Abstract
Mood disorders cause much suffering and lost productivity worldwide, compounded by the fact that many patients are not effectively treated by currently available medications. The most commonly prescribed antidepressant drugs are the selective serotonin (5-HT) reuptake inhibitors (SSRIs), which act by blocking the high-affinity 5-HT transporter (SERT). The increase in extracellular 5-HT produced by SSRIs is thought to be critical to initiate downstream events needed for therapeutic effects. A potential explanation for their limited therapeutic efficacy is the recently characterized presence of low-affinity, high-capacity transporters for 5-HT in brain [i.e., organic cation transporters (OCTs) and plasma membrane monoamine transporter], which may limit the ability of SSRIs to increase extracellular 5-HT. Decynium-22 (D-22) is a blocker of these transporters, and using this compound we uncovered a significant role for OCTs in 5-HT uptake in mice genetically modified to have reduced or no SERT expression (Baganz et al., 2008). This raised the possibility that pharmacological inactivation of D-22-sensitive transporters might enhance the neurochemical and behavioral effects of SSRIs. Here we show that in wild-type mice D-22 enhances the effects of the SSRI fluvoxamine to inhibit 5-HT clearance and to produce antidepressant-like activity. This antidepressant-like activity of D-22 was attenuated in OCT3 KO mice, whereas the effect of D-22 to inhibit 5-HT clearance in the CA3 region of hippocampus persisted. Our findings point to OCT3, as well as other D-22-sensitive transporters, as novel targets for new antidepressant drugs with improved therapeutic potential.
Introduction
Dysfunction of the serotonin (5-HT) system is thought to underlie many affective disorders, primary among them, depression (Charney et al., 1981). The most commonly prescribed antidepressant medications are selective serotonin reuptake inhibitors (SSRIs). SSRIs act to block activity of the high-affinity serotonin transporter (SERT) and prevent the uptake of 5-HT from extracellular fluid into nerve terminals (Blakely et al., 1998). The ensuing increase in extracellular 5-HT is considered to be an important element in triggering the downstream events needed to produce therapeutic effects. However, a major problem in the treatment of depression is that many patients experience modest or no therapeutic benefit (Kirsch et al., 2008), indicating the need for alternative approaches to treat individuals who respond poorly to SSRIs.
Organic cation transporters (i.e., OCT1, OCT2, and OCT3) and the plasma membrane monoamine transporter (PMAT) are low-affinity transporters for 5-HT, but unlike SERT, have a high capacity to transport 5-HT (Gründemann et al., 1998; Engel et al., 2004; Amphoux et al., 2006; Koepsell et al., 2007; Baganz et al., 2008; Duan and Wang, 2010). Decynium-22 (D-22) (originally described by Schömig et al., 1993), is a blocker of OCTs and PMAT, which inhibits 5-HT uptake into cell lines expressing OCT1, OCT2, OCT3, or PMAT as well as 5-HT uptake into brain synaptosomes (Engel et al., 2004; Engel and Wang, 2005; Gasser et al., 2006; Schömig et al., 2006; Duan and Wang, 2010; Hagan et al., 2011). These reports raise the possibility that low-affinity, high-capacity D-22-sensitive transporters for 5-HT may account, at least in part, for the poor clinical efficacy of SSRIs by preventing extracellular 5-HT levels reaching those required to trigger the cascade of downstream events needed for therapeutic benefit (Daws et al., 2013).
Support for this idea comes from studies in mice genetically modified to lack SERT or to express half as many SERTs as wild-type mice. In these SERT mutant mice, administration of D-22 inhibits 5-HT clearance from extracellular fluid in hippocampus and produces antidepressant-like effects (Baganz et al., 2008). In contrast, D-22 does not produce these effects in wild-type mice, perhaps not surprisingly, since these mice have a full complement of functioning SERT controlling 5-HT uptake. Thus it appears that D-22-sensitive uptake mechanisms become more important in regulating 5-HT uptake and behavior when SERT function is genetically inactivated or impaired (Baganz et al., 2008). This raises the possibility that D-22-sensitive transporters for 5-HT may also play a more prominent role in 5-HT clearance when the high-affinity SERT is pharmacologically inactivated. If so, by limiting the increase of 5-HT in extracellular fluid that follows treatment with an SSRI, D-22-sensitive transporters could provide a mechanistic basis for poor therapeutic outcome in many patients. Thus, blockade of D-22-sensitive transporters might be a novel way to increase the therapeutic efficacy of currently available antidepressant drugs. Here we report that D-22 augments the ability of the SSRI, fluvoxamine, to inhibit 5-HT uptake and to produce antidepressant-like effects, providing support for D-22-sensitive transporters as novel targets for new antidepressant drugs.
Materials and Methods
Animals.
Adult (>60-d-old; 25–30 g) male C57BL/6 mice, or OCT3 knock-out (KO) mice (bred on a C57BL/6 background, originally developed by Zwart et al. (2001) and generously provided to us by Drs. Kim Tieu, Bruno Giros, and Sophie Gautron) were used for all experiments. All animal procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All measures were taken to limit the number of animals used and to minimize animal discomfort.
High-speed chronoamperometry.
In vivo chronoamperometry was performed according to methods described in detail previously (Daws and Toney, 2007; Baganz et al., 2008). We assemble our own carbon fiber electrodes and a detailed description can be found in Williams et al. (2007). Recording electrode/micropipette assemblies were constructed using a single carbon fiber (30 μm diameter; Specialty Materials), which was sealed inside fused silica tubing (Schott, North America). We based our procedure for electrode construction on modifications of published methods (Gerhardt, 1995; Perez and Andrews, 2005). Carbon fiber electrodes were coated with Nafion (5% solution; Aldrich Chemical), to prevent interference from anionic substances in extracellular fluid as previously described (Daws and Toney, 2007). Electrodes were tested for sensitivity to the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA; 250 μm) and calibrated with accumulating concentrations of 5-HT (0–3 μm). Only electrodes displaying a selectivity ratio for 5-HT over 5-HIAA greater than 500:1 and a linear response (r2 ≥ 0.9) to 5-HT were used.
The electrochemical recording assembly consisted of a Nafion-coated, single carbon fiber electrode attached to a four-barreled micropipette such that their tips were separated by ∼200 μm. Barrels were filled with combinations of either 5-HT (200 μm), D-22 (10 μm), fluvoxamine (400 μm), a combination of D-22 and fluvoxamine, or phosphate-buffered saline (PBS). We elected to use fluvoxamine primarily to allow direct comparison with over a decade of chronoamperometry data reported in the literature, including those from our own lab, as well as others who have used fluvoxamine routinely (Daws et al., 1998; Benmansour et al., 1999, 2002, 2012; Montañez et al., 2003; Daws et al., 2005; Baganz et al., 2008). All compounds were prepared in 0.1 m PBS with 100 μm ascorbic acid added as an antioxidant and the pH adjusted to 7.4. The electrode-micropipette recording assembly was lowered into the CA3 region of the dorsal hippocampus (anteroposterior, −1.94 from bregma; mediolateral, +2.0 from midline; dorsoventral, −2.0 from dura) (Franklin and Paxinos, 1997) of anesthetized mice. For all experiments, mice were anesthetized by intraperitoneal injection (2 ml/kg body weight) of a mixture of chloralose (35 mg/kg) and urethane (350 mg/kg). A tube was inserted into the trachea to facilitate breathing and mice were then placed into a stereotaxic frame. Body temperature was maintained at 36−37°C by a water circulated heating pad.
High-speed chronoamperometric recordings were made using the FAST-12 and FAST-16 systems (Quanteon). Oxidation potentials consisted of 100 ms pulses of +0.55 V. Each pulse was separated by a 900 ms interval during which the resting potential was maintained at 0.0 V. Voltage at the active electrode was applied with respect to an Ag/AgCl reference electrode positioned in the extracellular fluid of the ipsilateral superficial cortex. The oxidation and reduction currents were digitally integrated during the last 80 ms of each 100 ms voltage pulse.
