Abstract
Expression of MCP-1 in the central nervous system (CNS) is associated with various neuroinflammatory diseases, including multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). In this study, we found that MCP-1 was decreased in the CNS but increased in the gut following oral administration of myelin basic protein (MBP) correlating with protection from EAE. To study the trafficking and the fate of T cells during oral tolerance, MBP-specific TCR transgenic (Tg) CD4+ T cells were labeled using 5, 6-carboxy-succinimidyl-fluorescein-ester (CFSE) and transferred intravenously to syngeneic B10.PL recipients before feeding with either MBP or PBS. We observed that the CFSE-labeled T cells traffic to the peripheral lymphoid tissue and the Peyer’s patches (PP). The labelled T cells proliferate in vivo in both the lymph node and the PP 48 hours after MBP feeding, but the cells are maintained in the PP longer than in the LN. CFSE-labeled cells in the PP have high levels of CD69 and Fas expression which is accompanied by increased apoptosis after MBP feeding. Our observations suggest that oral administration of autoantigen induces an elevation of MCP-1 in the gut, early T cell trafficking and activation in the periphery and the PP, followed by deletion of autoreactive T cells in the PP.
Keywords: Autoimmunity, EAE/MS, Tolerance, T Cell Receptors, Peyer’s Patch
1. Introduction
Experimental autoimmune encephalomyelitis (EAE) shares many features in common with multiple sclerosis (MS). Nearly all therapies that have been tested in MS clinical trials were first tested in EAE, which is a T cell-mediated autoimmune disease of the central nervous system (CNS). EAE is induced by immunization with myelin components including myelin basic protein (MBP), proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG) or myelin derived peptides [1]. Immunization of SJL (H-2s) or B10.PL (H-2u) mice with MBP or MBP-derived peptides in adjuvant induces a relapsingremitting or chronic form of EAE [2]. MBP T cell receptor (TCR) transgenic (Tg) mice bred on to the B10.PL background, recognize the NAc1-11 immunodominant epitope of MBP and are highly susceptible to EAE induction [3].
We and others have shown that the oral administration of MBP prior to encephalitogenic challenge results in protection from clinical signs and histopathologic changes of EAE in rats [4–6], in B10.PL mice [7–8] and in MBP TCR Tg mice [10,11]. The primary mechanisms of oral tolerance include anergy, deletion [5, 10–13], and active suppression mediated by regulatory T cells including CD4+CD25+ [14, 15, 18], Th3 [16], or Tr1 cells[17].
Recently, CC chemokines have been shown to play a role both in the pathogenesis of EAE and in the induction of oral tolerance [19–20]. It has been noted that CCL2/MCP-1 and its receptor CCR2 are expressed in the CNS, associated with neuroinflammatory diseases, such as MS and EAE [21]. MCP-1 has been shown to play a role in development of antigen specific oral tolerance [20], through induction of IL-4, which potentiates the development of Th2 cells [19–20]. Chemokine production within the gastrointestinal compartment and mucosal lymphoid tissue is thought to direct leukocyte trafficking and/or differentiation of T helper lymphocytes, which subsequently influences peripheral immune responses.
We previously reported that CC chemokines are increased in the CNS in MBP TCR Tg mice with spontaneous EAE [23]. In this study, we hypothesize that autoreactive T cells are attracted to the site of oral Ag deposition where they will interact with specific antigen. We determined CC chemokine production in the gut as well as in the CNS during the course of disease with or without oral administration of MBP. To study the migration of antigen-specific T cells, small numbers of TCR transgenic T cells were labeled with 5,6-carboxy-succinimidyl-fluorescein-ester (CFSE) and adoptively transferred into normal syngeneic recipients [22–25] followed by antigen feeding. Following the CFSE labeled cells in vivo, we observed that the cells behaved differently in the lymph node and PP compartments. Our results suggest that the PP represents a preferential site for antigen-specific T cell deletion in oral tolerance.
