Abstract
Background
The American Heart Association (AHA) has defined the concept of ideal cardiovascular health in promotion of their 2020 Strategic Impact Goals. We examined if adherence to ideal levels of the seven AHA cardiovascular health metrics was associated with incident cancers in the Atherosclerosis Risk In Communities (ARIC) study over 17-19 years of follow-up.
Methods and Results
After exclusions for missing data and prevalent cancer, 13,253 ARIC participants were included for analysis. Baseline measurements were used to classify participants according to seven AHA cardiovascular health metrics. Combined cancer incidence (excluding non-melanoma skin cancers) from 1987-2006 was captured using cancer registries and hospital surveillance; 2880 incident cancer cases occurred over follow-up. Cox regression was used to calculate hazard ratios for incident cancer. There was a significant (p-trend< .0001), graded, inverse association between the number of ideal cardiovascular health metrics at baseline and cancer incidence. Participants meeting goals for 6-7 ideal health metrics (2.7% of the population) had 51% lower risk of incident cancer than those meeting goals for 0 ideal health metrics. When smoking was removed from the sum of ideal health metrics, the association was attenuated with participants meeting goals for 5-6 health metrics having 25% lower cancer risk than those meeting goals for 0 ideal health metrics (p-trend = .03).
Conclusions
Adherence to the seven ideal health metrics defined in the AHA 2020 goals is associated with lower cancer incidence. The AHA should continue to pursue partnerships with cancer advocacy groups to achieve reductions in chronic disease prevalence.
Keywords: ideal cardiovascular health, cancer, prevention
In 2010 the American Heart Association (AHA) announced the following strategic impact goal: “By 2020, to improve the cardiovascular health of all Americans by 20% while reducing death from cardiovascular diseases and stroke by 20%.”1 To accomplish this goal, the concept of ideal cardiovascular health was defined according to seven health behaviors or factors, which include smoking, physical activity, obesity, dietary intake, total cholesterol, blood pressure and blood sugar . The idea of working to attain the ideal goals for these seven health factors and behaviors is now being promoted through the use of the My Life Check™ online health assessment tool and the Life's Simple Seven™ health campaign from the AHA and American Stroke Association.
Following the announcement of these goals, research in a variety of populations has demonstrated that meeting the goals for a higher number of ideal health metrics is associated with more favorable health outcomes. In the Atherosclerosis Risk In Communities (ARIC) study, there was a strong graded relationship between the number of ideal health metrics met at baseline (when participants were age 45-64) and incident cardiovascular disease (CVD) over 20 years of follow-up2. The Cardiovascular Risk in Young Finns Study demonstrated that the number of ideal cardiovascular health metrics present in childhood predicts subsequent cardiometabolic health in adulthood3. Finally, analyses of the National Health And Nutrition Examination Survey demonstrated that the number of ideal metrics met was significantly and inversely related to mortality from all causes and mortality from diseases of the circulatory system 4.
Although the health metrics identified by the AHA Strategic Planning Task Force and Statistics Committee were selected primarily due to their strong associations with CVD1, many of the metrics, such as diet 5-7, physical activity 8-11, BMI12-14, and smoking 15-17, are also established risk factors for many types of cancer. Due to these shared risk associations, we investigated whether the number of achieved ideal cardiovascular metrics (as defined by the AHA) is also significantly inversely associated with incident cancer. We chose to pursue this analysis in the ARIC Study, which has information on both incident cancer and cardiovascular disease in a large population-based, bi-racial, geographically diverse cohort, to facilitate informal comparisons between the association of ideal cardiovascular health metrics with incident CVD and cancer.
Methods
The ARIC study is a multicenter prospective study originally conceived to investigate cardiovascular disease.18 White and black men and women aged 45 to 64 years were recruited in 1987 to 1989 from 4 communities: Forsyth County, North Carolina; Jackson, Mississippi; suburban areas of Minneapolis, Minnesota; and Washington County, Maryland. A total of 15,792 subjects participated in the baseline examination. Three triennial follow-up examinations were performed. The institutional review board at each field center approved the study, and all participants gave informed consent, which included consent for follow-up disease occurrence.
