Abstract
Objective
Chronic sinusitis is the most prevalent chronic disease in the United States in adults aged 18 to 44 years, with approximately 250,000 operations performed annually. Although often successful, sinus surgery fails in greater than 15% of patients. Adhesion formation is a common complication and cause for subsequent revision surgery. Here, the authors evaluate a sprayable chitosan/starch-based sinus sealant and demonstrate its ability to reduce adhesion formation both in vitro and in 2 animal models.
Study Design
Randomized, controlled, animal trials.
Setting
Academic medical center (fibroblast experiments) and animal laboratories (sheep and rabbit studies).
Subjects and Methods
This sinus sealant was applied to human cultured fibroblasts obtained from surgically removed polyps to examine its ability to inhibit fibroblast migration and proliferation. The sinus sealant was applied to New Zealand White rabbits (n = 20) in an established cecal-sidewall abrasion model and to sheep (n = 10) in a sinus surgical adhesion model to examine its ability to reduce adhesion formation.
Results
This sinus sealant inhibited migration and proliferation of human cultured fibroblasts and reduced the total adhesion score from 4.9 to 0.3 for a total reduction of 94% (95th percentile confidence interval [CI], 78%, 100%; P < .001) in a well-established rabbit cecal-sidewall model commonly used for adhesion testing. Moreover, this sealant reduced adhesion formation from 80% to 10% for a total reduction of 70% (95th percentile CI, 57%, 93%; P = .003) in a sheep sinus adhesion surgical model.
Conclusion
This chitosan-based sealant demonstrates promise for reducing adhesion formation in sinus surgery.
Keywords: animal model, adhesion, chitin/chitosan, ENT surgery, fibroblast
Chronic sinusitis is characterized by persistent inflammation in the paranasal sinus cavities and represents a constellation of disease processes that lead to disruption in mucociliary flow and ostial obstruction.1 Chronic sinusitis is the most prevalent chronic disease in adults aged 18 to 44 years, affecting 13% of Americans.2 Sinus surgery is performed more than 250,000 times annually in the United States, at an average cost of over $7000 per person.3,4 Although this operation is often highly successful, greater than 15% of patients require a repeat operation wherein one of the primary causes for failure is postoperative adhesion formation.3,5,6 Currently, there are no proven therapies commonly accepted by otolaryngologists that can reduce postoperative adhesion formation as an aftermath of human endoscopic sinus surgery.7
Chitosan is a linear polysaccharide produced by deacetylation of chitin, a long-chain polymer that is the main component of the cell walls of fungi, and found in numerous classes of animals, including insects and crustaceans.8 It is a biocompatible and slowly biodegradable polymer that has been widely used in medicinal applications, including bandages and drug delivery systems.9,10 Chitosan has demonstrated biomedically useful properties, including its ability to rapidly clot blood,11 hypoallergenicity,12 antimicrobial effects,13 and solubility in the acidic environment of epithelial wound healing.14 As a result, chitosan-based compounds appeared attractive for use intraoperatively during endoscopic sinus surgery,15 an environment where intraoperative and postoperative sterility is difficult to obtain and where traditional techniques for obtaining intraoperative hemostasis, such as electrocautery and/or thrombin-based hemostatic agents, have often led to increased postoperative adhesion formation.16 Chitosan in gel formulations has been assessed for abdominal adhesion reduction17 and sinus wound healing in sheep,18 and it recently has shown promising results in an Australian human study19; however, there are no current Food and Drug Administration (FDA)–cleared chitosan formulations indicated for use during human endoscopic sinus surgery.
Ideally, a medical device with antiadhesive properties for use during human endoscopic sinus surgery would also exist as a formulation that was visible during administration and that would also remain adherent to freshly operated sinus surfaces for the duration necessary for reepithelization to occur. A sprayable chitosan-based sealant, given the trade name NovaGen gel, was specifically developed for this intended use.
Herein, we tested the hypothesis that this sprayable sealant formulation would be effective in reducing intraoperative adhesions during sinus surgery by examining its ability to inhibit cultured fibroblast migration, reduce total adhesion score in a well-established rabbit model,20,21 and reduce adhesion formation in a sheep surgical model22 that recapitulates the pathophysiology of adhesion formation during human endoscopic sinus surgery.
