment for this condition is administration of recombinant human growth hormone (rhGH) made by prokaryotes. Since nonglycosylated human growth hormone is a biologically active protein, prokaryotic expression systems are preferred for its production.
Materials and Methods:
Different strains of E.coli were transformed by plasmid containing human growth hormone gene and cultured in different conditions. After induction by IPTG, recombinant human growth hormone production was assessed using ELISA, dot blotting and western blotting techniques.
Results:
High levels of rhGH were produced using E.coli prokaryotic protein production system.
Conclusion:
This simple and cost effective production process could be recruited for large scale production of rhGH.
Keywords: E.coli strain, ELISA, recombinant human growth hormone, recombinant protein expression, western blotting
INTRODUCTION
Human growth hormone (hGH) is a single-chain polypeptide hormone mainly synthesized by the acidophilic somatotrophs of the anterior pituitary gland.[1–3] hGH is encoded by the GHN gene in a gene cluster located at chromosome 17q22–24 which contains an array of five closely related genes.[4] There are several forms of hGH, but the predominant one contains 191 amino acids residues and two disulfide bonds and has a molecular mass of about 22 kDa.[1] GH has been shown to be synthesized locally, by lymphoid cells in regional lymph nodes.[5] It is released into the blood circulation where it participates in a wide range of biological functions including protein synthesis, cell proliferation, lactation, immune regulation, and metabolism of proteins, carbohydrates and lipids.[6–11] It has therapeutic applications in the treatment of dwarfism, bone fractures, skin burns, bleeding ulcers and AIDS[6,7] and has been studied in a variety of medical conditions and genetic syndromes such as children with Down's syndrome, Noonan's syndrome[2,12,13] and Prader-Willi syndrome.[14]
Growth hormone (GH) has important effects in stimulating the metabolism of bone, cartilage and muscle,[15] and somatic growth during childhood. Cardiomyopathy, decrease in myocardial mass with systolic and diastolic dysfunction both at rest and during exercise, has been reported in GH deficiency (GHD) adults. It was reported that GHD adolescents had reduced diastolic filling, cardiac size and disordered cardiac function.[16]
Until the mid-1980s, the only source of hGH was from human cadaver tissue. The use of pituitary-derived hGH was prohibited when its association with Creutz feldt-Jakob disease was proved. Recombinant DNA technology has facilitated a safe and abundant production of rhGH in various heterologous systems, without risk of transfer of human pathogens, eliminating the requirement for pituitary-derived preparation.[9] Advancement in recombinant DNA technology has made possible the expression of proteins in host cells, such as E. coli[17]). Recombinant hGH (rhGH) is now largely used to treat GH deficient short-stature children to final height and as therapy of adults with GHD,[1] acceleration of wound healing, as well as an increase in insulin-like growth factor (IGF)-1 levels in the blood from low to normal. rhGH increases IGF-1, osteocalcin, type I pro-collagen pro-peptide (PICP) and bone density, when administered to children with GHD.[18] Preliminarily studies also suggest that human GH combined with lactulose could prevent and cure severe hepatitis complicated by multiple organ dysfunction.[11] Since nonglycosylated hGH is a biologically active protein, prokaryotic expression systems are preferred in the production of recombinant hGH.[7] In order to achieve optimal productivity, the growth and production phase must be separated; therefore, recombinant organisms with inducible promoters are preferred.[3,7] Escherichia coli (E. coli) is one of the most widely used hosts for production of heterologous proteins. The strong, inducible promoter systems such as lPL, lPR, trc and T7, commonly used in recombinant E. coli, are advantageous for the over production of recombinant proteins at high cell density fermentation.[6,7]
In the present study, over-expression of rhGH under chemical induction in E. coli using recombinant DNA technology was achieved. The rhGH production at different medium compositions and bacterial strains were compared and optimal medium and strain for over production of human growth hormone were defined.