2. Materials and Methods
2.1. Animals
Vα4/Vβ8.2 MBP TCR Tg mice [3] were extensively backcrossed onto the B10.PL background, bred and housed in a clean SPF facility at The Ohio State University. Mice were screened by flow cytometry using peripheral blood leukocytes labeled with monoclonal antibodies directed against the clonotypic TCR (G19) or Vβ8.2 and CD4. The clonotypic antibody (G19) was a gift from Dr. Juan J. Lafaille. Transgenic animals were used in experiments at 6–8 weeks of age.
2.2. Neuroantigens
MBP was extracted from guinea pig spinal cords (Harlan Bioproducts for Science, Inc., Indianapolis, IN) using the method of Swanborg [26].
2.3. Induction of oral tolerance
Mice were deprived of food but not water for 4–6 h before oral administration of Ag. Mice were then given a total of 20 mg MBP suspended in 0.5 ml of PBS or PBS alone administered by gastric intubation. Immunization for EAE followed 7 days after the single feeding.
2.4. Induction of EAE
Mice were injected intradermally with 200 µg guinea pig MBP combined with complete Freund’s adjuvant (CFA) containing 200 µg Mycobacterium tuberculosis, Jamaica strain, over 4 sites on the flank. Mice also received two i.p. injections of 200ng pertussis toxin (List Biological, Campbell, CA), at the time of MBP injection and 48 hours later. Animals were observed daily for clinical signs and scored as follows: limp tail or waddling gait with tail tonicity 1+, waddling gait 2+, partial hind limb paralysis 3+, full hind limb paralysis 4+, death 5+.
2.5. Analysis of MCP-1 by ELISA
Deeply anesthetized mice were perfused with 50 ml of 0.2% D-glucose in PBS (pH 7.4) via the left ventricle. The brain, spinal cord, and small intestine were removed and homogenized in 2 ml of PBS (pH 7.4). Supernatants obtained by centrifugation at 10,000xg for 10 min were frozen at −70 °C until assay [27]. MCP-1 levels were quantitated using ELISA (R&D Systems, Minneapolis, MN). Tissue chemokine levels were expressed relative to total protein, measured using a protein quantification kit (Pierce, Rockford, IL).
2.6. Purification of CD4+ T cells
Single-cell suspensions were prepared from peripheral (inguinal, axillary, brachial, cervical, deep cervical, popliteal, periaortic) lymph nodes (pLNs), mesenteric lymph nodes (MLN) and spleens of naive MBP TCR Tg mice. CD4+ cells were purified by positive selection using magnetic bead columns (Miltenyi Biotec Corp., Auburn, CA). Cells were washed, counted, and incubated with magnetic bead conjugated anti-mouse CD4 (L3T4) (Miltenyi) for 15 min at 4 °C. Magnetically labeled CD4+ cells were eluted from selection columns (Miltenyi). Purity of CD4+ cells was routinely >95%.
2.7. CFSE labeling of CD4+ T cells and adoptive transfer
Purified CD4+ T cells from pooled LN/MLN and spleen cells (1:1) from naïve Vα4/Vβ8.2 mice were incubated with 5 µM CFSE (Molecular Probes, Eugene, OR) for 15 min. Cells were washed and transferred to sex matched B10.PL mice by i.v. injection (5–8×106 labeled cells/mouse). Labeling efficiency was determined to be greater than 98% by flow cytometery.
2.8. PP cell preparation
PP were excised from the wall of the small intestine, slit using a surgical blade and then teased gently in RPMI 1640 medium containing 10% fetal bovine serum (FBS). The cell suspension was passed through a stainless steel screen to remove cell debris and washed twice.
2.9. Flow cytometric analysis
Single cell suspensions from the pLNs and the PP were stained for Vβ8.2, CD69 and Fas (Pharmingen, San Diego, CA) with PE-conjugated mAb or CD4 with Cy-chrome-conjugated mAb respectively using three-color flow cytometry. Isotype control mAbs (Pharmingen) were matched for fluorochrome and used to set cursors. Lymphocytes were gated based on forward versus side scatter and a total of 200,000–500,000 events were analyzed on an EPICS XL flow cytometer (Coulter, Miami Lakes, FL).
