Abstract
CNS myelination and the maturation of the myelinating cells of the CNS, namely oligodendrocytes, are thought to be regulated by molecular mechanisms controlling the actin cytoskeleton. However, the exact nature of these mechanisms is currently only poorly understood. Here we assessed the role of calcium/calmodulin-dependent kinase type II (CaMKII), in particular CaMKIIβ, in oligodendrocyte maturation and CNS myelination. Using in vitro culture studies, our data demonstrate that CaMKIIβ is critical for the proper morphological maturation of differentiating oligodendrocytes, an aspect of oligodendrocyte maturation that is mediated to a large extent by changes in the cellular cytoskeleton. Furthermore, our data provide evidence for an actin-cytoskeleton-stabilizing role of CaMKIIβ in differentiating oligodendrocytes. Using Camk2b knock-out and Camk2bA303R mutant mice, our data revealed an in vivo functional role of CaMKIIβ in regulating myelin thickness that may be mediated by a non-kinase-catalytic activity. Our data point toward a critical role of CaMKIIβ in regulating oligodendrocyte maturation and CNS myelination via an actin-cytoskeleton-regulatory mechanism.
Introduction
During development, oligodendrocytes, the myelinating cells of the CNS, undergo a lineage progression during which bipolar progenitors give rise to cells with an extended process network that then transition into mature oligodendrocytes, generating the myelin sheath (Pfeiffer et al., 1993; Baumann and Pham-Dinh, 2001). The morphological aspects of this progression are to a large extent regulated by changes in the cellular cytoskeleton (Bauer et al., 2009). However, the exact mechanisms by which the cellular cytoskeleton regulates oligodendrocyte maturation and CNS myelination are currently only poorly understood.
One of the molecular players that emerges as an important regulator of the actin cytoskeleton is calcium/calmodulin-dependent kinase type IIβ (CaMKIIβ). CaMKIIβ belongs to a family of highly conserved serine/threonine kinases, which in mammals is encoded by four different genes, Camk2a, Camk2b, Camk2g, and Camk2d, giving rise to four isozymes, CaMKIIα, CaMKIIβ, CaMKIIγ, and CaMKIIδ (Tombes et al., 2003). Structure–functionally, CaMKII monomers are composed of four domains, a kinase catalytic, an autoinhibitory (regulatory), an association (oligomerization) domain, and a central variable domain that is subject to alternative splicing and located distal to the autoinhibitory domain (Hudmon and Schulman, 2002). Interestingly, CaMKIIβ has been found to also possess a distinctive actin-binding domain (Okamoto et al., 2009) that has been implicated in mediating actin filament stabilization/bundling (Shen et al., 1998; Fink et al., 2003; O'Leary et al., 2006; Okamoto et al., 2007; Lin and Redmond, 2008). Although primarily characterized in neurons, CaMKII genes, including CaMKIIβ, appear to also be expressed by cells of the oligodendrocyte lineage (Cahoy et al., 2008). Therefore, we examined here the role of CaMKII and in particular CaMKIIβ in regulating oligodendrocyte maturation and myelination.
Materials and Methods
Animals.
Sprague Dawley female rats with early postnatal litters were obtained from Harlan Laboratories. Camk2b knock-out (Camk2b−/−) and Camk2bA303R mice, both in the F2 129P2-C57BL/6 background (van Woerden et al., 2009; Borgesius et al., 2011), were generated and bred at Erasmus University Medical Center. Animal studies were approved by the institutional animal care and use committee at Virginia Commonwealth University or a Dutch ethical committee for animal experiments.
Primary oligodendrocyte cultures.
Primary oligodendrocytes were isolated from postnatal day 3 (P3) rat brains by A2B5 immunopanning (Barres et al., 1992) and then cultured in differentiation medium for at least 48 h (Lafrenaye and Fuss, 2010). Under these conditions, the majority of cells represented postmigratory, premyelinating oligodendrocytes because they expressed the O4 antigen (Sommer and Schachner, 1982; Warrington et al., 1993; data not shown).
For CaMKII inhibition experiments, cells were cultured for 44–48 h, followed by incubation with: KN-93 or its inactive analog KN-92 (EMD; Millipore), myristoylated autocamtide-2 related inhibitory (Myr-AIP) or myristoylated control (scrambled AIP sequence) peptide (Enzo Life Sciences), or KN93 or KN92 in combination with jasplakinolide (Enzo Life Sciences).
For siRNA-mediated gene silencing, cells were cultured for 20–24 h and then transfected with siGLO Green transfection indicator along with either an siRNA SMARTpool directed against rat Camk2a, Camk2b, Camk2g, or Camk2d or a control nontargeting siRNA SMARTpool (all from Thermo Fisher Scientific; Lafrenaye and Fuss, 2010).
Oligodendrocyte morphology analysis.