Methods
Sprayable, chitosan-based sealant characterization, development, and biocompatibility testing
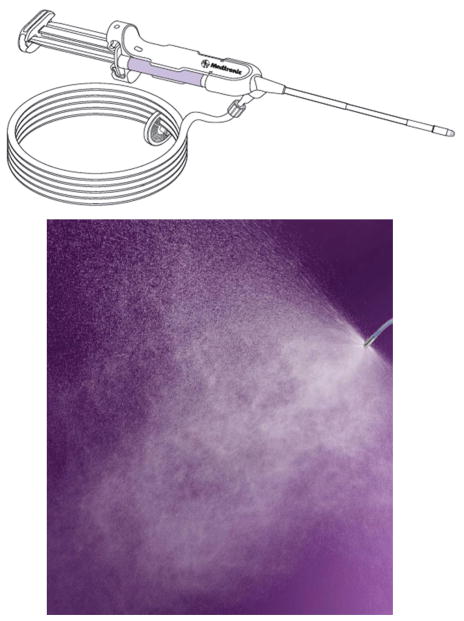
A biocompatible formulation that could be readily manufactured, sterilized, quickly hydrated and soluble at the time of surgery, visible, and easily delivered by the surgeon was prepared by the Medtronic research and development process. A sprayable, starch-based gel formulation was compounded with the active chitosan compound (Figure 1) to create the final sealant (Figure 2). A spray medical device was then developed to allow for precise delivery of the final compound to the sinus cavities (Figure 3).
Figure 1.
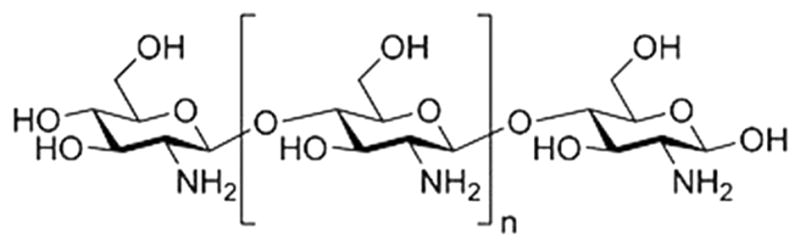
Biochemical formulation of chitosan, the active compound used in this sealant. The chitosan active compound was selected for its potential as an antiadhesive during sinus surgery.
Figure 2.
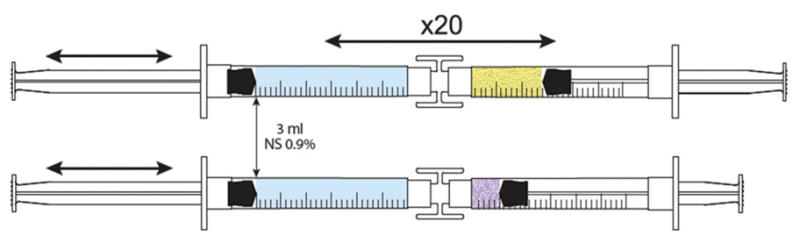
Chitosan was compounded with a starch-based gel to make the final preparation.
Figure 3.
A spray medical device was then developed to allow precise delivery of the compound to sinus cavities.
This sealant then underwent and passed biocompatibility testing for its intended use as a surface device with breached or compromised tissue contact with prolonged contact between 24 hours and 30 days of exposure.
Human fibroblast harvest and isolation
Human fibroblasts were isolated from surgically obtained nasal polyp tissue from patients (n = 6) with chronic sinusitis. Patients with cystic fibrosis, allergic fungal sinusitis, or a neutrophilic infiltrate into polyp tissue, thus indicating an ongoing acute infectious process, were excluded. The specimens were obtained from discarded pathological specimens under institutional review board–approved protocols at the University of Virginia, Charlottesville, Virginia. Informed consent was obtained prior to the patients’ surgery and after the risks, benefits, alternatives, and possible consequences of the studies were explained. Surgical tissue was minced with scissors and trypsinized using 25 mL trypsin-EDTA (Invitrogen, Carlsbad, California). Fibroblasts were pelleted and resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah), 10,000 U/mL penicillin (Invitrogen), and 10 μg/mL streptomycin (Invitrogen). Fibroblasts were plated in duplicate in 35 × 10-mm culture dishes (Becton Dickson, Franklin Lakes, New Jersey), then incubated in 5% CO2 at 37°C and grown until confluent. One day prior to assays, the medium for fibroblasts was replaced with serum-free RPMI 1640 medium.