2.10. Cell deletional analysis
PP cells were incubated with anti-Vβ8-PE for detection of the transgenic TCR. After washing, the cells were labeled with Annexin-V-Alexa 568 (Roche, Mannheim, Germany). Apoptotic cells were detected by flow cytometry using Annexin-V-Alexa 568 expression on CFSE+Vβ8+ cells, collecting 200,000 events per sample.
2.11. Statistical analysis
A non-parametric ANOVA with Tukey’s post-hoc test was performed to determine differences between vehicle-fed and MBP-fed groups. Groups were considered significantly different at p<0.05.
3. Results
3.1. Induction of increased MCP-1 in the gut by oral MBP correlates with protection from EAE
MCP-1 has been shown to play a prominent role in CNS inflammation in EAE, as well as a regulatory role in the induction of oral tolerance [20, 21, 30]. In this study, we hypothesized that MCP-1, induced in the small intestine by the oral administration of MBP, directs the autoreactive cells to migrate to the gut, thereby reducing the trafficking of autoreactive cells to the CNS and protecting mice from EAE. The data (Figure 1A) shows that control B10.PL mice, after immunization with MBP plus adjuvants, develop acute EAE (lasting from approximately day 15 to day 38) followed by disease remission around day 40–50 and subsequent relapses (starting around day 51). In contrast, mice are protected by feeding MBP 7 days prior to immunization (Figure 1A). It was noted that MCP-1 was decreased in both brain (Figure 1B) and spinal cord (Figure 1C) in MBP-fed mice; however, MCP-1 was increased in the small intestine (Figure 1D) of MBP-fed mice on days 9 and 12 after MBP immunization. We also determined MIP-1α and RANTES in the CNS and the gut, but there were no significant differences between MBP-fed and vehicle-fed groups (data not shown). Taken together, the data suggest that the induction of increased MCP-1 in the gut by feeding autoAg may play a role in the protection of mice from EAE.
Figure 1. Increased MCP-1 production in the gut is accompanied by protection of mice from EAE after oral MBP.

B10.PL mice (n = 10/group) were fed vehicle or 20 mg MBP 7 days before immunization with MBP/CFA/PT, and were scored daily for disease. The mean score of each group is shown (A). On the indicated day after immunization, brain (B), spinal cord (C), and small intestine tissue (D) were collected from individual mice and homogenized as described in Materials and Methods. The supernatants from individual mice were assayed by ELISA. Values are expressed as the concentration of chemokine/g protein.*: p<0.05 by ANOVA.
3.2. T cells traffic to both the lymph node and Peyer’s patch after oral administration of MBP
Are autoreactive T cells attracted to the gut in response to increased CC chemokine levels? To answer this question, we employed a transfer system [28] to avoid influences of immunization and adjuvant. CD4+ T cells (containing 88.6 ±7.4% Vβ8+G19+ Tg cells) were purified from naïve MBP TCR Tg mice, labeled with CFSE and transferred i.v. into sex- and age-matched B10.PL mice. One day later, the recipients were fed 20 mg MBP or PBS. We determined the percentage of CFSE+ cells in the CD4+ T cell population from the lymph node and PP on the indicated day after feeding. Figure 2 shows that CFSE-labeled Tg cells traffic to the peripheral LNs (Figure 2A) and to the PP (Figure 2B) as a result of antigen feeding. This same pattern is observed in the spleen and mesenteric LNs (data not shown). The labeled T cells peak in the LN at 48 hours and in the PP on day 7 after feeding (Figure 2B). We found very few CFSE+ cells amongst the intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) (data not shown), and therefore we focused on the PP.
Figure 2. Evidence for trafficking of CFSE-labeled Tg cells to the periphery and the PP.

CFSE-labeled CD4+ Tg cells (10 × 106) were injected i.v. into B10.PL mice. 24 hours later, the mice were fed 20 mg MBP or PBS. On the indicated day after feeding, transferred CFSE+ cells in lymph node (LN) and Peyer’s patch (PP) from each group of mice were analyzed by flow cytometry. Data are represented as % of total CD4+ T cells which are CFSE+ and is shown from one of three representative experiments.