Oligodendrocyte morphology was assessed by determining the process index (total area found to be O4-positive minus the area occupied by the cell body) as described previously (Dennis et al., 2008). For the generation of representative images, confocal laser scanning microscopy was used (LSM 510 META NLO; Carl Zeiss). Images represent 2D maximum projections of stacks of 0.4 μm optical sections.
CaMKIIβ-F-actin colocalization analysis.
Cells of the oligodendroglia cell line CIMO (Bronstein et al., 1998) were nucelofected (Lonza) with a plasmid encoding GFP-CaMKIIβ (Okamoto et al., 2004) and F-actin was visualized using Acti-Stain 555 phalloidin (Cytoskeleton).
PCR and Western blot analysis.
For the determination of alternative splicing profiles, end-point RT-PCR analysis was performed using the following gene-specific primer pairs: Camk2a: Forward: 5′-TGGCCACCAGGAACTTCTCCGGAGG-3′ and Reverse: 5′-TGCGGCAGGACGACGGAGGGCGCCCCAGA-3′); Camk2b: Forward: 5′-CACGGAATTTCTCAGTGGGCAGACAG-3′ and Reverse: 5′-CGCAGCTCTCACTGCAGCGGGGCCAC-3′; Camk2g: Forward: 5′-CGCTCCGGAAAGGGTGCCATCCTCACAACCATGC-3′ and Reverse: 5′-TCCGGAGCGTCTCCTCTGACTGACTGGTGCGAGG-3′; and Camk2d: Forward: 5′-CGCTCCGGAACGAGAAATTTTTCAGCAGCCAAGA-3′ and Reverse: 5′-TCCGGATCCTGGCTTGATGGGGACTGTTGGGGAC-3′.
For the determination of relative mRNA expression levels, qRT-PCR was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad) using the following gene-specific primer pairs: Camk2a: Forward: 5′-ACGGAAGAGTACCAGCTCTTCGAGG-3′ and Reverse: 5′-CCTGGCCAGCCAGCACCTTCAC-3′; Camk2b: Forward: 5′-GTCGTCCACAGAGACCTCAAG-3′ and Reverse: 5′-CCAGATATCCACTGGTTTGC-3′; Camk2g: Forward: 5′-ACGCAAGTTCAACGCCCGGAGAA-3′ and Reverse: 5′-AGGCTCTTGGCAGCTTGCCCG-3′; Camk2d: Forward: 5′-TGCCGTCTCTGAAGCACCCCA-3′ and Reverse: 5′-ACCAAGTAATGGAAGCCCTCTTCGG-3′; Mpb (exon 2 containing isoforms): Forward: 5′-ACTTGGCCACAGCAAGTACCATGGACC-3′ and Reverse: 5′-TTGTACATGTGGCACAGCCCGGGAC-3′; Mpb (all isoforms): Forward: 5′-GTGACACCTCGTACACCCCCTCCAT-3′ and Reverse: 5′-GCTAAATCTGCTGAGGGACAGGCCT-3′; Plp: Forward: 5′-CCACACTAGTTTCCCTGCTCACCT-3′ and Reverse: 5′-GGTGCCTCGGCCCATGAGTT-3′; Cyclophilin (as reference gene): Forward: 5′-GGAGACGAACCTGTAGGACG-3′ and Reverse: 5′-GATGCTCTTTCCTCCTGTGC-3′.
For comparing the expression levels of the different Camk2 genes, R0 values were determined as described by Peirson et al. (2003). To determine relative expression levels, the ΔΔCT method was used (Livak and Schmittgen, 2001).
For Western blot analysis, anti-CaMKIIβ (Life Technologies) and anti-GAPDH antibodies (Millipore) were used. Bound antibodies were detected using enhanced chemiluminescence in combination with VersaDoc imaging (Bio-Rad).
Electron microscopic analysis.
Spinal cord tissue was prepared and analyzed by electron microscopy as described previously (Dupree et al., 1998; Marcus et al., 2006; Forrest et al., 2009). Numbers of axons were determined manually per field of view (14.6 μm2). G-ratios were determined as described previously (Dupree et al., 1998; Marcus et al., 2006; Forrest et al., 2009).
Results
Camk2b is the predominant Camk2 gene expressed by differentiating oligodendrocytes
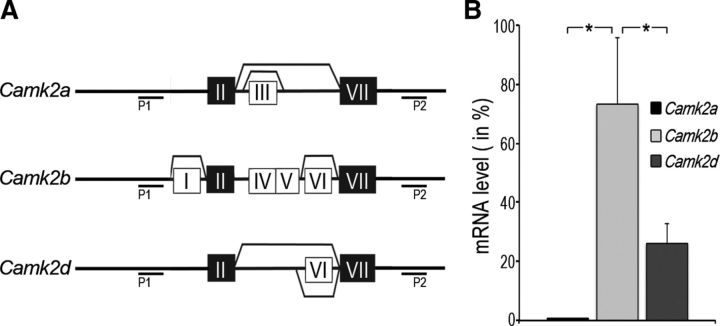
To determine the extent and alternative splicing pattern of Camk2 gene expression in differentiating oligodendrocytes, end-point RT-PCR analysis was performed using gene-specific primer pairs spanning the variable domain (Hudmon and Schulman, 2002; Tombes et al., 2003; Fig. 1A). Sequence analysis of the resulting amplification products revealed the expression of Camk2a, Camk2b, and Camk2d, but not Camk2g. All of the three oligodendrocyte-derived genes were found to give rise to multiple alternatively spliced isoforms in a gene-specific fashion. Interestingly, the majority of Camk2b isoforms contained the alternatively spliced exon I of the variable domain, which has been implicated in conferring actin-binding/stabilizing properties to CaMKIIβ (O'Leary et al., 2006).