Of the 15,792 ARIC participants, we excluded anyone lacking any one of the measurements necessary to classify the participant on all seven ideal health metrics (n = 1536). Additionally, we excluded anyone who did not give permission for their data to be used in non-cardiovascular disease research (n = 11), and participants who self- reported a race other than white or African American (n = 42). Participants were queried about their history of cancer at baseline and participants who reported a personal history of cancer at baseline were excluded (n = 950), resulting in a final sample size of 13,253.
Exposure measurements
Home interviews and medical examinations were conducted at each study visit. Baseline exposure information was used to classify all participants on seven ideal cardiovascular health metrics outlined in the AHA 2020 goals1 (please see a complete description of the seven ideal cardiovascular health metrics in Table 2 of the AHA 2010 Scientific Statement “Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association's Strategic Impact Goal Through 2020 and Beyond” available at http://my.americanheart.org/professional/StatementsGuidelines/ByPublicationDate/PreviousYears/2010-Publications_UCM_322319_Article.jsp). We refer to these simply as “ideal health metrics”. Per the AHA 2020 report, four ideal health metrics (never smoking or quitting more than 12 months ago; having body mass index (BMI) < 25 kg/m2; having 4-5 components of a healthy diet score, and having at least 75 min/week of vigorous physical activity [or 150 min/week of moderate or moderate + vigorous activity]) were identified as ideal health behaviors and three ideal health metrics (having untreated total cholesterol less than 200 mg/dl, having untreated blood pressure less than 120mm Hg systolic and 80 mm Hg diastolic, and having untreated fasting serum glucose less than 100 mg/dl) were identified as ideal health factors. Specific methods to classify ARIC participants according to the seven ideal health metrics have been described in-depth previously, and every effort was made to use identical classifications for this analysis 2. Briefly, diet was assessed by a slightly modified 66-item Harvard food frequency questionnaire19 (modified for application in a bi-racial cohort 20) and then categorized based on achievement of the five AHA ideal cardiovascular health diet components. Physical activity was reported with the Baecke questionnaire21, and smoking status was derived from interviews. Use of antihypertensive, cholesterol-lowering, and glucose-lowering medications within the past 2 weeks of baseline interview were self-reported or taken from prescription bottles. Fasting plasma total cholesterol was measured by enzymatic methods. Serum fasting glucose was measured by a hexokinase/glucose-6-phosphate dehydrogenase method. Sitting blood pressure was measured 3 times using a random-zero sphygmomanometer and the average of the 2nd and 3rd measurements used for analysis. BMI (kg/m2) was computed from weight while wearing a scrub suit and standing height.
Table 2. Incident combined cancer rates by number of ideal health metrics: The ARIC Study, 1987-2006.
| # Ideal health metrics | Total sample % (n= 13253) | # Cancer cases | Incidence rate per 1000 person-years* | Hazard Ratio (95% C.I)*† |
|---|---|---|---|---|
| 0 | 2.8 | 95 | 17.3 | 1.0 (referent) |
| 1 | 15.7 | 475 | 14.3 | 0.79 (0.64-0.98) |
| 2 | 25.9 | 815 | 14.3 | 0.79 (0.64-0.98) |
| 3 | 26.3 | 779 | 13.4 | 0.74 (0.59-0.91) |
| 4 | 17.8 | 463 | 12.3 | 0.67 (0.54-0.84) |
| 5 | 8.8 | 203 | 11.3 | 0.61 (0.48-0.79) |
| 6-7 | 2.7 | 50 | 9.0 | 0.49 (0.35-0.69) |
adjusted for age, sex, race, and ARIC center
trend test for this association; Hazard Ratio per 1 increase in number of ideal heath metrics = 0.92, p-trend < .0001
Ascertainment of incident cancer
The ascertainment of incident cancer cases in ARIC has been described previously22. Incident cancer cases from 1987-2006 were obtained by linking to cancer registries. ARIC hospital surveillance was used to identify additional cancer cases. For participants who had hospital ICD codes for cancer but were not in cancer registries, including those who may have moved, records of hospitalized events were obtained on a yearly basis. Primary site and date of cancer diagnosis were obtained. For analysis, we combined all incident cancer cases, except for cases of non-melanoma skin cancer. We conducted secondary analyses on female breast, colorectal, prostate, and lung cancer as these are the four most common types of incident non-skin cancer observed both in the ARIC cohort and the United States population. If a participant had more than one type of incident cancer during follow-up, the earliest date of cancer incidence was chosen for analysis of the combined endpoint.