We also followed the CFSE+ cells in vivo to determine their levels of proliferation in the pLN and the PP. As shown in Figure 3A, in vivo proliferation is not evident one day after MBP feeding in either vehicle-fed or MBP-fed groups, as indicated by the retention of the CFSE label in Vβ8+ cells. However, decreased intensity of CFSE staining appeared on day 2 and day 3 after MBP feeding in both the pLN and the PP, indicating that the transferred cells were dividing in both organs. Interestingly, by day 7, in vivo proliferation was observed in nearly all transferred cells in the PP but not the pLN from the MBP-fed group (Figure 3A, B). The results show that autoreactive T cells traffic (Figure 2) and proliferate (Figure 3) in both the peripheral LN and the PP at early timepoints (days 2 and days 3) following MBP feeding. The shift in CFSE+ cell number and proliferative activity from the lymph node to the PP suggests an active migration of antigen-specific cells to the PP. The PP may be a relevant site for activation induced cell death for autoreative T cells activated by orally delivered MBP.
Figure 3. In vivo proliferation of CFSE+ Tg cells in LN and PP after MBP feeding.

Lymphocytes from the same experiment described in Figure 2 were analyzed by flow cytometry. (A) Histograms were generated by gating on CFSE+Vβ8+ cells, and proliferating cells are indicated to the left of the vertical line. Percentages refer to the proportion of cells that have proliferated. (B) The percentages indicate the proportion of cells that have proliferated (dim-Vβ8+CFSE+) of CFSE+ cells from recipient mice. Data are shown from one of three representative experiments.
3.3. Increase in activation and deletion of autoreactive T cells in the Peyer’s patches
To determine the relationship between in vivo proliferation and activation, we analyzed the activation marker CD69 on CFSE+ cells 7 days after MBP feeding. Figure 4 shows that expression of CD69 is elevated in the PP greater than in the LN in the MBP-fed group. Therefore, the in vivo proliferation of autoreactive T cells (Figure 3) is accompanied by activation in the PP at the day 7 timepoint after MBP feeding.
Figure 4. Enhancement of CD69 and Fas expression on CFSE+ cells in the PP on day 7 after MBP feeding.
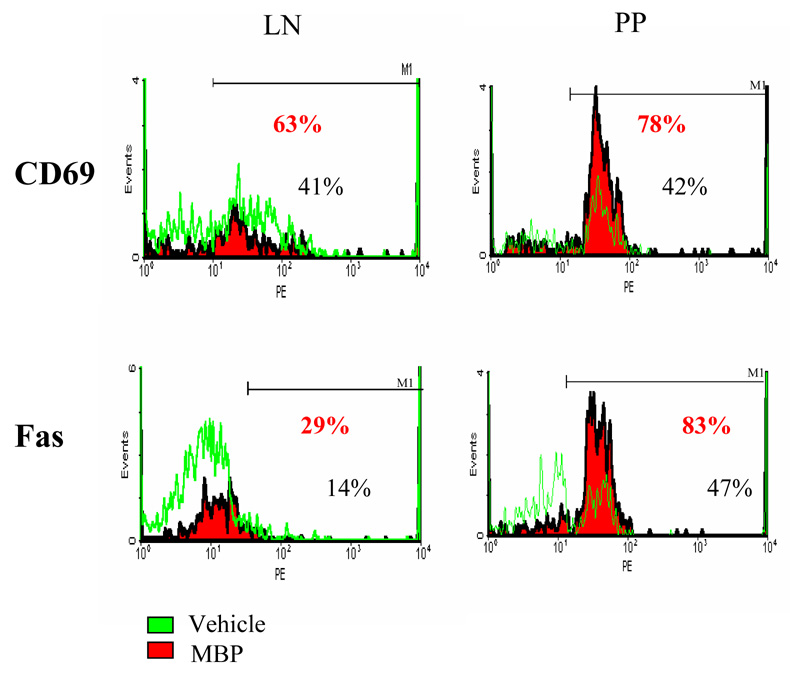
CD69 and Fas expression in PP cells were analyzed by flow cytometry, gating on CFSE+ cells. Data are shown from one of three representative experiments.