Fibroblast proliferation and migration assay
On the day of scratch wounding, a 2-mm experimental wound was made into the fibroblast monolayer by scratching the monolayer with a pipette tip. The chitosan-based active component; the final sprayable, chitosan-based sealant; and delivery sprayers were provided by Medtronic Surgical Technologies (Jacksonville, Florida). The active chitosan compound was dissolved in RPMI 1640 media at concentrations of 1.56 mg/mL, 3.13 mg/mL, and 6.25 mg/mL, the highest concentration being the amount of active compound in the spray gel formulation. Cells were reincubated, and at 0, 24, 48, and 72 hours after chitosan delivery, an image of the culture dish was obtained with an Olympus BX51 microscope (Center Valley, Pennsylvania). The ability of chitosan to inhibit fibroblast proliferation and migration was assessed by examining the lack of migration and proliferation into the scratched area (percentage of wound healed). Percentage wound-healing calculations were made using Image J software (National Institutes of Health, Bethesda, Maryland). The assays were then repeated using the sprayable sealant formulation. The sealant was sprayed onto the treatment plates from a height of approximately 4 inches and left in place on the culture plates for 5 minutes to allow gelation to occur. After 5 minutes, fresh RPMI 1640 medium was added to the sealant, and tissue culture plates were returned to the incubator. Images of the plates were taken at 0, 24, 48, and 72 hours. For both assays that were performed separately, data presented represent the average ± standard error of the mean from the average of duplicate cultures for 6 individual fibroblast surgical isolates.
Rabbit cecal and sidewall adhesion model
Rabbits were maintained at the North American Sciences Associates, Inc. (NAMSA, Northwood, Ohio), an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)–accredited facility. The study protocol was reviewed and approved by the NAMSA Ohio Division Institutional Animal Care and Use Committee (IACUC). Sample size was estimated a priori using Mead’s resource equation method.
On the day of operative procedures, 20 female New Zealand White rabbits (Myrtle’s Rabbitry, Inc, Thompsons Station, Tennessee), were anesthetized. A 12-cm skin incision was made along the midline of the ventral abdomen. The cecum was identified, exteriorized, and abraded by wiping the entire serosal surface with a sterile dry gauze sponge until punctate bleeding was achieved. Cecal wall integrity was preserved in all 20 animals. Bilateral defects were made to the sidewalls of each rabbit. The defects were created by cutting and removing a 2 × 4.5-cm window of peritoneum and layer of overlying abdominal muscle. For the test cohort, 3 mL of the sealant was sprayed onto the peritoneal wall to coat and cover the sidewall defects. For the control cohort, no additional material was applied. Sidewall defect sites were photographed, the sidewall peritoneum and cecum were returned to normal anatomic positioning, and the abdominal incision was closed in layers. Each animal was fitted with an Elizabethan collar to restrict access to the wound site. After 14 days, necropsies were performed, and the presence and location of any remaining test article were noted and adverse reactions were recorded. Adhesions to the sidewall defect sites were scored for extent and strength by an independent veterinarian per a previously validated scoring system.20,21 Extent of adhesions was determined by the proportion of the surface area of the experimentally wounded mucosal site to the proportion that developed any adhesions. Adhesion extent was scored as 0: 0% adhesions, 1 = 1% to 25%, 2 = 26% to 50%, 3 = 51% to 75%, and 4 = 76% to 100%. Adhesion strength was scored as 0 = no adhesions; 1 = friable adhesions; 2 = immature adhesions, easy to break; and 3 = mature adhesions, difficult to break. A total adhesion score (extent + strength) was calculated for each animal.
Sheep sinus adhesion model
Sheep were maintained at the Sinclair Research Center (Columbia, Missouri), an AAALAC-accredited facility. The animal study protocol was approved by the Sinclair Research Center Missouri Division IACUC prior to conducting the study. Sample size estimation was performed a priori using Mead resource equation method. The sealant and delivery sprayers were provided by Medtronic Surgical Technologies. Ten female domestic sheep (Sinclair Bio-Resources, LLC, Columbia, Missouri) weighing 48.5 to 85.5 kg at surgery were housed outdoors and in a large animal shelter. Animals were group-housed according to the needs of each stage of the study. The surgical procedure was staged via 2 separate operations. Staging was performed to minimize prolonged anesthesia time (to decrease intraoperative complication risk) and to allow for completion of experimental wounding for all 10 animals over a single 36-hour period to minimize procedural and postoperative course variability.