Statistical Methods
All statistical analyses were performed in SAS, version 9.2. If a participant was classified as having a given ideal health metric at baseline, the participant was coded as 1 for this metric (others were coded as 0). The total number of ideal health metrics was summed for each individual, resulting in a score of 0 (having no ideal health metrics at baseline) to 7 (having all seven ideal health metrics at baseline). Because so few ARIC participants had all seven ideal health metrics, participants having six or seven ideal health metrics (a score of 6 or 7) were grouped together for analysis. Poisson regression was used to calculate age, sex, race and ARIC center adjusted rates (and 95% C.I.) for combined cancer incidence. Adjusted hazard ratios for combined cancer incidence by ideal health metrics were calculated using Cox proportional hazards models. Individuals who died or were lost to follow-up were censored in Poisson and Cox analyses. Tests of trend for hazard ratios across ideal health metrics were performed by including the ordinal ideal health metric variable modeled as a continuous variable in Cox models. We tested the proportional hazard assumption for the association of ideal health metrics with incident cancer using an interaction of the ideal health variable with follow-up time and found the assumption was not violated (p = .59). Survival functions by number of ideal health metrics were calculated using the life-table method in PROC LIFETEST. Secondary analyses were performed examining associations of ideal health metrics with types of incident cancer individually.
Results
Table 1 presents the characteristics of the 13,253 ARIC participants reporting no history of cancer at baseline, by gender. The proportions of participants (both genders combined) who had ideal levels of individual health metrics were very similar to the proportions reported previously in the 12,744 ARIC participants free of cardiovascular disease at baseline 2: 71.5 % had ideal levels of (not) smoking, 33.2% had ideal levels of BMI, 36.9 % had ideal levels of total cholesterol, 5.3 % had ideal diet, 37.9 % had ideal levels of physical activity, 51.8 % had ideal levels of blood sugar, and 41.6% had ideal levels of blood pressure. When the total number of ideal metrics was summed, most individuals had 2 or 3 ideal health metrics, with only 16 individuals (0.1%) having all seven ideal health metrics.
Table 1. Characteristics of baseline participants without history of cancer reported at baseline, by gender: The ARIC Study, 1987-1989.
| Female (n = 7223) | Male (n=6030) | |
|---|---|---|
| Age* | 53.7 (5.7) | 54.6 (5.8) |
| African American (%) | 28.2 | 21.4 |
| ARIC Center(%) | ||
| Forsyth County, NC | 25.4 | 26.4 |
| Jackson, MS | 24.8 | 18.4 |
| Minneapolis, MN | 24.5 | 28.4 |
| Washington County, MD | 25.3 | 26.8 |
| Number of ideal health metrics present (%) | ||
| 0 | 2.7 | 3.0 |
| 1 | 15.4 | 16.0 |
| 2 | 23.8 | 28.4 |
| 3 | 25.1 | 27.7 |
| 4 | 19.3 | 16.1 |
| 5 | 10.4 | 6.8 |
| 6 | 3.2 | 1.9 |
| 7 | 0.1 | 0.1 |
mean(sd)
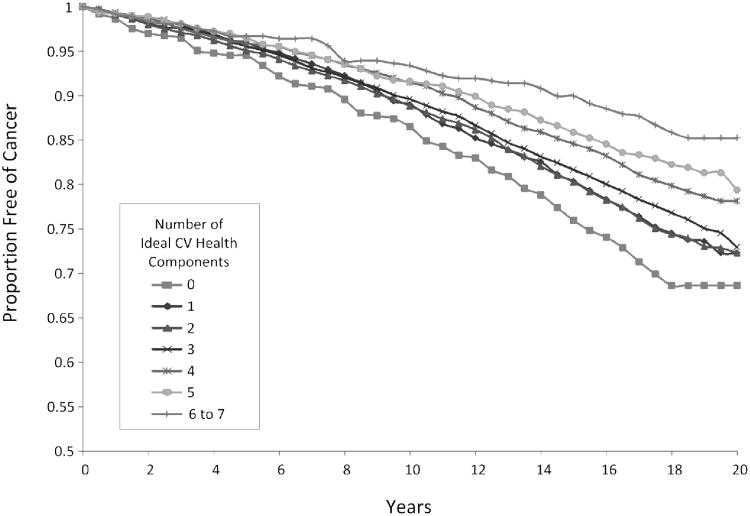