Figure 1.
Camk2b is the predominantly expressed Camk2 gene in differentiating oligodendrocytes. A, Alternative splicing profile of oligodendrocyte-derived Camk2 genes as determined by end-point RT-PCR analysis. Conserved, nonalternatively spliced “linker” exons within the variable region are depicted as black boxes labeled with the Roman numerals II and VII. Alternatively, spliced exons are depicted as white boxes labeled with Roman numerals. Lines indicate alternative splicing events. P1 and P2 indicate the locations of the two primers used for RT-PCR amplification. B, Camk2 mRNA expression levels as determined by qRT-PCR analysis. For the bar graph, total Camk2 mRNA levels were set to 100% and the values for each of the three genes were adjusted accordingly. Data represent means ± SEM (n = 3 independent experiments, *p < 0.05, Student's t test).
To determine the quantitative contribution of each of the three oligodendrocyte-derived Camk2 genes to overall Camk2 expression, qRT-PCR was performed using primer pairs located outside of the variable region and not affected by alternative splicing. This analysis revealed a quantitative expression of Camk2b > Camk2d > Camk2a (Fig. 1B).
Inhibition of CaMKII activity restrains the morphological maturation of differentiating oligodendrocytes
The above expression analysis suggested that CaMKII, and in particular CaMKIIβ, may play an important functional role in differentiating oligodendrocytes. To assess such a potential role of CaMKII, differentiating oligodendrocytes were treated with KN-93, a membrane-permeable pharmacological inhibitor of CaMKII activity, or its inactive derivative KN-92, and process morphology as a measure for oligodendrocyte maturation was determined (Dennis et al., 2008). Treatment with KN-93 caused a decreased process index (Fig. 2A,B) at 10 μm but not 1 μm. Such concentration dependency is in agreement with a half-maximal inhibition of CaMKII at a KN-93 concentration of ∼12 μm (Tombes et al., 1995). In addition, cells were treated with the membrane-permeable myristoylated-autocamtide-2-related inhibitory peptide, which mimics the CaMKII autoinhibitory domain (Ishida et al., 1995) and blocks activity at concentrations similar to KN-93 (Easley et al., 2006; Easley et al., 2008). Such treatment resulted similar to the KN-93 treatment in a decreased process index (Fig. 2C). CaMKII inhibition was not found to be associated with a change in cell viability (KN-92: 100 ± 9%; KN-93: 96 ± 12%).
Figure 2.
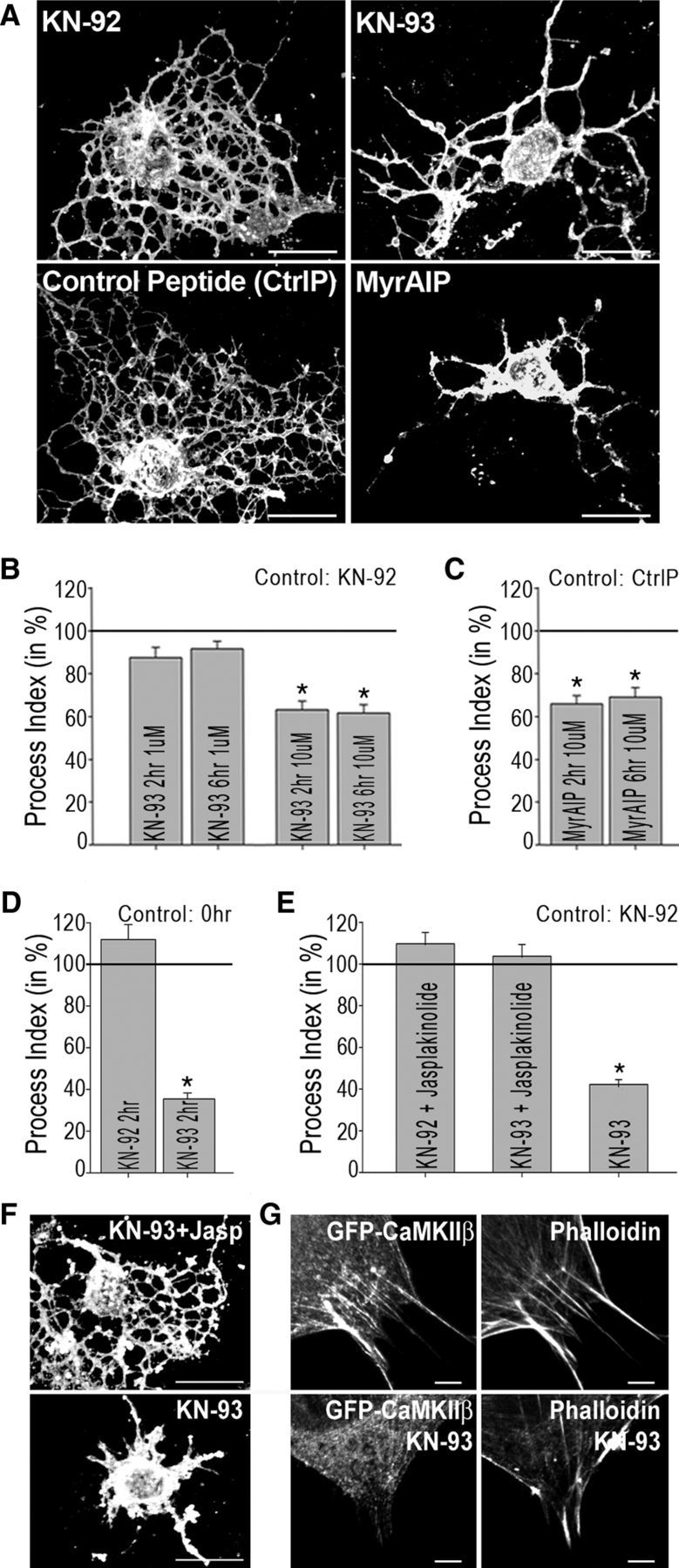