At the conclusion of the experiment, an electrolytic lesion was made to mark the placement of the electrode tip. The brain was removed, rapidly frozen on dry ice, and stored at −80°C until use. At this time brains were thawed to −15°C and sliced into 20-μm-thick sections for histological verification of electrode localization. Only data from mice in which the electrode was confirmed to be in the CA3 region of the hippocampus were included in the analyses.
Effects of local and systemic drug administration on 5-HT clearance.
Exogenous 5-HT was applied into the CA3 region of the hippocampus by pressure ejection (5–25 psi for 0.25–3.0 s). Advantages of this approach are that clearance can be measured without an associated “release” component and that measurements can be made in vivo with excellent temporal (milliseconds) resolution. Serotonin was pressure ejected into hippocampus to achieve concentrations at the recording electrode of ∼0.5 μm. Once reproducible 5-HT electrochemical signals were obtained, drug or vehicle was applied either locally into the CA3 region of hippocampus, or intraperitoneally, before the next application of 5-HT. For locally administered drugs, 5-HT was pressure ejected 2 min after drug or vehicle to allow sufficient time for drugs to diffuse to the recording site. Serotonin was applied again at 10 min intervals after drug or vehicle until the signal returned to predrug status. The 10 min time interval ensured that each signal produced by local application of 5-HT had completely cleared before the next ejection of 5-HT, drug or vehicle. For systemic administration, exogenous 5-HT was applied 30 min following intraperitoneal administration of drugs or vehicle so as to correspond to the timing used in the tail suspension test (TST). Two signal parameters were analyzed: the peak signal amplitude and the T80 time course parameter. T80 is defined as the time for the signal to decline by 80% of the peak signal amplitude.
TST.
The TST was conducted based on the original description by Steru et al. (1985). In dose–response studies, mice received an intraperitoneal injection of D-22 (0.01–0.32 mg/kg), fluvoxamine (0.32–32 mg/kg), or saline. In drug interaction studies, mice received an intraperitoneal injection of either D-22 (0.1 or 0.32 mg/kg) or saline 60 min before testing, and received a second intraperitoneal injection of either fluvoxamine (10 mg/kg) or saline 30 min before testing; immediately after each injection, they were placed in an observation cage. Thirty minutes after the second injection, the distal end of the tail was fastened to a flat aluminum (2 × 0.3 × 10 cm) bar using adhesive tape at a 90 degree angle to the longitudinal axis of the mouse tail and the aluminum bar, with a distance of 3–4 cm between the base of the tail and the edge of the bar. A hole opposite the taped end of the bar was used to secure the bar to a hook in the ceiling of a visually isolated white test box (40 × 40 × 40 cm). Each mouse was suspended by its tail for 6 min, allowing the ventral surface and front and hindlimbs to be video recorded using a digital camera facing the test box. Total time immobile was measured (in seconds) during the entire 6 min test period. Immobility was defined as the absence of initiated movements, and included passive swaying of the body. A mouse was excluded from the experiments if it climbed and held to its tail or the aluminum bar for a period of 3 s or longer.
Serotonin syndrome.
Mice were given saline or a combination of D-22 and fluvoxamine, using the same doses and injection-test intervals as used in the drug interaction study in the TST. In addition, separate groups of mice received 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT, 10 mg/kg), 5-hydroxy-l-tryptophan (5-HTP, 100 mg/kg), or their vehicles (saline and 5% Tween 80, respectively). Each of the five groups received two intraperitoneal injections, 30 min apart, as follows: (1) D-22 followed by fluvoxamine, (2) saline followed by 8-OH-DPAT, (3) saline followed by 5-HTP, (4) saline followed by saline and (5) saline followed by 5% Tween 80. Immediately after the first injection, animals were placed in a transparent observation cage with a mirror positioned beneath it to allow simultaneous viewing of both the side and ventral surface of the mouse. Mice were observed for possible occurrence of elements of the 5-HT syndrome during five, 6 min periods, starting at 15, 30, 60, 120, and 240 min after the second injection. Each animal was recorded on video until immediately after the observation period that started at 120 min. The animal was then returned to the home cage where it was observed at 240 min. During each 6 min period mice were observed for the occurrence of the following signs: hindlimb abduction, low body posture, Straub tail, backward movement, tremor, head weaving, and forepaw treading, according to methods adapted from Fox et al. (2008). The presence (1) or absence (0) of each sign was assessed during each min of the 6 min observation period, allowing an animal to receive, for each of the signs, a score of 0–6 at each observation period and an overall score of 30 for all five observation periods.
HPLC analysis of D-22 in brain.
HPLC was used to assay for D-22 in brain. D-22 (10 mg/kg) was injected intraperitoneally and mice were killed 30 min later. Brain tissue for samples, calibrators, and controls were weighed into polypropylene tubes, then 10 times the volume of a 70% acetonitrile solution was added. Samples were homogenized by sonication and then 500 μl of each was placed in clean polypropylene tubes. Twenty microliters of diazepam (10 μg/ml) for internal standard was added to each sample. Next, samples were shaken for 10 min, centrifuged at 3200 g for 20 min at 18°C in a Beckman Allegra X-15R centrifuge. The supernatant was decanted into clean tubes, and dried under a stream of nitrogen. Samples were resuspended with 100 μl of mobile phase buffer, and microfilterfuged at 3200 g for 10 min. Fifty microliters of sample was injected into the HPLC system.
The HPLC system included a Waters model 510 pump, a Waters model 717 sample injector, a Waters model 2487 UV detector for HPLC equipped with a deuterium lamp (320/520 nm), and an Alltima 150 mm × 4.6 mm C18 column (5 U). Samples were analyzed at a fixed wavelength of 520 nm, a time constant of 1 s, and a range of 0.1 AUFS on the UV detector. The mobile phase contained 65% methanol, and 35% of a solution of 12 mm KH2PO4, pH 6.7. The flow rate of the mobile phase was 1.5 ml/min. D-22 eluted at 8.7 min and diazepam at 10.1 min. The peak area ratio of D-22 and the internal standard diazepam were determined using the Waters Empower chromatography software. D-22 concentration was determined by comparing peak area ratio (D22/diazepam) against the linear regression of ratios of calibrator samples from a 6 point calibrator curve. The concentrations of D-22 in brain were expressed as pg D-22/mg brain wet weight.
High purity-grade acetonitrile and methanol were purchased from Burdick and Jackson. Water used in the assay was purified with a Milli-Q Water System (Millipore). Potassium phosphate was obtained from Fisher Scientific. Hydrochloric acid was obtained from JT Baker Chemical. The internal standard, diazepam, was obtained from Sigma.
Statistics.
Statistical analyses were performed using Prism 5.04 (GraphPad). Data were analyzed using one-way ANOVA, followed by Newman–Keuls or Dunnett's multiple-comparison tests. All data were expressed as mean ± SEM, and p < 0.05 was considered statistically significant.
Drugs.
Serotonin, 5-HIAA, fluvoxamine, urethane, α-chloralose, D-22, 8-OH-DPAT HBr, and 5-HTP were purchased from Sigma-Aldrich. All compounds were injected intraperitoneally in a volume of 5–20 ml/kg. Doses are expressed as mg salt weight per kg body weight.