MATERIALS AND METHODS
Bacterial Strains and Plasmid
The E. coli host strains used in this study were TOP10 (Invitrogen, USA), XL1-blue and JM109 (Cinagen, Iran). The host-plasmids system used for the production of hGH was pTrcHis/ZRG (pTrcHis Topo, Invitrogen, USA) consists of the structural gene for rhGH, trc promoter for high-level expression in E. coli, the lacO sequence for induction by IPTG and 6x His tag for purification of the recombinant protein. All experiments were repeated at least three times.
Transformation
5 μl of the plasmid containing gene for rhGH was added into 200 μl of competent cells (Top10, XL1-blue and JM109 strains of E. coli) and mixed gently, incubated on ice for 20 min, heat shocked for 90 s at 42°C without shaking, immediately transferred the tubes to ice and then added 250 μl of room temperature SOC medium (Invitrogen, USA), caped the tubes tightly and shaked the tubes horizontally at 37°C for 45 min. Subsequently, the cell suspensions were centrifuged at 4000 rpm, discarded supernatant and added 100 μl of room temperature SOC medium, resuspended the cells and spread 10 μl from each transformation on a LBA (Luria–Bertani Agar, Biomark, India) plate containing 100 μg⁄ml ampicilline (for XL1-blue 100 μg⁄ml ampicilline plus 12.5 μg⁄ml tetracycline) and incubated overnight at 37°C.
Media, Cultivation and Expression:
E. coli strains were grown on LB medium which contained (g/l): yeast extract, 5; Tryptone, 10; NaCl, 5 (pH = 7.4) and 4YT medium which contained (g/l): yeast extract, 5; Tryptone, 8; NaCl, 5 (pH = 7.4). Both media contained 100 μg⁄ml ampicilline (for XL1-blue 100 μg⁄ml ampicilline plus 12.5 μg⁄ml tetracycline).
For each strain, 10 ml of LB containing appropriate antibiotics was inoculated with a single recombinant E. coli colony, grown overnight at 37°C with shaking (200 rpm), the next day, 200 ml of LB and 4YT containing 100 μg/ml ampicilline were inoculated with 10 ml of the overnight culture. The cultures were grown at 37°C with vigorous shaking to about an OD600 = 0.7. Fifty milliliter of culture suspension was removed (T0) and IPTG (Gibco, USA) was added to a final concentration of 1 mM and culture was continued at 32°C with shaking for 5 h (T5).
rhGH Extraction
Induced (T5) and non-induced (T0) cells and media were harvested by centrifugation at 4000 rpm at room temperature. Each 50 ml cell pellets were resuspended in 1 ml solution containing 20 mM sodium phosphate buffer, 500 mM sodium chloride, pH = 7.8 and lysed by the addition of lysozyme (Sigma, USA) to a final concentration of 100 μg/ml, and left at 37°C for 15 min. The cell suspension was then sonicated by applying 5 s on a medium intensity setting while holding on ice. Cell debris was then removed by centrifugation at 14000 rpm, 4°C for 20 min.
Dot Blotting, SDS-PAGE and Western Blotting
For primary detection of production of rhGH by E. coli strains, dot blot technique was performed. Purified commercial recombinant hGH (Genotropin, Pharmacia, Sweden) was used (1 mg/ml) as positive control and serial dilutions (1, 1/10, 1/100, 1/1000 and 1/10000) were prepared from that. 2 μl of each sample including cell lysates, supernatant of media from both transfected and non-transfected cells (negative control) and rhGH (positive control) were transferred on a nitrocellulose membrane, left at room temp for 30 min to dry and then blocked using 10% skim milk in PBS. Membranes were washed three times with PBS containing 0.05% Tween 20 (Sigma), incubated with primary antibody (10A7 anti human GH antibody, gift from professor Richard Ross, Sheffield University, 1/2000 dilution) in 5% skimmed milk in PBS for 90 min, washed three times, incubated with secondary antibody (sheep anti-mouse IgG HRP conjugate, Amersham, 1/2000 dilution) for 90 min, washed three times, incubated with ECL (Amersham) for 1 min and exposed to radiography film in dark room before development and fixation of the films.