Over the 17-19 years of follow-up for which cancer outcomes were available, 2880 ARIC participants developed incident cancer. There were 418 incident lung cancer cases, 322 incident colorectal cancer cases, 613 incident prostate cancer cases, and 526 incident female breast cancer cases. Supplementary Table 1 presents the number of incident cancers 1989-2006 by demographic subgroups. Table 2 presents the adjusted incidence rates and hazard ratios for combined cancer according to the number of ideal health metrics. There was an inverse graded combined cancer incidence rate in relation to a larger number of ideal health metrics; participants with 3 ideal health metrics had 25% lower risk of incident cancer and participants with 6-7 ideal health metrics had over 50% lower risk of incident cancer than those with 0 ideal health metrics. In the proportional hazards regression model adjusting for age, sex, race, and ARIC center, the trend of lower cancer incidence with higher numbers of ideal health metrics was statistically significant (p-trend < .0001). Results were similar when cases of cancer occurring in the first three years after follow-up were removed from the analysis. Figure 1 presents survival curves for combined cancer by sum of ideal health metrics in ARIC. When the smoking metric was removed from the sum of ideal health metrics (resulting in a possible total of 0 to 6 ideal health metrics for each individual) the observed trend of lower cancer incidence with a larger number of ideal health metrics was attenuated; participants with 3 ideal health metrics had 2% lower risk of incident cancer and participants with 5-6 ideal health metrics had 25% lower risk of incident cancer than those with 0 ideal health metrics. The trend test between number of ideal health metrics and incident cancer was still statistically significant (p-trend = .03). Supplementary Figure 1 presents survival curves for combined cancer incidence by sum of ideal health metrics (with the ideal smoking metric removed). After about 10 years of follow-up, separation is seen between the curves for those with 4 or 5-6 ideal health metrics, compared with those having 0-3 ideal health metrics. When the association of all seven ideal health metrics was examined with breast, lung, and colorectal incident cancers individually (see Supplementary Table 2), a trend of lower cancer incidence with a larger number of ideal health metrics was observed for breast cancer (p-trend = .11), lung cancer (p-trend < .0001) and colorectal cancer (p-trend = .0092). When the association of all seven ideal health metrics was examined with prostate cancer, a modest but significant trend of higher cancer incidence with larger number of ideal health metrics was observed (p-trend = .02).
Figure 1.
Survival curves for combined cancer incidence by total number of ideal health metrics, ARIC 1987-2006.
Cumulative cancer-free survival according to number of ideal cardiovascular health metrics, ARIC study 1987-2006
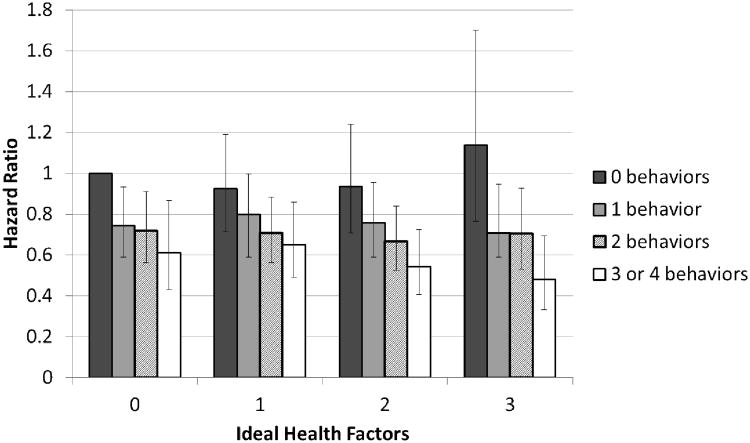
Figure 2 displays adjusted hazard ratios for combined cancer incidence by both number of ideal health behaviors (diet, smoking, physical activity and BMI) and factors (blood pressure, blood sugar, and total cholesterol) with individuals having 0 ideal heath factors and 0 ideal health behaviors being the referent group. A pattern of lower cancer incidence is generally observed across higher numbers of ideal health behaviors, while no consistent pattern in cancer incidence was observed across number of ideal health factors. In all categories of ideal health factors, the hazard ratio for combined cancer incidence in individuals with 3-4 ideal health behaviors compared to those with 0 ideal health behaviors was significantly less than 1. Participants with 3 ideal health factors and 3-4 ideal health behaviors were .48 times less likely to develop cancer than those with 0 ideal health factors and 0 ideal health behaviors.