Results
Intrahippocampally applied D-22 enhances the ability of the SSRI fluvoxamine to inhibit 5-HT clearance from extracellular fluid
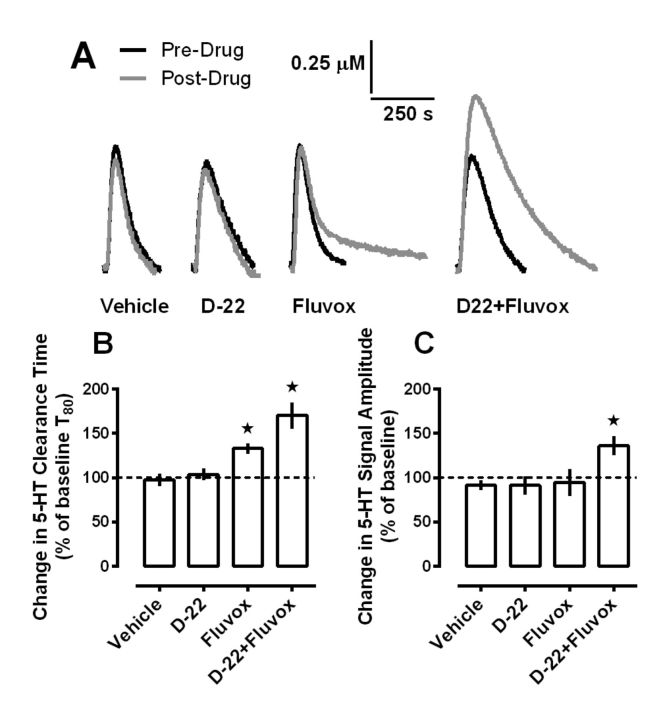
In wild-type mice, we previously found that D-22 could inhibit 5-HT clearance from CA3 region of hippocampus, but only when extracellular 5-HT levels were in a range that recruited low-affinity, high-capacity transporters for 5-HT (Baganz et al., 2008, 2010). At lower, SERT-recruiting 5-HT concentrations, D-22 had no effect on 5-HT clearance, a result not especially surprising given the high affinity of SERT for 5-HT. However, in the presence of an SSRI, we reasoned that D-22-sensitive transporters may exert a more prominent role in 5-HT uptake and therefore, limit the ability of SSRIs to prolong the time 5-HT remains in extracellular fluid. This led us to hypothesize that D-22 should enhance the inhibiting effect of an SSRI on 5-HT clearance. To test this hypothesis we used high-speed chronoamperometry, according to well established protocols (Daws and Toney, 2007; Baganz et al., 2008, 2010), to measure clearance of locally applied 5-HT from the CA3 region of the hippocampus of anesthetized mice, before and after intrahippocampal administration of fluvoxamine, D-22, both drugs given together, or vehicle. The amount of fluvoxamine delivered (54 pmol) was based on previous dose–response studies showing maximal inhibition of 5-HT clearance at this dose (Baganz et al., 2010). The amount of D-22 delivered (1.4 pmol) is the maximum that can be delivered locally without interfering with the properties of the carbon fiber recording electrode. D-22 (1.4 pmol) has no effect on 5-HT clearance in the CA3 region of hippocampus in wild-type mice but inhibits 5-HT clearance in mice lacking SERT, suggesting that the 5-HT clearance inhibiting effect of D-22 is mediated at sites other than SERT, putatively OCT3 (Baganz et al., 2008). Serotonin was pressure ejected to achieve reproducible signals with amplitudes of ∼0.5 μm, a concentration that we have previously found to recruit predominantly SERT-mediated 5-HT clearance (Daws et al., 2005; Baganz et al., 2008, 2010). Once reproducible signals were obtained, drug or vehicle was locally ejected into hippocampus and then 2 min later 5-HT was applied again. Representative traces are shown in Figure 1A. As expected, and consistent with other reports using similar methodology (Daws et al., 1998, 2000, 2005; Benmansour et al., 1999, 2002), fluvoxamine prolonged 5-HT clearance time (Fig. 1A,B), without producing any significant effect on signal amplitude (Fig. 1A,C). Likewise, replicating our previous study (Baganz et al., 2008), D-22 was without effect on 5-HT clearance time or signal amplitude (Fig. 1A–C). However, D-22 robustly increased the ability of fluvoxamine to inhibit 5-HT clearance. This effect was apparent not only in terms of prolonging 5-HT clearance time (Fig. 1A,B), but also in terms of increasing the amplitude of the 5-HT signal (Fig. 1A,C). Baseline (i.e., predrug) T80 values did not differ significantly (p > 0.20) among groups and were 101 ± 9, 116 ± 8, 107 ± 15, and 114 ± 6 s for vehicle, D-22, fluvoxamine, and D-22+fluvoxamine groups, respectively. One-way ANOVA revealed an effect of treatment on the percentage change from baseline 5-HT clearance time, driven by significant effects of fluvoxamine, and the combination of fluvoxamine and D-22 to slow 5-HT clearance (F(3,35) = 12.07; p < 0.0001; Fig. 1A,B). Likewise, baseline (i.e., predrug) signal amplitude values did not differ significantly (p > 0.50) among groups and were 0.66 ± 0.04, 0.56 ± 0.04, 0.65 ± 0.04, and 0.58 ± 0.06 μm for vehicle, D-22, fluvoxamine, and D-22+fluvoxamine groups, respectively. One-way ANOVA revealed an effect of treatment on the percentage change from baseline in 5-HT signal amplitude, driven by the marked ability of fluvoxamine, in combination with D-22, to increase signal amplitude (F(3,35) = 3.85; p < 0.05; Fig. 1A,C).
Figure 1.
Local blockade of D-22-sensitive transporters enhances fluvoxamine-induced inhibition of 5-HT clearance in mouse hippocampus in vivo. A, Representative oxidation currents (converted to micromolar values) produced by pressure ejection of 5-HT into the CA3 region of the hippocampus before (black line) and 2 min after (gray line) local application of saline, D-22 (1.4 pmol), fluvoxamine (54 pmol), or fluvoxamine and D-22 given together. Raw tracings are superimposed for ease of comparison. Note the marked increase in both clearance time and signal amplitude after local application of D-22 and fluvoxamine when given together. B, The effect of hippocampally applied fluvoxamine to slow 5-HT clearance is enhanced by concurrent administration of D-22; one-way ANOVA with Newman–Keuls post hoc comparison, *p < 0.05 versus vehicle. C, Hippocampally applied fluvoxamine, in combination with D-22, increases 5-HT signal amplitude; one-way ANOVA with Newman–Keuls post hoc comparison, *p < 0.05 versus vehicle. Data are expressed as mean ± SEM percentage of baseline values with n = 9–10 for each drug treatment.
It is worth noting that we conducted these in vivo electrochemical studies in the hippocampus for three key reasons. First, it is a limbic structure considered to be important in the therapeutic response to antidepressant drugs (Campbell and Macqueen, 2004). Second, it is a region where mechanisms contributing to 5-HT clearance in vivo have been best characterized to date (Daws et al., 1998, 2000, 2005; Baganz et al., 2008). Third, and especially relevant to the present study are reports that D-22-sensitive OCTs and PMAT are located in the hippocampus. Indeed, the availability of selective antibodies has made it possible to map the distribution of OCT subtypes and PMAT in brain (Amphoux et al., 2006; Dahlin et al., 2007; Vialou et al., 2007; Baganz et al., 2008; Lamhonwah et al., 2008; Cui et al., 2009; Gasser et al., 2009; Bacq et al., 2012).
The finding that locally administered D-22 can markedly enhance fluvoxamine-induced inhibition of 5-HT clearance in hippocampus suggests that the combination of these drugs might produce similar effects on 5-HT clearance when administered systemically and, importantly, enhance the antidepressant-like effect of fluvoxamine. The next set of experiments was designed to test this hypothesis.
D-22 enhances fluvoxamine-induced antidepressant-like effects in the TST
The ability of intrahippocampally applied D-22 to enhance fluvoxamine-induced inhibition of 5-HT clearance supports the idea that this drug combination might also produce greater antidepressant-like effects than either drug given alone. To this end, we studied the effects of systemic administration of D-22 and fluvoxamine in the TST, which measures antidepressant-like activity in mice (Steru et al., 1985; Cryan et al., 2005). We then examined how time spent immobile in the TST related to drug-induced inhibition of 5-HT clearance in hippocampus.