For SDS-PAGE and western blotting, samples were applied into a 12% acryl amide gel. Proteins were stained with Coomassie Brilliant Blue dye. Proteins were also transferred into a nitrocellulose membrane and primed with anti-GH antibody and continued as stated for dot blotting.
Determination of rhGH Concentration by ELISA
Concentration of the rhGH in samples was measured by an enzyme-linked immunosorbent assay (ELISA) using a commercially available test kit (Radim) according to manufacturers’ protocols, briefly: 20 μl of each samples was added to the pre-coated wells, then 200 μl of enzyme-linked conjugates was dispensed instilled into each well and incubated for 60 min at 37°C. 200 μl of freshly prepared substrate solution was added to the wells and incubated for 20 min at 37°C. Between each step, wells were washed three times with washing buffer. After blocking the reaction, the absorbance was measured at 450 nm with an automated ELISA reader. The concentration of hGH was calculated from the OD 450 nm values based on the hGH ELISA standard curve.
Purification of rhGH Protein
The cell lysates and other samples were applied to a His•Bind Quick 300 Cartridge column (Novagen, USA) for protein purification according to manufacturer's recommendations, briefly: the column was washed with 2 ml of binding Buffer (8x binding buffer contained NaCl 4 M, Tris-Hcl 160 mM, Imidazol 40 mM 20 mM, pH = 7.9). Column was loaded with samples containing rhGH and then washed with 5 ml binding buffer, followed by washing with 2.5 ml wash buffer (8x wash buffer contained NaCl 4 M, imidazol 480 mM, Tris-Hcl 160 mM, pH = 7.9). Bound proteins were eluted with elution buffer (4x elution buffer contained imidazol 4 M, NaCl 2 M, Tris-Hcl 80 mM, pH = 7.9) in 1 ml fractions. 1 ml fractions were collected and the purity of the samples was confirmed by SDS-PAGE. The specificity and molecular weight of the rhGH was confirmed by Western blotting method using a specific monoclonal antibody against hGH.
RESULTS
Transformation
The expression plasmid pTrcHis/ZRG was used to transform different E. coli strains including Top10, XL1-blue and JM109. Transformation efficiency and plasmid stability were assessed. The Top10 strain proved to be the best strain for transformation with this plasmid.
Figure 1.
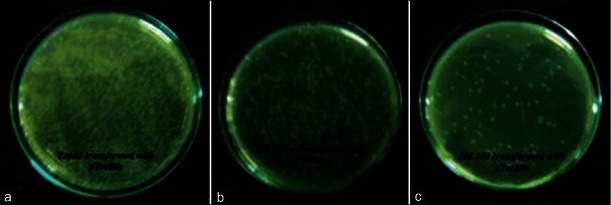
Comparison of transformation efficiency between different E.coli strains (A) Top10 (B) XL1-blue and (C) JM109
rhGH Expression and Detection
Recombinant E. coli was grown in LB and 4YT mediums containing appropriate antibiotics. Cells were harvested 5h after induction of protein production by IPTG and rhGH production was assessed in the samples. Dot blot analysis showed that all strains have the ability to produce human growth hormone as it was detectable in both cell lysate and medium [Figure 2].
Figure 2.