Inhibition of CaMKII activity in differentiating oligodendrocytes restrains the establishment of an expanded process network. A, F, Representative images of differentiating oligodendrocytes immunostained with the O4 antibody and treated for 6 h as indicated. Scale bars, 20 μm. B–E, Bar graphs representing quantitative analyses of process indices as described by Dennis et al. (2008). Cells in B, D, E were treated with the pharmacological CaMKII inhibitor KN-93 or its inactive derivative KN-92 as control, and cells in C were treated with the myristoylated autoinhibitory CaMKII peptide (Myr-AIP) or a myristoylated control peptide (CtrlP). Cells in E were cotreated with the actin stabilizing peptide jasplakinolide (10 μm) where noted and analyzed after 6 h of treatment. Otherwise, final concentrations and duration of treatments are indicated within the bar graphs. In B, C, E, experimental conditions were compared with control-treated cells cultured for an equivalent period of time. In D, experimental conditions were compared with control-treated cells at time-point 0. For all bar graphs, the mean values for control cells were set to 100% (horizontal gray line) and experimental values were calculated accordingly. At least 25 cells per condition and experiment were analyzed in three independent experiments (i.e., a total of at least 75 cells per condition). Data represent experimental means ± SEM (*p < 0.05, Student's t test). G, Representative images of CIMO cells transfected with a plasmid encoding GFP-CaMKIIβ and stained for F-actin (phalloidin). Scale bars, 5 μm.
Morphological maturation of oligodendrocytes occurs as a dynamic process that is characterized by process extension and retraction events (Kachar et al., 1986; Fox et al., 2006). As shown in Figure 2D, process indices were found to be significantly decreased 2 h after initial KN-93 treatment compared with the process indices found at the beginning of the treatment. Therefore, the decreased morphological maturation seen in response to CaMKII inhibition is likely due to an increase in process retraction events rather than an inhibition of process outgrowth.
Retraction of cellular processes has been associated with destabilization of the actin cytoskeleton (Easley et al., 2006). KN-93 has been well described to inhibit not only CaMKII's kinase catalytic activity, but also CaMKIIβ's actin-binding/stabilizing activity (Sumi et al., 1991; Lin and Redmond, 2008). Accordingly, a marked reduction in F-actin-CaMKIIβ co-localization was noted upon KN-93 treatment (Fig. 2G). Furthermore, and in support of an actin-destabilizing effect of KN-93 treatment in differentiating oligodendrocytes, cotreatment with jasplakinolide, which specifically and rapidly blocks actin filament disassembly (Boggs and Wang, 2004), abolished the effect of KN-93 on the oligodendrocyte's process network (Fig. 2E,F). No evidence for a change in cellular viability was noted.