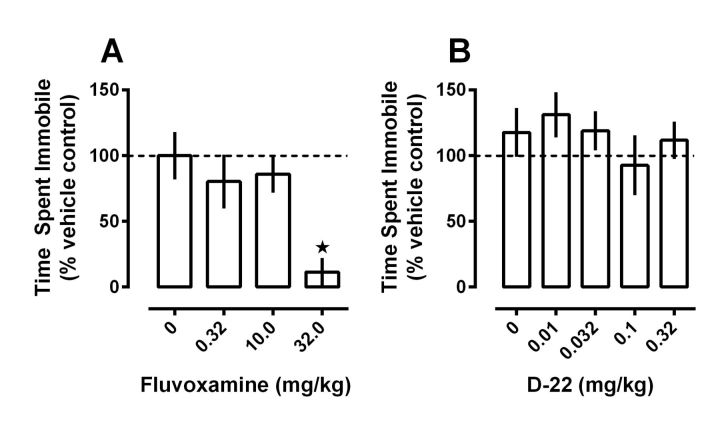
Consistent with the literature (Fujishiro et al., 2002; Cryan et al., 2005), fluvoxamine, given 30 min before testing, dose dependently reduced time spent immobile in the TST (Fig. 2A; F(3,28) = 5.75, p < 0.01). Previously, we found that 0.001 mg/kg D-22 decreased immobility in SERT-deficient mice, but not in wild-type mice, and decreased immobility more extensively at 60 min than at 30 min after intraperitoneal administration (Baganz et al., 2008). Here, we examined the effects of D-22 60 min after intraperitoneal administration in wild-type mice at doses ranging from 0.01 to 0.32 mg/kg. At these doses, D-22 did not significantly affect immobility (Fig. 2B; p > 0.60). This result was not especially surprising, given the presence of fully functioning, high-affinity SERT in these mice, which serve to maintain extracellular 5-HT at concentrations below those where low-affinity, high-capacity transporters for 5-HT can exert more influence. Doses >0.32 mg/kg, which in preliminary experiments appeared to decrease locomotion, were not tested.
Figure 2.
Fluvoxamine, but not D-22, dose dependently reduces immobility time in the TST. Time spent immobile was measured in seconds, and was expressed as a percentage of the corresponding vehicle control group. Time spent immobile for vehicle control groups did not differ significantly (p > 0.50) and were 116 ± 19 and 118 ± 19 s for fluvoxamine and D-22 control groups, respectively. A, Fluvoxamine, administered 30 min before testing, dose dependently decreased time spent immobile, producing a near maximal effect at the highest dose tested. B, D-22, given 60 min before testing, had no significant effect on time spent immobile at any of the doses tested. Data are expressed as mean ± SEM, n = 7–9 for each dose tested; one-way ANOVA with Dunnett's multiple-comparison post hoc test, *p < 0.05.
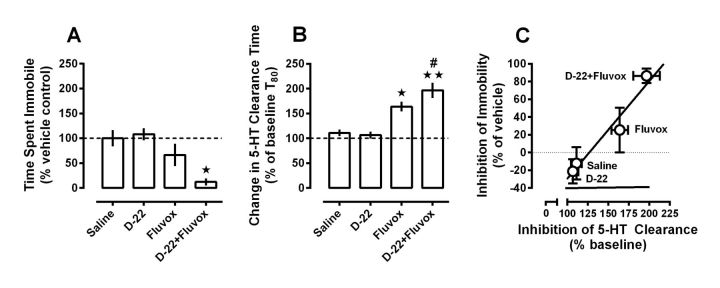
To test our hypothesis that D-22 would enhance the antidepressant-like effect of fluvoxamine, we selected a dose of each drug that did not produce significant effects on time spent immobile in the TST (i.e., 0.1 mg/kg D-22, and 10 mg/kg fluvoxamine given 60 and 30 min before testing, respectively). Consistent with the results shown in Figure 2, treatment with either D-22 (0.1 mg/kg i.p.) or fluvoxamine (10 mg/kg, i.p.) alone produced no statistically significant decrease in time spent immobile relative to saline-treated control mice (Fig. 3A). However, cotreatment with D-22 and fluvoxamine markedly decreased time spent immobile in the TST (one-way ANOVA, F(3,31) = 6.92, p < 0.05).
Figure 3.
D-22 enhances the antidepressant-like and 5-HT clearance-inhibiting effects of fluvoxamine following systemic administration in wild-type C57BL/6 mice. A, Doses of D-22 (0.1 mg/kg) and fluvoxamine (10 mg/kg), which by themselves did not significantly change time spent immobile in the TST compared with saline-injected control mice, markedly decreased immobility time when given together. Data are expressed as mean ± SEM percentage of time (s) immobile under saline control conditions (112 ± 18 s); n = 7–10 per treatment condition; one-way ANOVA, F(3,31) = 6.92, p < 0.05; Newman–Keuls post hoc test, *p < 0.01. B, In a separate group of mice, using the same doses and injection-test intervals used for the TST, systemic administration of D-22 enhanced fluvoxamine-induced inhibition of 5-HT clearance in hippocampus. Data are expressed as a percentage of baseline T80 values (i.e., prevehicle or drug injection T80 clearance times for 5-HT) ± SEM; n = 6–9 per treatment condition; one-way ANOVA, F(3,27) = 15.47, p < 0.05; Newman–Keuls post hoc test, *p < 0.01, **p < 0.001 versus saline; #p < 0.05 versus fluvoxamine. C, Regression analysis shows a strong, positive, correlation (r2 = 0.95, p = 0.025) between the ability of a given treatment to inhibit 5-HT clearance in hippocampus and to produce antidepressant-like effects in the TST.
To examine whether the marked antidepressant-like effect of the combination of D-22 and fluvoxamine was related to an increased ability of this drug combination to inhibit 5-HT uptake, we used high-speed chronoamperometry to study 5-HT clearance in the CA3 region of hippocampus under the same doses and injection-test intervals as used in the TST. Consistent with behavioral data in Figure 3A, D-22 had no effect on clearance of intrahippocampally applied 5-HT from extracellular fluid (Fig. 3B). Interestingly, systemically administered fluvoxamine (10 mg/kg), which did not produce statistically significant effects in the TST, robustly inhibited clearance of intrahippocampally applied 5-HT. D-22 enhanced fluvoxamine-induced inhibition of 5-HT clearance (one-way ANOVA, F(3,27) = 15.47, p < 0.05) and of time spent immobile in the TST (one-way ANOVA, F(3,31) = 6.92, p < 0.05). Thus our own data (Fig. 3C), and those of others (David et al., 2003), suggest a positive correlation between a drug's ability to inhibit 5-HT uptake and to produce antidepressant-like effects.
D-22 crosses the blood–brain barrier
The finding that both intrahippocampal and peripheral (i.p.) administration of D-22 produced similar inhibition of 5-HT clearance in hippocampus suggests that systemically administered D-22 probably exerts its effects on 5-HT clearance and behavior via central actions. To ensure that D-22 crossed the blood–brain barrier, we quantified D-22 in whole brains of three mice injected intraperitoneally with 10 mg/kg D-22 and three control mice injected with an equal volume of vehicle. Thirty minutes after the injection, the brain concentration of D-22 was 56 ± 27 pg/mg, confirming that D-22 reaches the brain following systemic administration.
D-22 enhances fluvoxamine-induced inhibition of 5-HT clearance without inducing elements of the serotonin syndrome
Drugs or drug combinations that produce excessive serotonergic activity can induce the serotonin syndrome (Sternbach, 1991; Kalueff et al., 2007). In humans, symptoms include hyperthermia, tachycardia, and rhabdomyolysis, which can result in death (Lane and Baldwin, 1997; Ener et al., 2003). Thus, an important consideration relating to our finding that D-22 enhances the ability of fluvoxamine to prevent 5-HT reuptake is that this drug combination conceivably could induce elements of the serotonin syndrome. In mice the serotonin syndrome consists of hindlimb abduction, low body posture, Straub tail, tremor, backward movement, head weaving, and forepaw treading (Fox et al., 2007). In the present study we treated mice with D-22 and fluvoxamine using the same doses and injection-test intervals as in the TST to examine whether this combination of D-22 and fluvoxamine, which produced maximal antidepressant-like activity, also induced elements of the serotonin syndrome. Different groups of mice were treated with either vehicle, 8-OH-DPAT (10 mg/kg i.p.), an agonist at 5-HT1A receptors that produces the serotonin syndrome in mice (Middlemiss and Fozard, 1983; Blackburn et al., 1984; Hensler and Truett, 1998; Fox et al., 2007), or 5-HTP (100 mg/kg i.p.), the precursor to 5-HT that also produces the serotonin syndrome in mice (Fox et al., 2007, 2008). Mice were then observed for 6 min periods, starting at 15, 30, 60, 120, and 240 min after the second injection. During each 6 min observation period, mice were scored at 1 min intervals for the presence or absence of serotonin syndrome elements, according to methods adapted from Fox et al., (2007). Results for the observation period starting 30 min after the second injection (i.e., the time corresponding to scoring in the TST) are shown in Table 1. As expected, the positive controls, 8-OH-DPAT and 5-HTP, produced hindlimb abduction and low body posture. In addition, 8-OH-DPAT produced Straub tail, tremor, and backward movement. Essentially none of these signs were observed in vehicle-treated animals. Importantly, the combination of D-22 and fluvoxamine also did not significantly produce any element of the serotonin syndrome, at any time during the 6 min test, 30 min following the second injection. Likewise, no elements of the serotonin syndrome were observed throughout the entire 4 h observation period following the combination of D-22 and fluvoxamine, suggesting that at these doses, the combination treatment does not produce side effects typically associated with excess activation of the serotonin system.