Stage 1
Two weeks prior to the wounding procedure, middle turbinates were resected. This was completed via intranasal endoscopic techniques similar to human turbinate resection. This procedure was repeated for the opposite side, and visualization of the frontal sinus outflow tract and ethmoid sinus cells was possible at the end of the first staged procedure. Each nasal cavity of each animal was irrigated daily with 60 mL sterile saline until the second staged procedure.
Stage 2
The second wounding procedure occurred 14 or 15 days after middle turbinate resection. The wounding procedure was conducted using intranasal endoscopic surgical techniques. Unilateral mini-trephines accessing the frontal sinus cavities were inserted. A mini-trephine drill was used to penetrate the frontal bone, and a metal trocar was placed into the frontal sinus. The frontal recess drainage pathway was observed via sterile saline irrigation through the trocar. Partial-thickness mucosal wounds were created superior and inferior to the ridge of bone (middle turbinate attachment site to the lateral nasal wall) that remained after middle turbinectomy and between the lateral aspect of each nasal wall and the ethmoidal turbinals (2 wound sites per side). To create the wounds, a microdebrider (Medtronic Surgical Technologies) with a 3-mm cutting bur was used. The size of the defects was measured intraoperatively with a demarcated suction tube and was consistently 4 × 2 cm (± 0.5 cm). The sealant was sprayed onto each of the wounded areas (2.5 mL per site) and into the frontal sinus (1 mL) on 1 side of the animal. The wounds on the opposite nasal cavity were not treated and served as a control. The laterality of the treated side was randomized, alternating between animals. Nasal cavities were irrigated daily with 60 mL sterile saline. On postoperative days 4, 7, 10, and 18 after the wounding, sites were endoscopically observed for adhesion formation and for the presence and appearance of sealant.
On postoperative day 18, necropsies were performed. Wound sites were exposed using a bone saw and rongeurs. Presence of adhesions was confirmed visually by the surgeon and a veterinarian. High-resolution digital images of the sites were taken. To limit variation in scores and bias, animals were scored only as either + or − for adhesions (regardless of single vs multiple adhesions), and a corresponding 1 or 0 was assigned.
Results
Fibroblast migration and proliferation
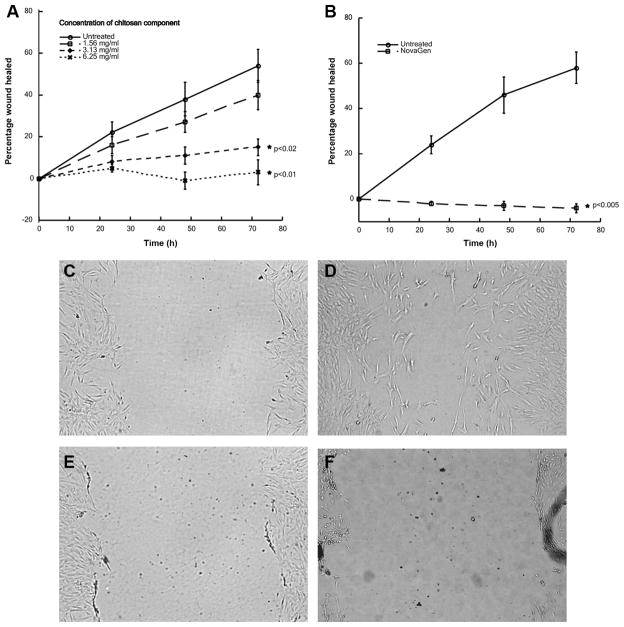
The active chitosan-based compound, which was dissolved in fibroblast culture medium, demonstrated a dose-dependent (0, 1.56, 3.13, and 6.25 mg/mL) ability to inhibit fibroblast migration and proliferation (Figure 4A; n = 6) over the tested 72-hour time course. Statistically significant differences in percentage closure (P < .02) were observed at the 3.13- and 6.25-mg/mL concentrations at 48 hours (0 mg/mL: 0.38% ± 0.08% vs 3.13 mg/mL: 0.11% ± 0.05% and 6.25 mg/mL: 0.03% ± 0.06%) and 72 hours (0 mg/mL: 0.54% ± 0.08% vs 3.13 mg/mL: 0.15% ± 0.07% and 6.25 mg/mL: −0.01% ± 0.04%). The final sealant formulation was then tested (n = 6) wherein the sealant was sprayed to form a confluent layer over a fibroblast monolayer from a height of 4 inches. The extent of wound closure was measured at 0, 24, 48, and 72 hours. Comparison of means between groups was made using the Student t test using the statistical program SigmaStat (SPSS, Inc, an IBM Company, Chicago, Illinois). Inhibition of fibroblast migration and proliferation by the sealant was statistically significant (P < .005) at all measured time points with the maximal effect observed at 72 hours (untreated: 0.58% ± 0.07% vs sealant: −0.04% ± 0.02%) (Figure 4B). Microscopy images are provided at 0 and 72 hours of untreated at 0 (Figure 4C, E) and 72 (Figure 4D, F) hours and treated wounded fibroblast monolayers, respectively, to illustrate the difference in migration and proliferation.