Apoptosis can occur through Fas/FasL interactions which have been shown to be critical for peripheral tolerance and lymphoid homeostasis (31). We previously reported that feeding high dose MBP to MBP TCR Tg mice induces early T cell activation and TCR downregulation which precedes deletion in the MLN [10]. In order to further elucidate the mechanisms of oral tolerance in the gut, we determined the expression of apoptosis markers such as Fas and Annexin-V in the CFSE+ T cells. Annexin-V is a Ca2+ dependent, phospholipid-binding protein with high affinity for phosphatidylserine, testing early stages of apoptosis. We observed higher levels of Fas expression (Figure 4) and Annexin-V (Table 1) on CFSE+ cells in the PP from MBP-fed mice on day 7 when compared with vehicle-fed controls. A similar extent of increase in CD69 and Fas was also observed in the lymph node of MBP-fed mice versus controls. However, Annexin-V staining of CFSE+ cells in the LN was not different between MBP-fed and vehicle-fed mice (data not shown). The results with Annexin staining in the Peyer’s patch confirm earlier studies by Chen et al [12] who showed a large number of TUNEL-positive cells in the dome area of the Peyer’s patches from mice fed high doses of ovalbumin. Taken together, the data suggests that oral administration of MBP induces proliferation of in vivo activated autoreactive T cells in the PP, that lead to the death of Ag-specific T cells. Therefore, these results suggest that the PP as well as the MLN represent sites for deletion of autoreactive T cells in oral tolerance.
Table 1.
Evidence for apoptosis of the transferred CFSE+ T cells after MBP feeding
| Tissue | Analysis | Treatment | |
|---|---|---|---|
| Vehicle-fed | MBP-fed | ||
| PP | aFas+/CFSE+ | 40.05% | 82.74% |
| bAnnexin-V+/CFSE+ | 9.17% | 41.30% | |
Fas expression in the PP cells was analyzed by flow cytometry by gating on CFSE+ cells. The percentages indicate the proportion of Fas+ cells on CFSE+ cells from recipient mice 7 days after feeding. Data is shown from one of two representative experiments.
Alexa-Annexin-V expression in the PP cells was analyzed by flow cytometry by gating on CFSE+ cells. The percentages indicate the proportion of Annexin-V+ cells on CFSE+ cells from recipient mice7 days after feeding. Data is shown from one of two representative experiments.
4. Discussion
MCP-1 or CC chemokine ligand 2 (CCL2) has been shown to be expressed in the CNS of MS patients and in mice with EAE [21]. MCP-1 can be induced in many cell types and has been reported to affect the migration of memory T lymphocytes, dendritic cells, natural killer cells and microglia [29]. Studies in EAE using neutralizing Ab, transgenic and knockout models imply a pathogenic role for MCP-1 in CNS inflammatory demylination [30,32,34]. Anti-MCP-1 treatment of mice, but not treatment with anti-MIP-1α, was shown to suppress the clinical severity of ongoing relapsing EAE [30]. On the other hand, MCP-1 has also been reported to regulate oral tolerance by downregulation of mucosal IL-12 expression and increased mucosal IL-4 production [20]. We propose that the function of MCP-1 is linked to its location in oral tolerance and in EAE: playing a pathogenic role in recruitment of encephalitogenic cells to the CNS but a regulatory role in recruitment of T cells to the gut induced by oral auto-Ag. Our data shows that oral administration of MBP induces less MCP-1 in the CNS and higher levels in the gut in the MBP-fed group relative to the vehicle-fed controls (Figure 1B and 1C). We elected to use vehicle-fed mice in these studies to properly control for periods of food deprivation as well as stress effects of feeding. Local increased chemokine production in the gut leads to T cell trafficking to the Peyer’s patch (Figure 2 and 4). Labeled Tg+ cells were not detected in the CNS of non-immunized mice in these experiments (data not shown), and activated Tg cells were significantly reduced in the CNS of Vα2.3/Vβ8.2 MBP TCR Tg mice directly fed MBP compared to vehicle-fed mice (Song et al unpublished data). Oral administration of autoAg likely leads to the recruitment of fewer activated T cells into the CNS.