Table 1.
D-22 together with fluvoxamine does not produce elements of the serotonin syndrome, unlike the 5-HT1A receptor agonist 8-OH-DPAT and the 5-HT precursor, 5-HTP
| Element of the 5-HT syndrome | Saline (Tween 80) | D-22 (0.1 mg/kg) + fluvoxamine (10 mg/kg) | 8-OH-DPAT (10 mg/kg) | 5-HTP (100 mg/kg) | F[4,18] | p value |
|---|---|---|---|---|---|---|
| Hindlimb abduction | 0.2 ± 0.2 (2.0 ± 1.5) | 0.2 ± 0.2 | 5.0 ± 1.0* | 4.2 ± 1.1* | 7.4 | 0.001 |
| Low body posture | 0 ± 0 (0 ± 0) | 0 ± 0 | 4.4 ± 1.2* | 4.2 ± 0.9* | 10.7 | 0.001 |
| Straub tail | 0 ± 0 (0 ± 0) | 0 ± 0 | 2.0 ± 0.9* | 1.6 ± 0.6 | 4.4 | 0.012 |
| Tremor | 0 ± 0 (0 ± 0) | 0 ± 0 | 1.6 ± 0.7* | 0.4 ± 0.3 | 4.0 | 0.017 |
| Backward movement | 0 ± 0 (0 ± 0) | 0.2 ± 0.2 | 1.0 ± 0.5* | 0 ± 0 | 3.4 | 0.031 |
| Head weaving | 0 ± 0 (0 ± 0) | 0 ± 0 | 0.6 ± 0.6 | 0 ± 0 | 0.9 | 0.495 |
| Forepaw treading | 0 ± 0 (0 ± 0) | 0 ± 0 | 0.2 ± 0.2 | 0 ± 0 | 0.9 | 0.495 |
Data are expressed as mean score ± SEM; n = 5 per treatment group, except 5% Tween 80 vehicle, n = 3; one-way ANOVA, followed by multiple comparisons with Newman–Keuls test.
*Significantly different from saline, 5% Tween 80 vehicle and from D-22 + fluvoxamine.
Enhancement by D-22 of fluvoxamine-induced antidepressant-like effects in the tail suspension test is attenuated in OCT3 knock-out mice
Our data show that D-22 can enhance the antidepressant-like activity of fluvoxamine. D-22 has affinity for OCT1, 2, and 3 subtypes as well as PMAT (Amphoux et al., 2006; Schömig et al., 2006; Koepsell et al., 2007; Duan and Wang, 2010). Our next studies therefore sought to gain insight into which of these transporters might be necessary for this action of D-22. OCT1 has limited ability to transport 5-HT and is not densely expressed in hippocampus (Amphoux et al., 2006; Schömig et al., 2006; Koepsell et al., 2007). OCT2 expression in brain is confined primarily to subventricular regions, though it is expressed in hippocampus (Wu et al., 1998, 1999; Amphoux et al., 2006; Bacq et al., 2012). OCT3 and PMAT are richly expressed in limbic regions, including hippocampus (Gründemann et al., 1998; Dahlin et al., 2007; Baganz et al., 2008; Gasser et al., 2009). Based on these reports and because our previous studies support a role for OCT3 in mediating the effects of D-22 on immobility time in the TST (Baganz et al., 2008, 2010), we turned to OCT3 KO mice to investigate the requirement of this transporter for D-22 to enhance the effects of fluvoxamine in the TST. Behavioral and neurochemical characterization of OCT3 KO mice has been reported previously (Vialou et al., 2008, Cui et al., 2009; Wultsch et al., 2009).
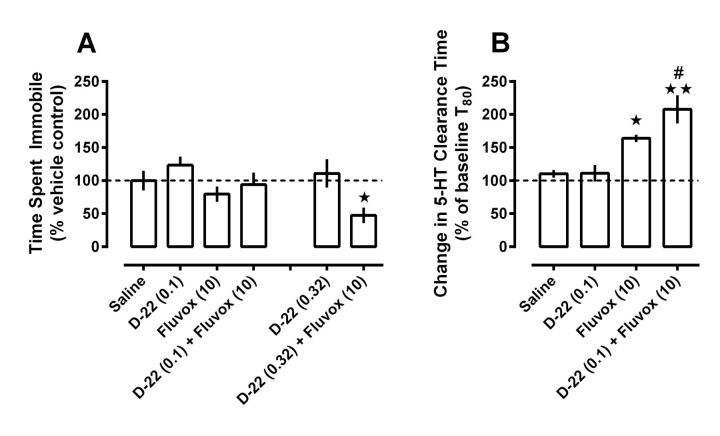
Using the same dosing and timing regimen as before, we found that like wild-type C57BL/6 mice, D-22 (0.1 mg/kg) and fluvoxamine (10 mg/kg), when given alone to OCT3 KO mice, did not produce significant effects on time spent immobile compared with saline-treated mice. However, in contrast to wild-type mice, we found that D-22 failed to enhance the ability of fluvoxamine to reduce time spent immobile in the TST in OCT3 KO mice (Fig. 4A). These results suggest a key role for OCT3 in mediating this effect of D-22.
Figure 4.
D-22 enhancement of the antidepressant-like effect of fluvoxamine in the TST is attenuated in OCT3 KO mice, whereas D-22 enhancement of the 5-HT clearance inhibiting effect fluvoxamine remains intact. A, Doses of D-22 (0.1 and 0.32 mg/kg) and fluvoxamine (10 mg/kg) by themselves did not significantly change time spent immobile in the TST compared with saline-injected control mice. In contrast to wild-type mice (Fig. 3A), a dose of 0.1 mg/kg D-22 did not decrease immobility time when given together with fluvoxamine; however, a significant reduction in immobility time resulted when a higher dose of D-22 (0.32 mg/kg) was given with fluvoxamine. Data are expressed as mean ± SEM percentage of time (s) immobile under saline control conditions (127 ± 19 s); n = 8–11 per treatment condition; one-way ANOVA, F(3,35) = 3.17, p < 0.015; Newman–Keuls post hoc test, *p < 0.05, from saline and D-22 counterparts. B, In a separate group of mice, using the injection-test intervals as for the TST, systemic administration of D-22 (0.1 mg/kg) enhanced fluvoxamine (10 mg/kg)-induced inhibition of 5-HT clearance in hippocampus. Data are expressed as a percentage of baseline T80 values (i.e., prevehicle or drug injection T80 clearance times for 5-HT) ± SEM; n = 6–7 per treatment condition; one-way ANOVA, F(3,25) = 12.34, p < 0.01; Newman–Keuls post hoc test, *p < 0.05, **p < 0.001 versus saline; #p < 0.05 versus fluvoxamine.