Figure 4.
Human nasal polyp fibroblast migration and proliferation model. (A) Dose-response inhibition of fibroblast migration and proliferation by chitosan. (B) Inhibition of fibroblast migration and proliferation by chitosan-based sealant. Microscopy images at 0 and 72 hours of untreated at 0 (C, E) and 72 (D, F) hours and treated wounded fibroblast monolayers, respectively, show complete inhibition of migration and proliferation in sealant-treated fibroblasts.
Rabbit cecal and sidewall adhesion model
We surgically created cecal abrasions and bilateral sidewall defects in New Zealand White rabbits (n = 20). Nineteen of 20 rabbits survived to the end of the 14-day observation period. One rabbit in the treatment group was euthanized at postoperative day 10 because of a full-thickness chin lesion attributed to irritation from the Elizabethan collar and scored 4 days early. Rabbits were randomized to control (n = 10) or sealant treatment (n = 10). All 10 control animals had adhesion formation present at 1 or both sites (16/20 sites, 80%). One in 10 (10%) sealant-treated rabbits had adhesions, with adhesions present in 1 site only (1/20 sites, 5%). Wound sites were scored by an independent veterinarian for adhesion extent and adhesion strength, and a total adhesion score was calculated. Statistical analyses were performed using the statistical program SigmaStat (SPSS, Inc). A Mann-Whitney rank sum test was performed. Photographs of each sidewall defect were taken (Figure 5). Animals treated with the sealant had significantly decreased adhesion extent, strength, and total adhesion scores (Table 1). Mean total adhesion score was reduced by 94% (95th percentile confidence interval [CI], 78%, 100%; P < .001) with sealant treatment compared with untreated controls (0.3 vs 4.9, respectively).
Figure 5.
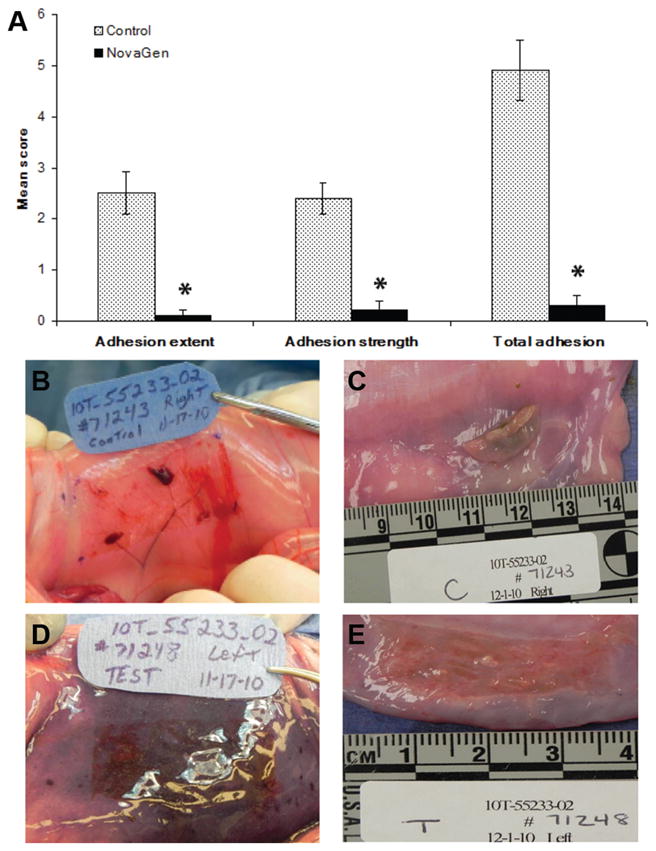
Rabbit cecal and sidewall adhesion model. (A) Sealant treatment of wounds resulted in significantly lower adhesion scores, which reduced the calculated total adhesion score by 94% compared with untreated controls. *P < .001. (B) Control rabbit after wounding. (C) Large adhesion formed 14 days after surgery. (D) Rabbit treated with chitosan-based sealant. (E) Lack of adhesion formation 14 days after surgery.