In this study, we undertook a comparison of a peripheral and a mucosal lymphoid tissue site for trafficking of relevant cells and their ultimate function in those sites (peripheral lymph node and Peyer’s patch) during induction of oral tolerance. In the lymph node, antigen feeding resulted in an increased number of labeled antigen-specific cells migrating there within 48 hours which did not happen when vehicle was fed (Figure 2). During this same time frame, an equivalent number of labeled cells migrated to the Peyer’s patch whether mice were fed antigen or vehicle. In both sites, there was an equivalent amount of proliferative activity. By day 3 after antigen feeding, interesting differences started to emerge between the two sites. In the lymph node, both proliferation and numbers of labelled cells decreased to day 7 when relatively few proliferating cells were detected (Figure 3). In contrast, levels of proliferating cells continued to increase with time after feeding in the PP out to day 7. The preferential decrease in cell number and proliferative activity in the LN could reflect death of labelled cells in the LN or migration of cells to the PP. The increase observed in the PP later after antigen feeding would suggest the latter possibility. Migration of cells to the PP is also suggested by a lack of evidence for apoptosis in the LN as a result of antigen feeding.
The extent of T cell activation in the PP led us to explore the fate of those T cells and whether there was massive proliferation/expansion occurring or deletion (Figure 2, 3, 4 and Table 1). It is known that feeding large doses of antigen such as were fed in these studies (20 mg) results in anergy and deletion of antigen-specific T cell populations [10]. Therefore, the demonstration of activation-induced death in the PP as evidenced by Annexin positivity (Table 1) in the PP of MBP-fed mice showed that deletion was occurring. One earlier report [12] had shown apoptosis in the PP by TUNEL staining following ovalbumin feeding of an ovalbumin TCR transgenic mouse.
The transit of orally administered antigen through the gut mucosa is thought to follow one of two paths [33]. First in the case of large antigen doses, antigen can traverse the epithelium into the bloodstream, or go through the portal circulation to peripheral lymphoid tissue. Another pathway is the transport of Ag through the M cells into the PP, where it is taken up by dendritic cells (DCs). DCs present MBP to Ag-specific T cells which are activated and proliferate in the PP and mesenteric LN [10]. The data represented here shows an increase in Fas expression at the later timepoint that appears to promote T cell deletion in the gut. Suvannavejh et al reported that Fas-deficient mice have significantly elevated clinical scores of EAE and reduced remission rates [40]. These findings suggest that apoptosis plays a critical role for mediating remissions of relapsing EAE that indirectly support our data. Therefore, protection from EAE may result from a decrease in the number of activated T cells trafficking to the CNS [34], due to deletion of autoreactive cells in the gut and periphery.
It has been noted that M cells and PPs play important roles in the induction of oral tolerance, since oral tolerance is not observed in lymphotoxin α (TLα−/−) and β (TLβ−/−) knockout mice [35,36] as well as in mice treated with TLβ receptor Ig fusion protein, all of which lack PPs [37]. In contrast, Kraus et al reported that both high- and low-dose tolerance could be induced in the absence of PPs using a ligated small bowel loop system [38]. The authors observed a reduction in OVA-specific T cell proliferation, serum antibody responses and Th1 cytokines in PP-containing loops and in loops without PP in their OVA experimental system in BALB/C mice [38]. However, the authors did not study whether activation induced cell death played a role in oral tolerance in their OVA system (non-disease model). Our observation that the PP is a site for deletion of autoreactive T cells was based on oral administration of self-neuro-Ag in an autoimmune disease model using a cell transfer system.
The possibility exists that the PP may be a major site, but not the exclusive site for T cell deletion in oral tolerance. Our previous work has shown evidence for deletion occurring in the MLN around the same time as we observe deletion in the PP [10]. More importantly, it should be recognized that mechanisms other than deletion are likely operative in oral tolerance, such as T regulatory cells. One can envision that several mechanisms (deletion, anergy, immune deviaion, and T regulatory cells) may work in concert and at separate locations which serve to render an antigen-fed animal tolerant to the fed antigen.