To confirm that this result was not due to reduced sensitivity of OCT3 KO mice to fluvoxamine, we performed dose–response studies and found that, like in wild-type mice, 10 mg/kg fluvoxamine was the largest dose that did not have significant effects by itself in the TST in OCT3 KO mice. Immobility time following vehicle injection in OCT3 KO mice was 127 ± 19 s (n = 10) (compared with 112 ± 18 s in wild-type mice, Fig. 3, legend). Similar to wild-type mice, fluvoxamine at doses of 3.2 and 10.0 mg/kg did not significantly influence time spent immobile in OCT3 KO mice (123 ± 14 s, n = 8, and 101 ± 15 s, n = 11, respectively), but significantly reduced immobility time at a dose of 32.0 mg/kg (47 ± 14 s, n = 8, one-way ANOVA, F(3,33) = 4.81, p < 0.05). These data indicate that the inability of D-22 to enhance the antidepressant-like effect of fluvoxamine in the TST in OCT3 KO mice was not due to a reduction in sensitivity of these mice to fluvoxamine.
We next investigated if a higher dose of D-22 (0.32 mg/kg) would enhance the antidepressant-like effect of fluvoxamine (10 mg/kg). This dose of D-22 was the highest that did not decrease locomotion in wild-type mice. We found that this dose of D-22 did enhance the ability of fluvoxamine to reduce time spent immobile in OCT3 KO mice (Fig. 4A; one-way ANOVA, F(3,35) = 3.49, p < 0.05); however, the magnitude of this enhancement was not as pronounced as that produced by the lower dose of D-22 in wild-type mice (Fig. 3A). In OCT3 KO mice given D-22 (0.32 mg/kg) together with fluvoxamine, time spent immobile decreased to 60 ± 15 s (47 ± 12% vehicle control, n = 10), whereas in wild-type mice D-22 (0.1 mg/kg) given in combination with fluvoxamine decreased immobility time essentially maximally (to 14 ± 8 s, or 12 ± 7% vehicle control, n = 7). An unpaired t test showed this difference to be significant (t(15) = 2.29, p < 0.05). These data suggest that OCT3 plays a predominant role in mediating the ability of D-22 to enhance the antidepressant-like effect of fluvoxamine in the TST, but that D-22 can also produce this effect by actions at sites other than OCT3, albeit with lesser potency.
To examine whether the loss of antidepressant-like effect of the combination of D-22 (0.1 mg/kg) and fluvoxamine in the TST was associated with a reduced ability of the drug combination to inhibit 5-HT uptake in OCT3 KO mice, we used high-speed chronoamperometry to measure 5-HT clearance in the CA3 region of hippocampus under the same dose and injection-test interval as used previously. In contrast to the behavioral data, this drug combination inhibited 5-HT clearance in OCT3 KO mice similarly to that in wild-type mice (compare Figs. 3B, 4B). As we found in wild-type mice, D-22 (0.1 mg/kg) had no effect on 5-HT clearance, whereas fluvoxamine (10 mg/kg), which did not produce statistically significant effects in the TST, significantly inhibited clearance of intrahippocampally applied 5-HT in OCT3 KO mice. D-22 (0.1 mg/kg) enhanced fluvoxamine-induced inhibition of 5-HT clearance in OCT3 KO mice (one-way ANOVA, F(3,25) = 12.34, p < 0.05).
Baseline (i.e., predrug) T80 values in OCT3 KO mice did not differ significantly (p > 0.13) among treatment groups and averaged 86 ± 6 s (n = 26), a value comparable to that obtained in wild-type mice (90 ± 4 s, n = 69). Likewise, baseline (i.e., predrug) signal amplitude values did not differ significantly (p > 0.40) among OCT3 KO mice treatment groups and averaged 0.63 ± 0.03 μm, comparable to that in wild-type mice (0.64 ± 0.02 μm).
Discussion
The major finding of the present studies is that the 5-HT clearance inhibiting and antidepressant-like effects of the SSRI fluvoxamine can be significantly augmented by coadministration of D-22. These data suggest that D-22-sensitive transporters may limit the ability of SSRIs to increase extracellular 5-HT and thus, limit their ability to produce antidepressant effects. D-22-sensitive transporters, such as OCTs and PMAT, may be useful targets for the development of new antidepressant medications to improve the therapeutic utility of SSRIs.
Implications for understanding the relationship between the ability of SSRIs to inhibit serotonin clearance and to produce antidepressant-like effects
An especially intriguing finding was the significant effect of the combination of fluvoxamine and D-22 to increase both the time course for 5-HT clearance and signal amplitude produced by 5-HT locally applied into the CA3 region of hippocampus. Previously, using similar approaches, we and others have not routinely observed an increase in amplitude of the 5-HT signal following administration of fluvoxamine. This has been a puzzling observation given that one would expect blockade of a transporter to increase both the clearance time and signal amplitude of exogenously applied neurotransmitter. Indeed, this is true for dopamine (DA) signals following blockade of the DA transporter (DAT) with drugs such as cocaine and nomifensine in DAT-rich regions, such as striatum and nucleus accumbens (Zahniser et al., 1999). This paradoxical lack of effect of SSRIs to increase 5-HT signal amplitude may well be attributed to the presence of D-22-sensitive transporters (OCTs and PMAT) and their expression level relative to SERT in hippocampus. As discussed earlier, these low-affinity, high-capacity transporters may become more effective at clearing 5-HT when extracellular 5-HT concentrations rise. Thus, following fluvoxamine, D-22-sensitive transporters may serve to prevent the 5-HT signal amplitude from increasing. Consistent with this notion is the observation that the effect of fluvoxamine on 5-HT clearance is most pronounced toward the tail of the signal, i.e., when the 5-HT concentration has fallen below the “reach” of low-affinity, high-capacity D-22-sensitive transporters (Fig. 1A). Thus, the combined effect of an SSRI and blocker of D-22-sensitive transporters is to not only further increase the duration that 5-HT remains in the extracellular fluid, but also to further increase the concentration (Daws et al., 2013).
Increased extracellular levels of 5-HT are considered to be an important trigger for ultimate therapeutic benefit in humans. Supporting this idea, our findings in wild-type mice show that antidepressant-like activity in the TST is positively correlated with the ability of a drug, or drug combination, to inhibit clearance of 5-HT in CA3 region of hippocampus (Fig. 3C). However, this was not the case in OCT3 KO mice, where the ability of D-22 to enhance the antidepressant-like effect of fluvoxamine in the TST was greatly attenuated, and yet inhibition of 5-HT clearance in the CA3 region of hippocampus was equivalent to that in wild-type mice. Thus, OCT3 appears to play a prominent role in mediating effects of D-22 in the TST, but does not appear to be involved in mediating the effect of D-22 to enhance fluvoxamine-induced inhibition of 5-HT clearance in the CA3 region of hippocampus. Together, these results indicate that antidepressant-like effects of a given treatment in the TST are not necessarily related to its ability to inhibit 5-HT clearance in the CA3 region of hippocampus. This raises the possibility that D-22's augmentation of the antidepressant-like effect of fluvoxamine in the TST is mediated by brain regions other than, or in addition to the CA3 region of hippocampus, and/or by its activity at other sites, possibly other OCT subtypes or PMAT, where it may inhibit uptake of 5-HT as well as other biogenic amines considered important in antidepressant-like response, discussed below.
Implications for increasing the clinical effects of antidepressants
The lack of symptom relief in many patients treated with currently available antidepressants emphasizes the need for novel pharmacological approaches with improved efficacy to treat depression. Our findings support D-22-sensitive uptake mechanisms as additional targets for the discovery of novel antidepressant therapies to maximize blockade of 5-HT uptake in brain. However, in addition to 5-HT, D-22-sensitive OCTs and PMAT are also low-affinity, high-capacity transporters for norepinephrine (NE) and DA (Wu et al., 1998; Amphoux et al., 2006; Cui et al., 2009; Bacq et al., 2012). Thus, while SSRIs have been the focus of this study, our results may generalize to other classes of antidepressants, including selective NE reuptake inhibitors, which block NE uptake via the NE transporter (NET), dual 5-HT-NE reuptake inhibitors, and triple-uptake inhibitors, which block transport of 5-HT, NE, and DA via their high-affinity transporters, SERT, NET, and DAT. To date, antidepressants that block SERT, NET, and DAT are thought to have the greatest clinical efficacy (Sulzer and Edwards, 2005; Chen and Skolnick, 2007). This may be in part attributed to the promiscuity that exists among SERT, NET, and DAT, which are all capable of low-affinity transport of their non-native biogenic amines (Pacholczyk et al., 1991; Daws et al., 1998; Norrholm et al., 2007; Rice and Cragg, 2008; Daws, 2009), as well as to the likelihood that successful treatment of depression requires targeting multiple neurotransmitter systems. Regardless, our findings suggest that the presence of D-22-sensitive transporters might also limit therapeutic efficacy of antidepressants other than SSRIs, and that blockade of D-22-sensitive transporters might serve to enhance their therapeutic efficacy by enhancing their ability to inhibit uptake of NE and DA, as well as 5-HT. Consistent with this idea, Hagan et al. (2011), using rotating disk voltammetry to measure 5-HT uptake into whole brain synaptosomes, showed that complete blockade of 5-HT uptake could only be achieved after incubating synaptosomes with blockers of SERT, NET, and DAT together with D-22. Thus, in terms of elevating extracellular levels of 5-HT and other biogenic amines to those that effectively treat depression, several lines of evidence, including the present study, point to blockade of D-22-sensitive transporters as a promising strategy.