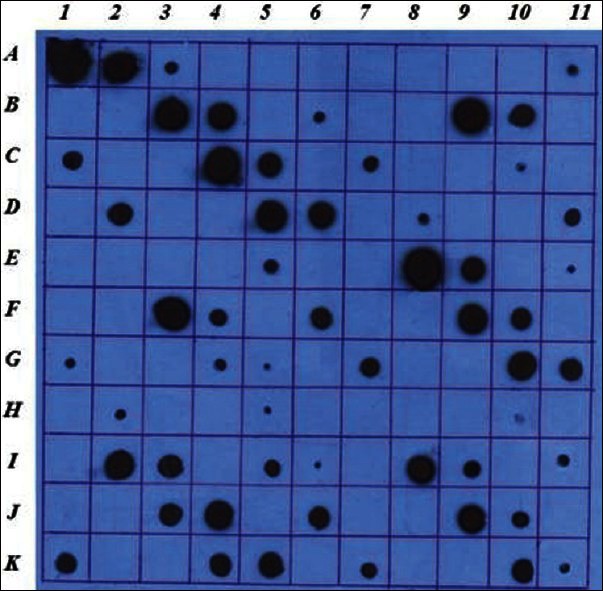
Dot blot analysis of E. coli strains expressed rhGH in LB and 4YT mediums. A1: GH 1 mgr/ml, A2: GH 100 μgr/ml, A3: GH 10 μgr/ml, A4: GH 1 μgr/ ml, A5: GH 100 ngr/ml, A6: PBS, A7: control – with out loading, A8: Top10 none transformed supernatant, A9: Top10 none transformed cell lysate, A10: Top10 transformed LB T0 supernatant, A11: Top10 transformed LB T0 cell lysate, B1: Top10 transformed LB T5 supernatant, B2: Top10 transformed LB T5 supernatant 1/10 diluted, B3: Top10 transformed LB T5 cell lysate, B4: Top10 transformed LB T5 cell lysate 1/10 diluted, B5: Top10 transformed 4YT T0 supernatant, B6: Top10 transformed 4YT T0 cell lysate, B7: Top10 transformed 4YT T5 supernatant, B8: Top10 transformed 4YT T5 supernatant 1/10 diluted, B9: Top10 transformed 4YT T5 cell lysate, B10: Top10 transformed 4YT T5 cell lysate 1/10 diluted, B11 to C11: Top10 repeat 2, D1 to E1: Top10 repeat 3. E2 to F4: repeat 1 of XL1-blue, F5 to G5: repeat 2, G6 to H6: repeat 3. H7 to I9: repeat 1 of JM109, I10 to J10: repeat 2 and J11 to K11: repeat 3.
Characterization of Produced rhGH
Based on sequence analysis of the inserted gene, molecular weight of rhGH was expected to be around 27 kd. This subject was confirmed by Western blotting. Analysis of the rhGH by SDS-PAGE and staining with a monoclonal antibody against hGH showed a major band corresponding to rhGH [Figure 3]. Subsequently, ELISA technique was used to quantify and compare the levels of rhGH produced by different strain in order to determine the best protein producing strain and culture conditions. Results showed that Top10 and LB medium were the best strain and media for rhGH production in which the maximum concentration of produced rhGH was about 100 mg/ml [Figure 4].
Figure 3.
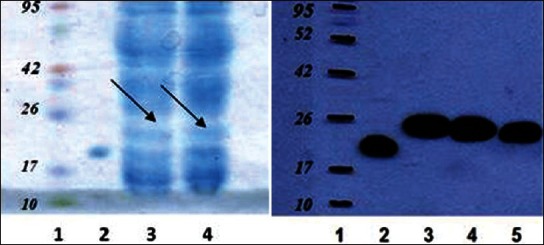
(A) SDS-PAGE analysis of bacterial lysate extract. Lane 1, molecular weight marker; Lane 2, standard GH, Lane 3,4 bacterial lysate extract before purification; (B) Western blot analysis of bacterial lysate extract. Lane 1, molecular weight marker; Lane 2, standard GH, Lane 3,4 bacterial lysate extract before purification; Lane 5, bacterial extract after purification by His·Bind Quick 300 Cartridg column. The protein ladder has been highlited using pen marker for better visulalization.
Figure 4.
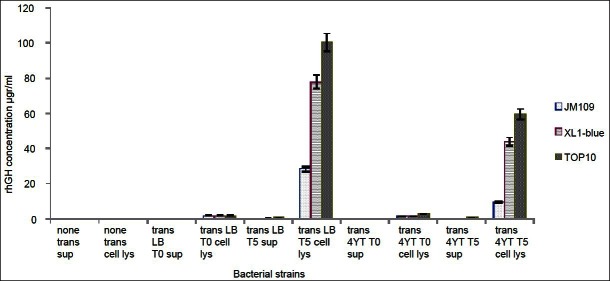
ELISA analysis of rhGH production. Different E. coli strains were transformed with plasmid containing sequence for rhGH and protein production was induced using IPTG. rhGH production was measured in supernatant and cell lysate using ELISA kits, trans: transformed, sup: supernatant, lys: lysate, LB: LB medium, 4YT: 4YT medium, T0: before induction and T5: 5h after induction.