Table 1.
Sprayable, Chitosan-Based Sealant in Significantly Reducing Adhesion Strength, Adhesion Extent, and Total Adhesion Scores Using a Previously Validated Adhesion Scoring System21
| Parameter | Control (n = 20) | Test (n = 20) |
|---|---|---|
| Adhesion extent | 2.5 ± 1.7 | 0.1 ± 0.4 |
| Adhesion strength | 2.4 ± 1.2 | 0.2 ± 0.7 |
| Total adhesion | 4.9 ± 2.8 | 0.3 ± 1.1 |
Values presented as mean ± SD.
Sheep sinus adhesion model
Adult female sheep (n = 10) underwent bilateral endoscopic sinus surgery with randomized unilateral intraoperative treatment with the sealant, and thus each animal served as its own control. No mortality or morbidity occurred over the course of the study. Endoscopic examinations were performed to assess material presence and adhesion formation at postoperative days 4, 7, 10, and 18. The sealant resided in the sheep sinus cavities between 4 and 10 days. Sinuses were scored as either + or − for adhesions, and statistical analysis via a Fisher exact test was performed using the statistical program SigmaStat. During the 18-day posttreatment period, adhesions were observed in 8 of 10 (80%) untreated sinus cavities and 1 of 10 (10%) sinus cavities treated with the sealant, resulting in a 70% (95th percentile CI, 57%, 93%; P = .003) reduction in adhesion formation (Figure 6).
Figure 6.
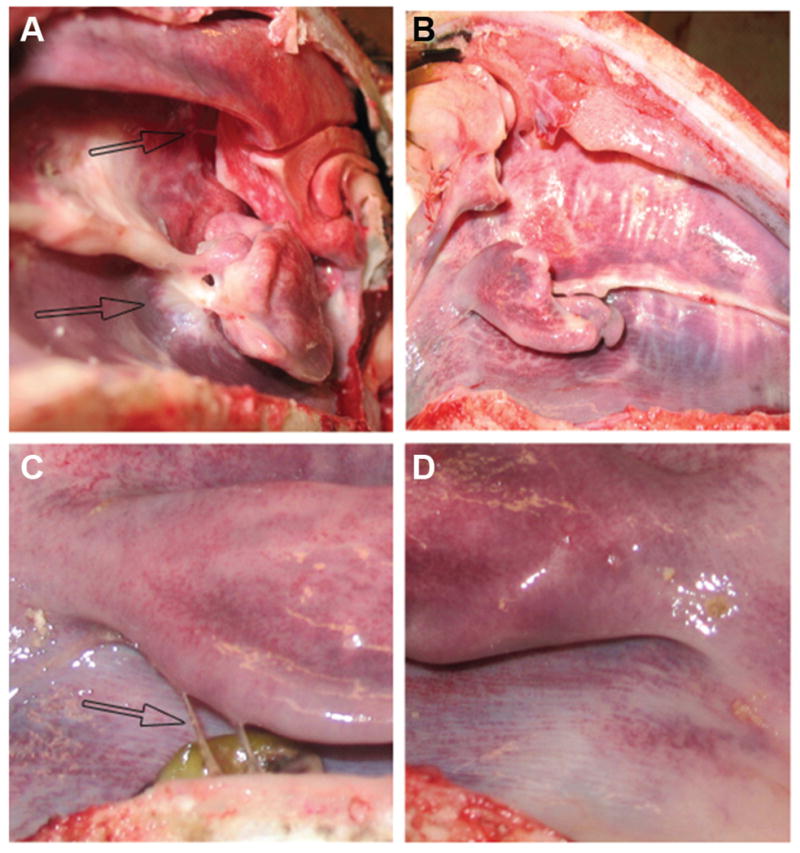
Experimental sheep sinus surgical model. Sealant treatment reduced adhesion formation by 70%. Black arrows indicate adhesions in the untreated sides of the sheep. (A) A control sheep nasal cavity showing large adhesion formation 18 days after surgery. (B) A sheep nasal cavity treated with chitosan-based sealant showing no adhesion formation. (C) Closeup of a nasal cavity showing two adhesions in a control animal 18 days after surgery. (D) Closeup of a nasal cavity treated with chitosan-based sealant showing no adhesion formation.