In addition to being a potent blocker of low-affinity, high-capacity 5-HT transporters, D-22 has α-1-adrenoceptor antagonist properties (Russ et al., 1996). α-1 antagonism is unlikely to mediate the enhancement by D-22 of fluvoxamine-induced inhibition of 5-HT clearance and antidepressant-like effects, because the α-1-adrenoceptor antagonist prazosin reportedly produces effects that are unlike those observed here with D-22. For example, prazosin decreases SSRI-induced increases of 5-HT levels (Rea et al., 2010), whereas D-22 enhanced the fluvoxamine-induced increase of 5-HT levels. In addition, prazosin increases immobility in the TST (Stone and Quartermain, 1999), whereas D-22 was inactive in this test when given alone at doses that enhanced effects of fluvoxamine. Finally, prazosin can block behavioral actions of antidepressants (Kostowski, 1985), whereas D-22 enhanced the antidepressant-like effects of fluvoxamine. Thus, it appears unlikely that α-1 antagonist properties underlie the ability of D-22 to enhance effects of fluvoxamine. Recently, the D-22 congener disprocynium-24 (D-24) has been reported to have antagonist properties not only at α-1 receptors, but also at α-2 receptors (Amphoux et al., 2010). It is presently unknown whether D-22 shares α-2 antagonist properties with D-24. If it does, these properties are unlikely to account for the effects of D-22 observed here, because the α-2 antagonist yohimbine does not affect 5-HT clearance (Ansah et al., 2003), and yohimbine increases immobility time in the TST (Ferrari et al., 1991). Together, there is evidence that D-22 has α-1-adrenoceptor antagonist properties, and evidence that α-1 antagonism produces effects that are opposite to those observed here with D-22. Thus, analogs of D-22 that lack α-1 antagonist properties may enhance effects of SSRIs even more markedly than D-22.
Although the idea of low-affinity, high-capacity transporters for 5-HT is not new, heralding back to the 1960s and 1970s when several groups reported promiscuous uptake of 5-HT by the so-called “uptake-2” transporter (Bertler et al., 1964; Fuxe et al., 1967; Lichtensteiger et al., 1967; Shaskan and Snyder, 1970), the identity of the transporter(s) has remained under some debate (Daws, 2009). In 2004, Schildkraut and Mooney proposed the extraneuronal monoamine transporter (uptake-2) as a target for development of more rapidly acting antidepressants, but still, the precise identity of “uptake-2” remained controversial. Since that time, several reports have emerged pointing to D-22-sensitive OCTs and PMAT as being primary uptake-2 (i.e., low-affinity, high-capacity) transporters for the biogenic amines (for review, see Daws, 2009). The challenge now will be to determine which of these might be the best to target for the discovery of new antidepressant drugs. While this field is still in its infancy OCT3 and more recently, OCT2, are emerging as leading candidates (Kitaichi et al., 2005; Baganz et al., 2008; Bacq et al., 2012). The markedly reduced potency of D-22 to enhance the antidepressant-like effect of fluvoxamine in the TST in OCT3 KO mice provides the first direct evidence that OCT3 is important for this action of D-22. Our behavioral and neurochemical results encourage further research into the role of D-22-sensitive transporters in regulating biogenic amine neurotransmission and behavior. Selective targeting of different OCT subtypes or PMAT may be a valid strategy to discover treatments with improved antidepressant efficacy, either as a cotreatment with existing antidepressant drugs or as novel 5-HT reuptake inhibitors with additional OCT or PMAT blocking properties.
Footnotes
This work was supported by National Institutes of Health Grants R01-MH064489 (L.C.D.), R01-MH093320 (L.C.D., W.K.), R03-MH086708 (G.G.G.), and National Alliance for Research on Schizophrenia and Depression Independent Investigator Award (L.C.D.). We would like to thank Dr. Marty Javors and Jesus Sanchez for HPLC analyses, and Mandakh Bekhbat for assistance with behavioral assays. Drs. Sophie Gautron, Bruno Giros, and Kim Tieu are gratefully acknowledged for generously providing OCT3 KO mice.
References
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Amphoux A, Millan MJ, Cordi A, Bönisch H, Vialou V, Mannoury la Cour C, Dupuis DS, Giros B, Gautron S. Inhibitory and facilitory actions of isocyanine derivatives at human and rat organic cation transporters 1, 2 and 3: a comparison to human alpha 1- and alpha 2-adrenoceptor subtypes. Eur J Pharmacol. 2010;634:1–9. doi: 10.1016/j.ejphar.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Ansah TA, Ramamoorthy S, Montañez S, Daws LC, Blakely RD. Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304) J Pharmacol Exp Ther. 2003;305:956–965. doi: 10.1124/jpet.102.047134. [DOI] [PubMed] [Google Scholar]
- Bacq A, Balasse L, Biala G, Guiard B, Gardier AM, Schinkel A, Louis F, Vialou V, Martres MP, Chevarin C, Hamon M, Giros B, Gautron S. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry. 2012;17:926–939. doi: 10.1038/mp.2011.87. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci. 2010;30:15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A. Comparison of the effects of estradiol and progesterone on serotonergic function. Biol Psychiatry. 2012;71:633–641. doi: 10.1016/j.biopsych.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertler A, Falck B, Owman C. Studies on 5-hydroxytryptamine stores in pineal gland of rat. Acta Physiol Scand. 1964;(Suppl 239):1–18. doi: 10.1111/j.1748-1716.1964.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Blackburn TP, Kemp JD, Martin DA, Cox B. Evidence that 5-HT agonist-induced rotational behaviour in the rat is mediated via 5-HT1 receptors. Psychopharmacology. 1984;83:163–165. doi: 10.1007/BF00429727. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44:169–178. doi: 10.1016/S0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Menkes DB, Heninger GR. Receptor sensitivity and the mechanism of action of antidepressant treatment. implications for the etiology and therapy of depression. Arch Gen Psychiatry. 1981;38:1160–1180. doi: 10.1001/archpsyc.1981.01780350094011. [DOI] [PubMed] [Google Scholar]
- Chen Z, Skolnick P. Triple uptake inhibitors: therapeutic potential in depression and beyond. Expert Opin Investig Drugs. 2007;16:1365–1377. doi: 10.1517/13543784.16.9.1365. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–1211. doi: 10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Bourin M, Jego G, Przybylski C, Jolliet P, Gardier AM. Effects of acute treatment with paroxetine, citalopram and venlafaxine in vivo on noradrenaline and serotonin outflow: a microdialysis study in Swiss mice. Br J Pharmacol. 2003;140:1128–1136. doi: 10.1038/sj.bjp.0705538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Toney GM. High-speed chronoamperometry to study kinetics and mechanisms for serotonin clearance in vivo. In: Michael AC, Borland LM, editors. Electrochemical methods for neuroscience. Boca Raton, FL: Taylor and Francis; 2007. [PubMed] [Google Scholar]
- Daws LC, Toney GM, Gerhardt GA, Frazer A. In vivo chronoamperometric measures of extracellular serotonin clearance in rat dorsal hippocampus: contribution of serotonin and norepinephrine transporters. J Pharmacol Exp Ther. 1998;286:967–976. [PubMed] [Google Scholar]
- Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- Daws LC, Moñtanez S, Owens WA, Gould GG, Frazer A, Toney GM, Gerhardt GA. Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperometry. J Neurosci Methods. 2005;143:49–62. doi: 10.1016/j.jneumeth.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Daws LC, Koek W, Mitchell NC. Revisiting serotonin reuptake inhibitors and the therapeutic potential of “uptake-2” in psychiatric disorders. ACS Chem Neurosci. 2013;4:16–21. doi: 10.1021/cn3001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ener RA, Meglathery SB, Van Decker WA, Gallagher RM. Serotonin syndrome and other serotonergic disorders. Pain Med. 2003;4:63–74. doi: 10.1046/j.1526-4637.2003.03005.x. [DOI] [PubMed] [Google Scholar]