Figure 2. Hazard ratios* of combined cancer according to the number of ideal health behaviors and ideal health factors, ARIC, 1987-2006.
*All hazard ratios are adjusted for age, sex, race and ARIC study center. The referent category (farthest left, above) is participants having 0 ideal heath factors (blood pressure, blood sugar, and total cholesterol) and 0 ideal health behaviors (diet, smoking, physical activity and BMI) at baseline. Across all categories of ideal health factors, the hazard ratio for combined cancer incidence in individuals with 3-4 ideal cardiovascular health behaviors compared to those with 0 idea health behaviors was significantly less than 1.
Discussion
In this prospective study, there was a significant, graded, inverse association between the number of ideal cardiovascular health metrics (as defined by the AHA) during middle age and combined cancer incidence (excluding non-melanoma skin cancers) over nearly twenty years of follow-up. This result is consistent with other analyses that have demonstrated a significant association between adherence to lifestyle guidelines similar to the behaviors endorsed by the AHA and lower incidence of cancer, such as an analysis in the Iowa Women's Health Study Cohort which showed a negative association between adherence to American Institute for Cancer Research guidelines and cancer incidence and mortality 23. Our objective was not to determine if this association is entirely independent of other known cancer risk factors, and thus we did not attempt to control for confounding with extensive adjustment. Instead, we sought simply to demonstrate that adherence to ideal cardiovascular health, as proposed by the American Heart Association, is associated with a lower incidence of cancer.
A previous paper from the ARIC study demonstrated a strong and graded association between the number of ideal health metrics at baseline and incident CVD over 20 years of follow-up 2. The association of ideal health metrics with incident CVD is stronger than that for incident combined cancer (for example: the HR for CVD comparing individuals with 5 ideal metrics at baseline to those with 0 was 0.182, the same HR for combined cancer was 0.61). However, the association of incident combined cancer with ideal health metrics was strongly significant and having 6 or more ideal health metrics was associated with a substantial (i.e. 51%) reduction in cancer risk.
To address the concern that the observed association was due solely to one component of ideal cardiovascular health (smoking) or one type of cancer (lung), we conducted additional analyses removing smoking from the score of ideal health metrics and repeated our analyses for the four most common incident cancers individually. The test of trend for the association of ideal health metrics with incident cancer was attenuated after smoking was removed from the score, but the association remained statistically significant and supplementary figure 1 demonstrates the survival curves for those with at least 4 ideal health metrics (representing about 15% of the sample) diverging from curves for those with fewer baseline ideal health metrics. In addition to lung cancer, there was a significant inverse relationship between number of ideal health metrics and colorectal cancer, and the association with breast cancer was also approaching statistical significance (p = .11). Nonetheless, it appears that ideal levels of smoking are responsible for driving a large portion of the negative association between ideal cardiovascular health and cancer incidence. Also, once smoking is removed from the score, only a small percentage of the sample was achieving the number of healthy behaviors or factors (4-6) that might notably reduce their risk of cancer.
We were surprised to observe a modest but significant trend of higher prostate cancer incidence with larger number of ideal health metrics. We hypothesize this association might be driven by the inclusion of smoking in the ideal health metrics score as, in a previous cohort study including over 250,000 men, smoking was inversely associated with non-advanced prostate cancer, but positively associated with fatal prostate cancer 24. We could not examine the association of ideal health metrics with incident prostate cancer by stage in ARIC, but, when we removed smoking from the score of ideal health metrics, the association of the score with higher prostate cancer incidence was no longer observed (p-trend = .40).