Downregulation of Camk2b expression restrains the morphological maturation of differentiating oligodendrocytes
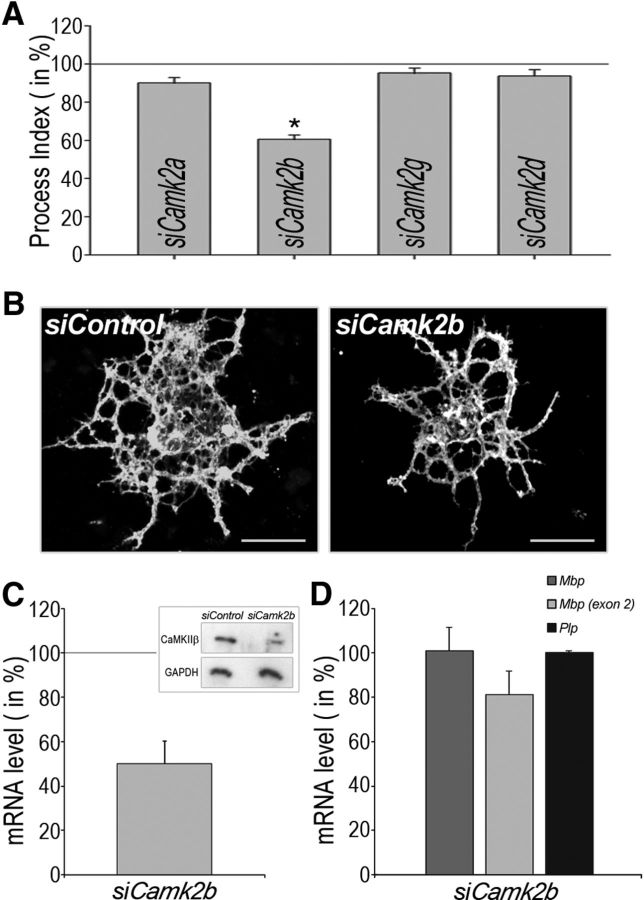
To determine the extent to which CaMKIIβ may be involved specifically in regulating the morphology of the oligodendrocyte's process network, an siRNA-mediated gene silencing approach was used. As shown in Figure 3A, B, treatment with an siRNA pool to Camk2b led to a significantly decreased process index. Under the conditions used, siRNA treatment resulted in significantly reduced mRNA levels for Camk2b (Fig. 3C), Camk2a (60 ± 4%), and Camk2d (74 ± 9%). Use of an siRNA pool to Camk2g served as a control because expression of Camk2g was undetectable in our original analysis (Fig. 1A). For cells treated with the siRNA pool to Camk2b, a reduction in CaMKIIβ protein levels could also be confirmed (Fig. 3C, inset). In neither case was the gene-specific downregulation of Camk2 expression associated with an increase in mRNA levels for any of the other Camk2 genes (data not shown).
Figure 3.
Knock-down of Camk2b expression in differentiating oligodendrocytes restrains the establishment of an expanded process network. A, Bar graph representing quantitative analyses of process indices (Dennis et al., 2008) upon siRNA-mediated knock-down of individual Camk2 genes as indicated. The mean value for cells treated with the control siRNA pool was set to 100% (horizontal gray line) and experimental values were calculated accordingly. At least 25 cells per condition and experiment were analyzed in four independent experiments (i.e., a total of at least 100 cells per condition). Data represent experimental means ± SEM (*p < 0.05, Student's t test). B, Representative images of differentiating oligodendrocytes immunostained with the O4 antibody and treated with a control (siControl) or Camk2b-specific (siCamk2b) siRNA pool. Scale bars, 20 μm. C, Bar graph depicting the Camk2b mRNA level upon siRNA-mediated knock-down of Camk2b. The mean value for cells treated with the control siRNA pool was set to 100% (horizontal gray line) and the experimental value was calculated accordingly. The experimental mean ± SEM (*p < 0.05, one sample t test) is shown. The inset depicts a representative Western blot. CaMKIIβ and GAPDH protein levels are shown for cells treated with a control (siControl) or Camk2b-specific (siCamk2b) siRNA pool. D, Bar graph depicting Mbp (total and exon 2 containing) and Plp mRNA levels upon siRNA-mediated knock-down of Camk2b. The mean value for cells treated with the control siRNA pool was set to 100% (horizontal gray line) and experimental values were calculated accordingly. Data represent experimental means ± SEM (*p < 0.05, one sample t test).
In vivo, morphological maturation of oligodendrocytes is associated with well described changes in gene expression (Pfeiffer et al., 1993; Baumann and Pham-Dinh, 2001; Emery, 2010). Under experimental conditions, however, molecular mechanisms regulating cellular morphology may be uncoupled from those that regulate gene expression (Osterhout et al., 1999; Buttery and ffrench-Constant, 1999; Kim et al., 2006; Lafrenaye and Fuss, 2010). To investigate a potential role of Camk2b in regulating gene expression in differentiating oligodendrocytes, expression levels for mRNAs encoding the major myelin genes myelin basic protein (Mbp) and proteolipid protein (Plp) (Fulton et al., 2010) were determined. No significant differences were noted (Fig. 3D). In addition, no difference was noted in the percentage of O4-positive cells that were also immunopositive for MBP (siControl 52 ± 4%, siCamk2b 49 ± 2%).