Discussion
Over the past 2 decades, the volume of endoscopic sinus procedures performed has significantly increased because of advances in endoscopic instrumentation and surgical training. Although these procedures have helped many patients, the long-term failure rate remains greater than 15% in published retrospective reports.3,5 Adhesion formation has been established as a major cause for failure during sinus surgery.6
Many medical devices have been employed in an attempt to reduce adhesion formation, with ineffective results.7 Many materials have successfully obtained hemostasis; however, many of these thrombogenic agents have also often been adhesogenic.16 Chitosan is a linear polysaccharide derived from chitin, a common long-chain polymer found throughout nature.8 Chitosan is biodegradable, nontoxic, and antimicrobial, and, importantly, it dissolves in acidic environments, such as a fresh operative wound site.14 These features of this polysaccharide have led chitosan to become a focus of biomaterial research. Chitosan is now commercially available as medical-grade material. Recent studies have shown the ability of chitosan to reduce adhesion formation in rabbits,17 improve mucosal wound healing following endoscopic sinus surgery in a sheep model of chronic sinusitis,18 and reduce adhesions in a human study.19 We therefore developed a sprayable, chitosan-based sealant with the goal of it being suitable for use as a medical device during human endoscopic sinus surgery.
As an initial investigation of potential efficacy, we tested this sprayable sealant on primary fibroblast cultures isolated from human polyps harvested from sinus surgery. We used a commonly employed scratch-wound model23 to investigate the antiadhesive properties of this sealant. This sealant completely inhibited fibroblast migration and proliferation. Furthermore, the active chitosan compound without the dye or carrier was able to inhibit fibroblast function in a dose-dependent manner, suggesting that the chitosan compound was responsible for the inhibition.
We therefore tested the therapeutic effectiveness of this sealant in 2 clinically relevant animal models. Initially, we used the New Zealand White rabbit cecal/sidewall abrasion model, a well-established model20 previously used to support antiadhesion claims in preclinical effectiveness studies reviewed by the Food and Drug Administration.21 In this model, this sealant gel reduced adhesion strength, extent, and total adhesion scores by 94%.
To better examine clinical relevance, we investigated the usefulness of this sealant as an antiadhesive in a sinus surgical adhesion model22 of 20 sheep. Sheep were selected because of their clinically relevant mammalian physiology, expansive sinus aeration, and nares with adequate diameter to perform intranasal endoscopic sinus surgery in a manner that mimics surgical procedures in humans. This sealant was found to have adequate visualization properties during the application of the material to allow the surgeon to see the areas being coated endoscopically. In addition, this sealant remained in sheep sinus cavities that received daily sinus irrigations between 4 and 10 days, reducing adhesion formation by 70%.
This initial feasibility study has important limitations. Animals were randomized to treatment sides to minimize the possibility of allocation bias. However, because of the purple composition of this sealant and the lack of availability of a similar placebo, neither the investigators nor the outcome assessors were blinded to the treatment group. Although the scoring of adhesions for both the rabbit studies and sheep studies was performed alongside an independent veterinarian, there is a possibility of detection bias for the study. Because of the cost and use of animals, the sample size was kept as small as necessary to detect a significant change, thus limiting the statistical power of this study. To minimize these limitations, the sheep study will be repeated using independent, double-blinded assessment strategies and larger samples of animals per cohort. Despite these limitations of this initial feasibility study, the sheep sinus surgical model closely recapitulates the pathophysiology of adhesion formation following human endoscopic sinus surgery, and the positive results from this study support further investigation of this sealant. Collectively, these data provide evidence of this sealant’s potential ability to reduce postoperative adhesions following human endoscopic sinus surgery.
Acknowledgments
We thank Lauren O. Bakaletz, PhD, D. Bradley Welling, MD, PhD, D. Amery, Bryan Jones, PhD, and D. Oliver for their assistance with editing this manuscript; K. Young and L. Tasse for assistance with the rabbit model; K. Horlen and T. Madsen for assistance with the sheep model; E. Sherman for delivery device design and assembling extended length devices for the sheep model; D. Guenther and K. Veleta for preparing the sealant samples; and M. Myntti and W. Chen for chemistry of final sealant formulation.
Funding source: Medtronic Corporation; NIH KL2RR025754 (Training and Development to Subinoy Das).
Footnotes
Author Contributions
Jennifer G. Medina, designed study, cowrote manuscript; John W. Steinke, performed fibroblast experiments, cowrote manuscript; Subinoy Das, performed sheep surgery, cowrote and supervised writing of manuscript.