Recently, Depaolo et al has reported that both MCP-1 and its receptor CCR2 are critical for the induction of oral tolerance since MCP-1−/− and CCR2−/− mice did not exhibit oral tolerance in response to OVA feeding [39]. The authors concluded that MCP-1 and CCR2 regulate Ag presentation, and their absence leads to a disruption in the balance of inflammatory and regulatory cytokines. In addition, Karpus et al reported that oral administration of antigen (PLP in SJL mice) was associated with an increase in intestinal mucosal MCP-1 and IL-4 [20]. It is possible that MBP feeding may also induce the production of suppressive cytokines such as IL-4, IL-10 and TGFβ in the gut of recipient mice. Our preliminary observations show IL-10 production in the PP on day 3 only in the MBP-fed group (data not shown), and the expression of CD25 on CFSE+ cells is not significantly different between MBP-fed and control groups (data not shown). Thus, the model suggests that the mechanisms of oral tolerance include both anergy/deletion and regulatory cells.
Recently, new evidence has emerged to indicate that self antigens (including MBP and MOG) may be post-translationally modified into new epitopes. Lolli et al [9] designed an antigenic probe for measuring IgM autoantibodies in the sera of MS patients. This probe recognized autoantibodies specific for myelin and oligodendrocyte antigens and was present in MS sera but not other autoimmune diseases, suggesting its use as a new biomarker for MS. This development suggests that a broader array of self antigens and modified self antigens should be targeted for therapeutic consideration in autoimmunity rather than a focus on nascent myelin antigens.
In summary, the present study provides evidence that T cell deletion by a mechanism of activation induced cell death in the gut plays an essential role in the induction of oral tolerance. We show that the PP is an important site for clonal deletion of autoreactive T cells in oral tolerance. Understanding the roles of the PP in the induction of tolerance pathways in EAE may provide new insights into immunotherapy for the treatment of MS.
Acknowledgments
This work was supported by NIH grants NS 23561, AI 35960; Fei Song was a fellow of the National Multiple Sclerosis Society (FA 1270-A-1). The authors thank Nicole Damico Powell for her help in preparing this manuscript.
Abbreviations used in this paper
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- MBP
myelin basic protein
- CNS
central nervous system
- PP
Peyer’s patches
- CFSE
5,6-carboxy-succinimidyl-fluorenscein-ester
- Ag
antigen
- GALT
gut-associated lymphoid tissue
- MOG
myelin oligodendrocyte glycoprotein
- PLP
proteolipid protein
- TCR
T cell receptor
- Tg
transgenic
- CFA
complete Freund’s adjuvant
- pLN
peripheral lymph node
- MLN
mesenteric lymph node
- IEL
intraepithelial lymphocytes
- LPL
lamina propria lymphocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit. Rev. Clin. Lab. Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 2.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 3.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 4.Bitar DM, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cell. Immunol. 1988;112:364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- 5.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J. Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 6.Miller A, Lider O, al-Sabbagh A, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. V. Hierarchy of suppression by myelin basic protein from different species. J. Neuroimmunol. 1992;39:243–250. doi: 10.1016/0165-5728(92)90258-m. [DOI] [PubMed] [Google Scholar]
- 7.Meyer AL, Benson JM, Gienapp IE, Cox KL, Whitacre CC. Suppression of murine chronic relapsing experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. J. Immunol. 1996;157:4230–4238. [PubMed] [Google Scholar]
- 8.Benson JM, Stuckman SS, Cox KLR, Wardrop M, Gienapp IE, Cross AH, Trotter JL, Whitacre CC. Oral Administration of Myelin Basic Protein Is Superior to Myelin in Suppressing Established Relapsing Experimental Autoimmune Encephalomyelitis. J. Immunol. 1999;162:6247–6254. [PubMed] [Google Scholar]