Systemic knock-out of Camk2b leads to significantly reduced myelination
To determine the extent to which Camk2b may regulate developmental myelination in vivo, ventral spinal cords of Camk2b−/− mice (van Woerden et al., 2009) were analyzed. As shown in Figure 4A–C, the myelin sheath g-ratio (axon diameter divided by the diameter of the entire myelinated fiber) was significantly increased at P21 in Camk2b−/− spinal cords. This effect on myelin thickness persisted up to at least 58 d of age (Fig. 4D,E) and was not associated with significant changes in the number of myelinated axons (P21: wild-type [WT] 39 ± 2/14.6 μm2, Camk2b−/− 41 ± 2/14.6 μm2; P58: WT 35 ± 2/14.6 μm2, Camk2b−/− 35 ± 1/14.6 μm2) or apparent signs of axonal damage (Fig. 4A). In addition, no significant changes in the number of oligodendrocytes were noted (P21: WT 100 ± 6%, Camk2b−/− 112 ± 5%).
Figure 4.
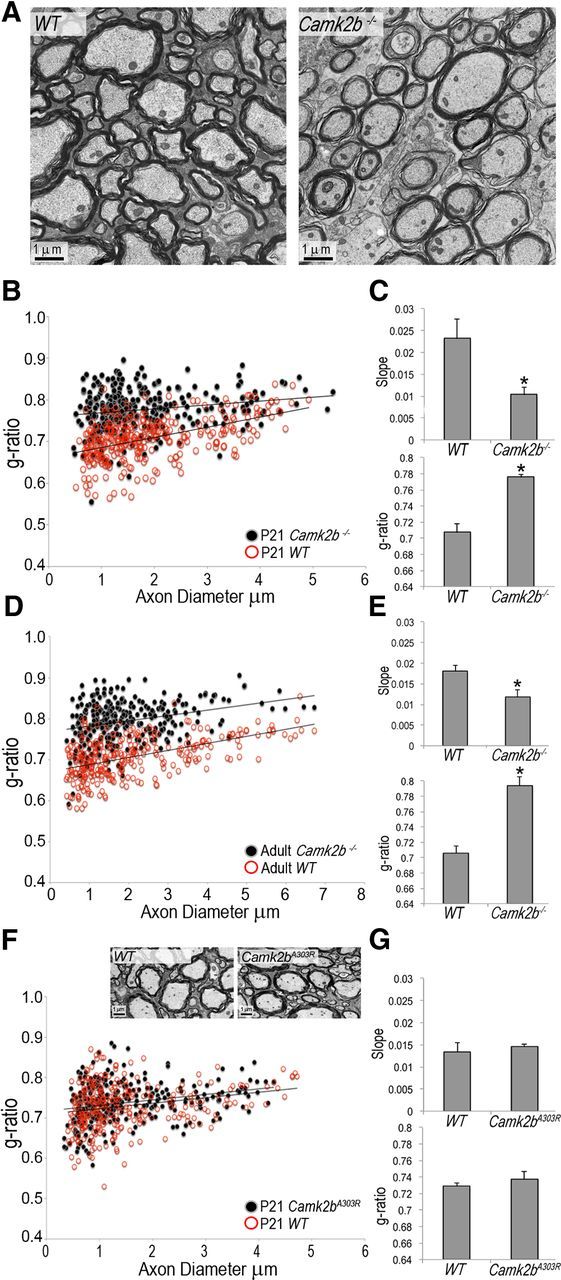
Knock-out of Camk2b leads to an increase in the g-ratio (decrease in the thickness) of the myelin sheath, whereas myelination appears to be unaffected in Camk2A303R mutant mice. A and inset in F, Representative electron micrographs of the ventral spinal cord of 21-d-old (P21) WT and Camk2b−/− mice in A or Camk2A303R mutant mice in F. Scale bars, 1 μm. B, D, F, Scatter plots depicting g-ratios versus axon diameters for P21 Camk2b−/− (B), adult Camk2b−/− (D), or P21 Camk2A303R (F; black filled circles) and WT littermate (red open circles) ventral spinal cords. The lines represent linear fits to pooled data from all mice for each genotype. One hundred axons per animal were measured and three animals per genotype were analyzed. C, E, G, Bar graphs depicting average slopes (g-ratio versus axon diameter) and average g-ratios from individual animals (n = 3). Asterisks indicate statistically significant differences between WT and knock-out/mutant mice (*p < 0.05, Student's t test).
To assess whether the mechanism by which CaMKIIβ regulates oligodendrocyte maturation and CNS myelination may be mediated by a nonenzymatic activity, developmental myelination was assessed in Camk2bA303R mutant mice. In these mice, the WT Camk2b gene has been replaced by the mutated Camk2bA303R gene (Borgesius et al., 2011). This mutation has been characterized to lead to a loss of calcium/calmodulin binding and kinase catalytic activation, but to preserve the ability of CaMKIIβ to bind to and bundle/stabilize actin filaments (Shen and Meyer, 1999; Fink et al., 2003; O'Leary et al., 2006; Lin and Redmond, 2008). As shown in Figure 4, F and G, Camk2bA303R mutant mice were devoid of the deficits in myelin thickness seen in Camk2b−/− mice.