DISCUSSION
Recombinant human growth hormone (rhGH) is a long chain amino acid molecule with a molecular weight of ± 22 kDa. Short stature in children due to growth hormone deficiency and chronic renal failure or Turner's syndrome is most often treated with human growth hormone. GH is one of the most widely used hormones in supplementation. Years of administration of this agent have proved its safety and efficacy in the therapy of various conditions associated with short stature.[19]
Expression of recombinant protein in E. coli allows its rapid and economical production in large amounts. We produced rhGH in high concentration with a simple method and compared the ability of different strains of E. coli and different components of medium and assessed the optimal condition for very high levels of protein production. We extracted the protein from supernatant as well as inclusion body. However, some new problems rose, especially in case of inactive protein synthesis and inclusion body formation. Plasmid stability, expression of target protein in high level, chemical induction system, easy isolation of the inclusion bodies from cells, lower degradation of the expressed protein, high level of target protein homogeneity in inclusion bodies, simple purification and possibility to reduce the number of purification steps are usually indicated as the main advantages of inclusion body formation.[1,7]
Shin et al. in 1998 produced rhGH in high level concentration in E.coli Bl-21 with this method and we produced rhGH in other strains of E. coli. Tabandeh et al. in 2004 produced rhGH in E.coli Bl-21 with heat induction method and we produced it with chemical induction.
Six histidine residues in the fusion partner facilitate the purification of the fusion protein by the highly selective metal affinity chromatography. The inducible promoter systems, such as trc, are advantageous for overproducing recombinant proteins in the bacteria.[6]
Separation of the two phases is especially useful when optimal growth conditions are not conducive for optimal product formation, which is frequently encountered in production of recombinant proteins from genetically engineered micro-organisms. In fed-batch cultures, the E.coli cells containing pTrcHis/ZRG were cultivated first to a high cell concentration (pre-induction, or growth phase) and then the trc promoter system was induced to synthesize rhGH protein (post-induction, or production phase) and the recombinant protein was produced at high-level in a high cell density culture.[6]
In conclusion, as shown in this article, high levels of rhGH production can be achieved in prokaryotic host in an inducible manner. The high expression level, affinity purification, and the maintenance of high expression level in the high density cell culture allow producing large quantities of rhGH which will enable us to produce this important hormone for therapeutic uses and a good source of hGH for further scientific investigation. This rhGH can be separated, purified and used for GHD treatment and other clinical applications. Normal flora bacteria can also be used for in situ production of rhGH in human body. The efficiency and safety of later method need further investigations. If proven to be effective, controllable and safe, this approach could be applied to other therapeutically important proteins which will result in a dramatic reduction in cost of treatment.