Disclosures
Competing interests: Subinoy Das is a consultant for Medtronic Corporation and received hourly compensation for performing sheep surgeries but has no direct financial conflict of interest (COI) in the successful commercialization of this medical device. John W. Steinke is a consultant for Medtronic Corporation and performed fibroblast studies under contract but has no direct financial COI in the successful commercialization of this medical device. Jennifer G. Medina is an employee of Medtronic Corporation–Biomaterials Research Division.
Sponsorships: Medtronic Corporation.
This article was presented at the 2011 AAO-HNSF Annual Meeting & OTO EXPO; September 11–14, 2011; San Francisco, California.
References
- 1.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 2.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat. 2009;10(242):1–157. [PubMed] [Google Scholar]
- 3.Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope. 2010;120:635–638. doi: 10.1002/lary.20777. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;144:440–445. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 5.Senior BA, Kennedy DW, Tanabodee J, Kroger H, Hassab M, Lanza DC. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151–157. doi: 10.1097/00005537-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan HH. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope. 1998;109:27–29. doi: 10.1097/00005537-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Orlandi RR, Lanza DC. Is nasal packing necessary following endoscopic sinus surgery? Laryngoscope. 2004;114:1541–1544. doi: 10.1097/00005537-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Karrer P, Hofmann A. Polysaccharide XXXIX. Über den enzymatischen Abbau von Chitin und Chitosan I. Helv Chim Acta. 1929;12:616–637. [Google Scholar]
- 9.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 10.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 11.Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater. 2004;69B:216–222. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- 12.Azab AK, Doviner V, Orkin B, et al. Biocompatibility evaluation of crosslinked chitosan hydrogels after subcutaneous and intraperitoneal implantation in the rat. J Biomed Mater Res A. 2007;83:414–422. doi: 10.1002/jbm.a.31256. [DOI] [PubMed] [Google Scholar]
- 13.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 14.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 15.Luppi B, Bigucci F, Cerchiara T, Zecchi V. Chitosan-based hydrogels for nasal drug delivery: from inserts to nanoparticles. Expert Opin Drug Deliv. 2010;7:811–828. doi: 10.1517/17425247.2010.495981. [DOI] [PubMed] [Google Scholar]
- 16.Chandra RK, Conley DB, Haines GK, Kern RC. Long-term effects of FloSeal packing after endoscopic sinus surgery. Am J Rhinol. 2005;19:240–243. [PubMed] [Google Scholar]
- 17.Zhou J, Elson C, Lee TD. Reduction in postoperative adhesion formation and re-formation after an abdominal operation with the use of N, O–carboxymethyl chitosan. Surgery. 2004;135:307–312. doi: 10.1016/j.surg.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Athanasiadis T, Beule AG, Robinson BH, Robinson SR, Shi Z, Wormald PJ. Effects of a novel chitosan gel on mucosal wound healing following endoscopic sinus surgery in a sheep model of chronic rhinosinusitis. Laryngoscope. 2008;118:1088–1094. doi: 10.1097/MLG.0b013e31816ba576. [DOI] [PubMed] [Google Scholar]
- 19.Valentine R, Athanasiadis T, Moratti S, Hanton L, Robinson S, Wormald PJ. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24:70–75. doi: 10.2500/ajra.2010.24.3422. [DOI] [PubMed] [Google Scholar]
- 20.Johns DB, Rodgers KE, Donahue WD, Kiorpes TC, diZerega GS. Reduction of adhesion formation by postoperative administration of ionically cross-linked hyaluronic acid. Fertil Steril. 1997;68:37–42. doi: 10.1016/s0015-0282(97)81472-0. [DOI] [PubMed] [Google Scholar]
- 21.Dunn R, Lyman MD, Edelman PG, Campbell PK. Evaluation of the SprayGel™ adhesion barrier in the rat cecum abrasion and rabbit uterine horn adhesion models. Fertil Steril. 2001;75:411–416. doi: 10.1016/s0015-0282(00)01677-0. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh D, Cowin A, Adams D, Rayner T, Wormald PJ. The effect of a dissolvable hyaluronic acid–based pack on the healing of the nasal mucosa of sheep. Am J Rhinol. 2002;16:85–90. [PubMed] [Google Scholar]
- 23.Steinke JW, Crouse CD, Bradley D, et al. Characterization of IL-4 stimulated nasal polyp fibroblasts. Am J Respir Cell Mol Biol. 2004;30:212–219. doi: 10.1165/rcmb.2003-0071OC. [DOI] [PubMed] [Google Scholar]