Discussion
Using in vitro tissue culture as well as in vivo knock-out and knock-in strategies, we identified CaMKIIβ as a critical component of the molecular mechanism regulating oligodendrocyte maturation and CNS myelination. More specifically, our data point toward a role of CaMKIIβ in regulating the oligodendrocyte's actin cytoskeleton via a mechanism that may not require its kinase catalytic activity, but may instead involve its actin-binding/stabilizing activity.
Our in vivo analysis of developmental myelination demonstrates that CaMKIIβ is involved in the regulation of myelin thickness. Together with our in vitro tissue culture studies, we propose that this regulatory role of CaMKIIβ is at least in part mediated by an oligodendrocyte-autonomous mechanism. In support of this idea, astrocytes are considered to not express considerable levels of Camk2b (Takeuchi et al., 2000; Vallano et al., 2000). In addition, CaMKIIβ protein levels in axons located within the ventral spinal cord have been described to be undetectable or very low (Terashima et al., 1994). Therefore, systemic Camk2b knock-out is unlikely to cause a predominantly axon-mediated effect on myelination within the CNS region investigated here.
The lack of a myelination deficit in the spinal cord of Camk2bA303R mutant mice strengthens the idea of a functional role of CaMKIIβ as an actin regulatory protein via its actin-binding activity. In neuronal dendritic spines, CaMKIIβ, via its actin-binding activity, is thought to stabilize the actin cytoskeleton and thus overall spine shape. At the same time, however, calcium signaling has been implicated in promoting the release of CaMKIIβ from the actin cytoskeleton, thereby opening a time window during which actin cytoskeleton remodeling events are favored (Okamoto et al., 2007; Okamoto et al., 2009). In analogy, oligodendrocyte maturation and CNS myelination may be regulated by CaMKIIβ-mediated alternating cycles of actin cytoskeleton stabilization and destabilization/remodeling. Because CaMKIIβ-mediated regulation is dependent on calcium-signaling events, it is worth mentioning that an increase in calcium signaling has been reported to stimulate oligodendrocyte process outgrowth and thus morphological maturation (Yoo et al., 1999). Furthermore, it has been shown recently that balanced activation and deactivation of the actin filament severing and depolymerizing factor cofilin regulates Schwann cell function during peripheral nervous system myelination (Sparrow et al., 2012). This finding supports the idea that efficient myelination may be critically dependent on a well balanced equilibrium between dynamic remodeling and kinetic stability of the actin cytoskeleton. Future studies will be necessary to better define the role of CaMKIIβ in regulating the actin cytoskeleton during oligodendrocyte maturation and CNS myelination.
Footnotes
This work was supported by the National Institutes of Health–National Institute of Neurological Disorders and Stroke, the National Multiple Sclerosis Society, and the European Leukodystrophies Association. Microscopy was performed at the Virginia Commonwealth University Department of Anatomy and Neurobiology Microscopy Facility, which is supported in part with funding from the National Institutes of Health–National Institute of Neurological Disorders and Stroke Center Core Grant #5 P30 NS047463. We thank Steve Pfeiffer and Yasunori Hayashi for providing the O4 hybridoma cells and the plasmid encoding GFP-CaMKIIβ, respectively, and Robert Tombes for stimulating discussions and critically reading the manuscript.