- 9.Lolli F, Mulinacci B, Carotenuto A, Bonetti B, Sabatino G, Mazzanti B, D'Ursi AM, Novellino E, Pazzagli M, Lovato L, Alcaro MC, Peroni E, Pozo-Carrero MC, Nuti F, Battistini L, Borsellino G, Chelli M, Rovero P, Papini AM. An N-glucosylated peptide detecting disease-specific autoantibodies, biomarkers of multiple sclerosis. Proc Natl Acad Sci U S A. 2005;102:10273–10278. doi: 10.1073/pnas.0503178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson JM, Campbell KA, Guan Z, Gienapp IE, Stuckman SS, Forsthuber T, Whitacre CC. T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. J. Clin. Invest. 2000;106:1031–1038. doi: 10.1172/JCI10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song F, Wardrop RM, Gienapp IE, Stuckman SS, Goverman J, Whitacre CC. Differences between two strains of myelin basic protein (MBP) TCR transgenic mice: implications for tolerance induction. J Autoimmun. 2002;18:27–37. doi: 10.1006/jaut.2001.0567. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 13.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28:59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 16.Gonnella PA, Chen YH, Waldner H, Weiner HL. Induction of oral tolerization in CD86 deficient mice: a role for CD86 and B cells in the upregulation of TGF-beta. J Autoimmun. 2006;26:73–81. doi: 10.1016/j.jaut.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Smith CE, Miller SD. Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. J Autoimmun. 2006;27:218–231. doi: 10.1016/j.jaut.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma R, Jarjour WN, Zheng L, Gaskin F, Fu SM, Ju ST. Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice. J Autoimmun. 2007;29:10–19. doi: 10.1016/j.jaut.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukacs NW, Chensue SW, Karpus WJ, Lincoln P, Keefer C, Strieter RM, Kunkel SL. C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am. J. Pathol. 1997;150:1861–1868. [PMC free article] [PubMed] [Google Scholar]
- 20.Karpus WJ, Kennedy KJ, Kunkel SL, Lukacs NW. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J. Exp. Med. 1998;187:733–741. doi: 10.1084/jem.187.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin, Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Dirden-Kramer B, Ito K, Ernst PB, Van Houten N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J. Immunol. 1999;162:5868–5875. [PubMed] [Google Scholar]
- 23.Song F, Wardrop R, Stuckman S, Gienapp I, Whitacre CC. T cell activation and proliferation in gut-associated lymphoid tissue (GALT) during oral tolerance induction. FASEB. 2000;14:A1198. [Google Scholar]
- 24.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J. Exp. Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanas E, Davey GM, Carbone FR, Heath WR. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J. Immunol. 2000;164:2890–2896. doi: 10.4049/jimmunol.164.6.2890. [DOI] [PubMed] [Google Scholar]
- 26.Swanborg RH, Swierkosz JE, Saieg RG. Studies on the species-variability of experimental allergic encephalomyelitis in guinea pigs and rats. J. Immunol. 1974;112:594–600. [PubMed] [Google Scholar]
- 27.Song F, Gienapp IE, Wang X, Whitacre CC. Activation of Vbeta8 T cells affects spontaneous EAE in MBP TCR transgenic mice. J. Neuroimmunol. 2002;123:112–122. doi: 10.1016/s0165-5728(01)00494-5. [DOI] [PubMed] [Google Scholar]
- 28.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 29.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 30.Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J. Neuroimmunol. 1998;92:98–108. doi: 10.1016/s0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17:583–588. doi: 10.1016/j.coi.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J. Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- 33.Song F, Whitacre CC. The role of the gut lymphoid tissue in induction of oral tolerance. Curr. Opin. Investig. Drugs. 2001;2:1382–1386. [PubMed] [Google Scholar]
- 34.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 36.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 37.Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR. Peyer's patches are required for oral tolerance to proteins. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3310–3315. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, Boros P, Mayer L. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J. Clin. Invest. 2005;115:2234–2243. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DePaolo RW, Rollins BJ, Kuziel W, Karpus WJ. CC chemokine ligand 2 and its receptor regulate mucosal production of IL-12 and TGF-beta in high dose oral tolerance. J. Immunol. 2003;171:3560–3567. doi: 10.4049/jimmunol.171.7.3560. [DOI] [PubMed] [Google Scholar]
- 40.Suvannavejh GC, Dal Canto MC, Matis LA, Miller SD. Fas-mediated apoptosis in clinical remissions of relapsing experimental autoimmune encephalomyelitis. J Clin Invest. 2000;105:223–231. doi: 10.1172/JCI8561. [DOI] [PMC free article] [PubMed] [Google Scholar]