- Engel K, Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68:1397–1407. doi: 10.1124/mol.105.016832. [DOI] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Cassinadri M, Tartoni PL, Tampieri A. Effects of B-HT 920 in the tail-suspension test. Pharmacol Res. 1991;24:75–81. doi: 10.1016/1043-6618(91)90067-8. [DOI] [PubMed] [Google Scholar]
- Fox MA, Jensen CL, Gallagher PS, Murphy DL. Receptor mediation of exaggerated responses to serotonin-enhancing drugs in serotonin transporter (SERT)-deficient mice. Neuropharmacology. 2007;53:643–656. doi: 10.1016/j.neuropharm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fox MA, Jensen CL, French HT, Stein AR, Huang SJ, Tolliver TJ, Murphy DL. Neurochemical, behavioral, and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology. 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. Sydney: Academic; 1997. [Google Scholar]
- Fujishiro J, Imanishi T, Onozawa K, Tsushima M. Comparison of the anticholinergic effects of the serotonergic antidepressants, paroxetine, fluvoxamine and clomipramine. Eur J Pharmacol. 2002;454:183–188. doi: 10.1016/S0014-2999(02)02557-8. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Hokfelt T, Ungerstedt U. Studies on uptake mechanisms in central monoamine neurones. Acta Pharmacol Toxicol. 1967;25(Suppl 4):8. doi: 10.1111/j.1600-0773.1967.tb03004.x. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA. Neuromethods: voltammetric methods in brain systems. In: Boulton AA., Baker GB, Adams RN, editors. Rapid chronocoulometric measurements of norepinephrine overflow and clearance in CNS tissues. Vol 27. Totowa, NJ: Humana; 1995. pp. 117–151. [Google Scholar]
- Gründemann D, Köster S, Kiefer N, Breidert T, Engelhardt M, Spitzenberger F, Obermüller N, Schömig E. Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. J Biol Chem. 1998;273:30915–30920. doi: 10.1074/jbc.273.47.30915. [DOI] [PubMed] [Google Scholar]
- Hagan CE, Schenk JO, Neumaier JF. The contribution of low-affinity transport mechanisms to serotonin clearance in synaptosomes. Synapse. 2011;65:1015–1023. doi: 10.1002/syn.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG, Truett KA. Effect of chronic serotonin-2 receptor agonist or antagonist administration on serotonin-1A receptor sensitivity. Neuropsychopharmacology. 1998;19:354–364. doi: 10.1016/S0893-133X(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaichi K, Fukuda M, Nakayama H, Aoyama N, Ito Y, Fujimoto Y, Takagi K, Takagi K, Hasegawa T. Behavioral changes following antisense oligonucleotide-induced reduction of organic cation transporter-3 in mice. Neurosci Lett. 2005;382:195–200. doi: 10.1016/j.neulet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Kostowski W. Possible relationship of the locus coeruleus–hippocampal noradrenergic neurons to depression and mode of action of antidepressant drugs. Pol J Pharmacol Pharm. 1985;37:727–743. [PubMed] [Google Scholar]
- Lamhonwah AM, Hawkins CE, Tam C, Wong J, Mai L, Tein I. Expression patterns of the organic cation/carnitine transporter family in adult murine brain. Brain Dev. 2008;30:31–42. doi: 10.1016/j.braindev.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Lane R, Baldwin D. Selective serotonin reuptake inhibitor-induced serotonin syndrome: review. J Clin Psychopharmacol. 1997;17:208–221. doi: 10.1097/00004714-199706000-00012. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Mutzner U, Langemann H. Uptake of 5-hydroxytryptamine and 5-hydroxytryptophan by neurons of the central nervous system normally containing catecholamines. J Neurochem. 1967;14:489–497. doi: 10.1111/j.1471-4159.1967.tb09548.x. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. 8-hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol. 1983;90:151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Montañez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–219. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Horton DB, Dwoskin LP. The promiscuity of the dopamine transporter: implications for the kinetic analysis of [3H]serotonin uptake in rat hippocampal and striatal synaptosomes. Neuropharmacology. 2007;53:982–989. doi: 10.1016/j.neuropharm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Perez XA, Andrews AM. Chronoamperometry to determine differential reductions in uptake in brain synaptosomes from serotonin transporter knockout mice. Anal Chem. 2005;77:818–826. doi: 10.1021/ac049103g. [DOI] [PubMed] [Google Scholar]
- Rea K, Folgering J, Westerink BH, Cremers TI. Alpha1-adrenoceptors modulate citalopram-induced serotonin release. Neuropharmacology. 2010;58:962–971. doi: 10.1016/j.neuropharm.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ H, Friedgen B, Königs B, Schumacher C, Graefe KH, Schömig E. Pharmacokinetic and alpha 1-adrenoceptor antagonistic properties of two cyanine-type inhibitors of extraneuronal monoamine transport. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:268–274. doi: 10.1007/BF00171057. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ, Mooney JJ. Toward a rapidly acting antidepressant: the normetanephrine and extraneuronal monoamine transporter (uptake 2) hypothesis. Am J Psychiatry. 2004;161:909–911. doi: 10.1176/appi.ajp.161.5.909. [DOI] [PubMed] [Google Scholar]
- Schömig E, Babin-Ebell J, Russ H. 1,1′-Diethyl-2,2′-cyanine (decynium22) potently inhibits the renal transport of organic cations. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:379–383. doi: 10.1007/BF00165387. [DOI] [PubMed] [Google Scholar]
- Schömig E, Lazar A, Gründemann D. Extraneuronal monoamine transporter and organic cation transporters 1 and 2: a review of transport efficiency. Handb Exp Pharmacol. 2006;175:151–180. doi: 10.1007/3-540-29784-7_8. [DOI] [PubMed] [Google Scholar]
- Shaskan EG, Snyder SH. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970;175:404–418. [PubMed] [Google Scholar]
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Alpha-1-noradrenergic neurotransmission, corticosterone, and behavioral depression. Biol Psychiatry. 1999;46:1287–1300. doi: 10.1016/S0006-3223(99)00234-6. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Edwards RH. Antidepressants and the monoamine masquerade. Neuron. 2005;46:1–2. doi: 10.1016/j.neuron.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Vialou V, Balasse L, Dumas S, Giros B, Gautron S. Neurochemical characterization of pathways expressing plasma membrane monoamine transporter in the rat brain. Neuroscience. 2007;144:616–622. doi: 10.1016/j.neuroscience.2006.09.058. [DOI] [PubMed] [Google Scholar]
- Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem. 2008;106:1471–1482. doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2) an organic cation/carnitine transporter. J Pharmacol Exp Ther. 1999;290:1482–1492. [PubMed] [Google Scholar]
- Wultsch T, Grimberg G, Schmitt A, Painsipp E, Wetzstein H, Breitenkamp AF, Gründemann D, Schömig E, Lesch KP, Gerlach M, Reif A. Decreased anxiety in mice lacking organic cation transporter 3. J Neural Transm. 2009;116:689–697. doi: 10.1007/s00702-009-0205-1. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther. 1999;289:266–277. [PubMed] [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter systems known as uptake-2 in Ort3/Slc22a3-deficient mice. Mol Cell Biol. 2001;21:4188–4196. doi: 10.1128/MCB.21.13.4188-4196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]