ACKNOWLEDGMENT
This study was conducted at the University of Isfahan and was supported by the Office of Graduate Studies of University of Isfahan.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sereikaite J, et al. Production of recombinant mink growth hormone in E.coli. Microb Cell Fact. 2006;5(Suppl 1):P15. [Google Scholar]
- 2.Vance ML, Mauras N. Growth hormone therapy in adults and children. N Engl J Med. 1999;341:1206–16. doi: 10.1056/NEJM199910143411607. [DOI] [PubMed] [Google Scholar]
- 3.Tabandeh F, et al. Growth kinetics and human growth hormone production of a heat inducible recombinantes Echerichia coli during batch fermentation. Iran J Sci Technol. 2004;28:11–7. [Google Scholar]
- 4.Zhan X, Giorgianni F, Desiderio D. Proteomics analysis of growth hormone isoforms in the human pituitary. Proteomics. 2005;5:1228–41. doi: 10.1002/pmic.200400987. [DOI] [PubMed] [Google Scholar]
- 5.Sommese L, Donnarumma G, de l’Ero C, Marcatili A, Vitiello M, Cipollaro D. Growth hormone modulates IL-{alpha} and IFN-{gamma} release by murine splenocytes activated by LPS or porins of Salmonella typhimurium. J Med Microbiol. 1996;45:40–7. doi: 10.1099/00222615-45-1-40. [DOI] [PubMed] [Google Scholar]
- 6.Shin N, Kim DY, Shin CS, Hong MS, Lee J, Shin HC. High-level production of human growth hormone in Escherichia coli by a simple recombinant process. J Biotechno. 1998;62:143–51. doi: 10.1016/s0168-1656(98)00054-6. [DOI] [PubMed] [Google Scholar]
- 7.Tabandeh F, Shojaosadati SA, Zomorodipour A, Khodabandeh M, Sanati MH, Yakhchali B. Heat-induced production of human growth hormone by high cell density cultivation of recombinant Escherichia coli. Biotechnol Lett. 2004;26:245–50. doi: 10.1023/b:bile.0000013714.88796.5f. [DOI] [PubMed] [Google Scholar]
- 8.Bozzola M, De Amici M, Zecca M, Schimpff RM, Rapaport R. Modulating effect of human growth hormone on tumour necrosis factor-alpha and interleukin-1beta. Eur J Endocrinol. 1998;138:640–3. doi: 10.1530/eje.0.1380640. [DOI] [PubMed] [Google Scholar]
- 9.Ghasemi F, et al. Using L-arabinose for production of human growth hormone in Escherichia coli, studying the processing of gIII: hGH precursor. Iran J Biotechnol. 2004;2:250–60. [Google Scholar]
- 10.Lebl J, Sediva A, Snajderova M, Pruhova S, Rakosnikova V. Immune system in adults with childhood-onset growth hormone deficiency: Effect of growth hormone therapy. Endocr Regul. 2000;34:169–73. [PubMed] [Google Scholar]
- 11.Ding H, Shan J, Zhang B, Ma HB, Zhou L, Jin R, et al. Combined human growth hormone and lactulose for prevention and treatment of multiple organ dysfunction in patients with severe chronic hepatitis B. World J Gastroenterol. 2005;11:2981–3. doi: 10.3748/wjg.v11.i19.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassidy S, Driscoll D. Prader#x2013;Willi syndrome. Eur J Hum Genet. 2008;17:3–13. doi: 10.1038/ejhg.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrel A, Myers SE, Whitman BY, Allen DB. Benefits of long-term GH therapy in Prader-Willi syndrome: A 4-year study. J Clin Endocrinol Metab. 2002;87:1581–5. doi: 10.1210/jcem.87.4.8414. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Ortiga R, Klibanski A, Tritos NA. Effects of Recombinant Human Growth Hormone Therapy in Adults with Prader-Willi Syndrome: A Meta-Analysis. Clin Endocrinol. 2011 doi: 10.1111/j.1365-2265.2011.04303.x. [DOI] [PubMed] [Google Scholar]
- 15.Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, et al. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol. 2010;588:341–51. doi: 10.1113/jphysiol.2009.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CL, He Y, Liang L, Zou CC, Hong F, Dong GP, et al. Effects of short-and long-acting recombinant human growth hormone (PEG-rhGH) on left ventricular function in children with growth hormone deficiency. Acta Paediatr. 2011;100:140–2. doi: 10.1111/j.1651-2227.2010.01994.x. [DOI] [PubMed] [Google Scholar]
- 17.Khodabandeh M, et al. Purification of large quantities of biologically active recombinant human growth hormone. Iran J Biotechnol (IJB) 2003:1. [Google Scholar]
- 18.Klein G, Wolf SE, Langman CB, Rosen CJ, Mohan S, Keenan BS, et al. Effect of therapy with recombinant human growth hormone on insulin-like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. J Clin Endocrinol Metab. 1998;83:21–4. doi: 10.1210/jcem.83.1.4518. [DOI] [PubMed] [Google Scholar]
- 19.Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metabo. 2010;95:167–77. doi: 10.1210/jc.2009-0178. [DOI] [PubMed] [Google Scholar]