References
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-G. [DOI] [PubMed] [Google Scholar]
- Bauer NG, Richter-Landsberg C, ffrench-Constant C. Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia. 2009;57:1691–1705. doi: 10.1002/glia.20885. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Boggs JM, Wang H. Co-clustering of galactosylceramide and membrane proteins in oligodendrocyte membranes on interaction with polyvalent carbohydrate and prevention by an intact cytoskeleton. J Neurosci Res. 2004;76:342–355. doi: 10.1002/jnr.20080. [DOI] [PubMed] [Google Scholar]
- Borgesius NZ, van Woerden GM, Buitendijk GH, Keijzer N, Jaarsma D, Hoogenraad CC, Elgersma Y. betaCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting alphaCaMKII to synapses. J Neurosci. 2011;31:10141–10148. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Hales TG, Tyndale RF, Charles AC. A conditionally immortalized glial cell line that expresses mature myelin proteins and functional GABA(A) receptors. J Neurochem. 1998;70:483–491. doi: 10.1046/j.1471-4159.1998.70020483.x. [DOI] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J, White MA, Forrest AD, Yuelling LM, Nogaroli L, Afshari FS, Fox MA, Fuss B. Phosphodiesterase-Ialpha/autotaxin's MORFO domain regulates oligodendroglial process network formation and focal adhesion organization. Mol Cell Neurosci. 2008;37:412–424. doi: 10.1016/j.mcn.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree JL, Coetzee T, Suzuki K, Popko B. Myelin abnormalities in mice deficient in galactocerebroside and sulfatide. J Neurocytol. 1998;27:649–659. doi: 10.1023/A:1006908013972. [DOI] [PubMed] [Google Scholar]
- Easley CA, Faison MO, Kirsch TL, Lee JA, Seward ME, Tombes RM. Laminin activates CaMK-II to stabilize nascent embryonic axons. Brain Res. 2006;1092:59–68. doi: 10.1016/j.brainres.2006.03.099. [DOI] [PubMed] [Google Scholar]
- Easley CA, 4th, Brown CM, Horwitz AF, Tombes RM. CaMK-II promotes focal adhesion turnover and cell motility by inducing tyrosine dephosphorylation of FAK and paxillin. Cell Motil Cytoskeleton. 2008;65:662–674. doi: 10.1002/cm.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/S0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Forrest AD, Beggs HE, Reichardt LF, Dupree JL, Colello RJ, Fuss B. Focal adhesion kinase (FAK): A regulator of CNS myelination. J Neurosci Res. 2009;87:3456–3464. doi: 10.1002/jnr.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Afshari FS, Alexander JK, Colello RJ, Fuss B. Growth conelike sensorimotor structures are characteristic features of postmigratory, premyelinating oligodendrocytes. Glia. 2006;53:563–566. doi: 10.1002/glia.20293. [DOI] [PubMed] [Google Scholar]
- Fulton D, Paez PM, Campagnoni AT. The multiple roles of myelin protein genes during the development of the oligodendrocyte. ASN Neuro. 2010;2:e00027. doi: 10.1042/AN20090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Kachar B, Behar T, Dubois-Dalcq M. Cell shape and motility of oligodendrocytes cultured without neurons. Cell Tissue Res. 1986;244:27–38. doi: 10.1007/BF00218378. [DOI] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenaye AD, Fuss B. Focal adhesion kinase can play unique and opposing roles in regulating the morphology of differentiating oligodendrocytes. J Neurochem. 2010;115:269–282. doi: 10.1111/j.1471-4159.2010.06926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci U S A. 2008;105:15791–15796. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53:372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- O'Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-K. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/S0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Cell that are O4 antigen-positive and O1 antigen-negative differentiate into O1 antigen-positive oligodendrocytes. Neurosci Lett. 1982;29:183–188. doi: 10.1016/0304-3940(82)90351-2. [DOI] [PubMed] [Google Scholar]
- Sparrow N, Manetti ME, Bott M, Fabianac T, Petrilli A, Bates ML, Bunge MB, Lambert S, Fernandez-Valle C. The actin-severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination. J Neurosci. 2012;32:5284–5297. doi: 10.1523/JNEUROSCI.6207-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291X(91)92031-E. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Yamamoto H, Fukunaga K, Miyakawa T, Miyamoto E. Identification of the isoforms of Ca(2+)/calmodulin-dependent protein kinase II in rat astrocytes and their subcellular localization. J Neurochem. 2000;74:2557–2567. doi: 10.1046/j.1471-4159.2000.0742557.x. [DOI] [PubMed] [Google Scholar]
- Terashima T, Ochiishi T, Yamauchi T. Immunohistochemical detection of calcium/calmodulin-dependent protein kinase II in the spinal cord of the rat and monkey with special reference to the corticospinal tract. J Comp Neurol. 1994;340:469–479. doi: 10.1002/cne.903400403. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Grant S, Westin EH, Krystal G. G1 cell cycle arrest and apoptosis are induced in NIH 3T3 cells by KN-93, an inhibitor of CaMK-II (the multifunctional Ca2+/CaM kinase) Cell Growth Differ. 1995;6:1063–1070. [PubMed] [Google Scholar]
- Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Vallano ML, Beaman-Hall CM, Mathur A, Chen Q. Astrocytes express specific variants of CaM KII delta and gamma, but not alpha and beta, that determine their cellular localizations. Glia. 2000;30:154–164. doi: 10.1002/(SICI)1098-1136(200004)30:2<154::AID-GLIA5>3.0.CO%3B2-S. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Hoebeek FE, Gao Z, Nagaraja RY, Hoogenraad CC, Kushner SA, Hansel C, De Zeeuw CI, Elgersma Y. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci. 2009;12:823–825. doi: 10.1038/nn.2329. [DOI] [PubMed] [Google Scholar]
- Warrington AE, Barbarese E, Pfeiffer SE. Differential myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. J Neurosci Res. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Krieger C, Kim SU. Process extension and intracellular Ca2+ in cultured murine oligodendrocytes. Brain Res. 1999;827:19–27. doi: 10.1016/S0006-8993(99)01282-2. [DOI] [PubMed] [Google Scholar]