The results presented in Figure 2 demonstrate that, as one might expect, incident combined cancer was significantly associated with ideal health behaviors (not smoking, physical activity, low BMI, and healthy diet) and not with ideal health factors (blood sugar, blood pressure, and total cholesterol). This result may be explained, in part, by the relatively low concordance between ideal diet and ideal health factors due to the particularly low prevalence of ideal diet as defined by the AHA. It is also important to note that some of the cardiovascular ideal health behavior definitions may not be optimal for cancer prevention; for example quitting smoking only 12 months ago and having an underweight BMI may not be the best classification of a healthy cancer behavior. However, we believe that the most important overall message from this paper is that adherence to the seven ideal (cardiovascular) health metrics as proposed by the AHA is associated not only with lower CVD incidence and total mortality, but also with lower cancer incidence. There are many health messages presented in the popular press and frequent (and sometimes contradictory) reports of novel risk factors for disease. These messages sometimes confuse consumers, leaving them unsure on the most important steps to take for disease prevention. We hope that emphasizing a unified approach from multiple chronic disease advocacy groups, promoting some common steps for disease prevention, will be particularly effective in helping the public to prevent chronic disease. This analysis demonstrates that promoting the ideal health metrics proposed by the AHA (and communicated to the public through the Life's Simple Seven™ campaign) could reduce both CVD and cancer incidence. Cancer advocacy groups are likely willing partners in the promotion of Life's Simple Seven™ perhaps with slight modifications; recent guidelines from the American Cancer Society on diet and nutrition are similar to elements of AHA's ideal cardiovascular health metrics as ACS sought to be “consistent with guidelines from the American Heart Association and the American Diabetes Association for the prevention of coronary heart disease and diabetes.”25 In addition, a group from the American Cancer Society recently published a report showing that adherence to American Cancer Society guidelines resulted in a reduction in CVD mortality 26.
Our study has several strengths. The ARIC study's prospective design allows for the examination of baseline ideal health factors with subsequent cancer diagnoses, analyses not possible in most cancer case-control studies. The large sample size and long-follow up of the ARIC study provided many cases of cancer, providing good power to detect associations with ideal health metrics. The use of cancer registries plus hospital records to capture cancer diagnoses allowed for good ascertainment of cancer cases. However, there was likely incomplete ascertainment of cancer cases in the Mississippi cohort as a state registry has not covered the Jackson, MS ARIC study center continuously. Additionally, cancer cases that migrated from the ARIC study areas and were not hospitalized as a result of their cancer may also not have been captured. As discussed in the previous analysis of incident CVD and ideal health metrics in ARIC, the use of ideal health metrics measured at baseline does not take into account changes in risk factor levels that occurred over the lengthy period of follow-up2. Also, self-reported diet and exercise likely have measurement error compared to objective measures of the same variables19, 27. We believe both these potential instances of exposure misclassification would most likely have occurred at random with respect to future cancer incidence and thus would be expected to bias our estimate of the association between ideal health metrics and incident combined cancer toward the null.
In conclusion, in the ARIC cohort, there was a significant inverse relation between the number of ideal cardiovascular health metrics at baseline, as defined by the AHA 1, and combined cancer incidence. These results should encourage the AHA in their efforts to partner with cancer and other chronic disease advocacy groups to promote the AHA 2020 goals in order to reduce the burden of CVD as well as other highly prevalent chronic diseases.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. Some cancer incidence data have been provided by the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Mental Health and Hygiene, 201 W. Preston Street, Room 400, Baltimore, MD 21201.
Funding Sources: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). We acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries (NPCR of the Centers for Disease Control and Prevention (CDC)) for the funds that helped support the availability of the cancer registry data.
Footnotes
No disclosures to report
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 2.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the american heart association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, Mikkila V, Jokinen E, Hutri-Kahonen N, Laitinen T, Kahonen M, Lehtimaki T, Raitakari OT, Juonala M. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: The cardiovascular risk in young finns study. Circulation. 2012;125:1971–1978. doi: 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the united states. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune D, Chan DS, Vieira AR, Rosenblatt DA, Vieira R, Greenwood DC, Norat T. Fruits, vegetables and breast cancer risk: A systematic review and meta-analysis of prospective studies. Breast cancer research and treatment. 2012;134:479–493. doi: 10.1007/s10549-012-2118-1. [DOI] [PubMed] [Google Scholar]
- 7.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: The women's health initiative randomized controlled dietary modification trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 8.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758–1764. doi: 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, Woods N, Ockene J. Recreational physical activity and the risk of breast cancer in postmenopausal women: The women's health initiative cohort study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 10.Wolin KY, Yan Y, Colditz GA. Physical activity and risk of colon adenoma: A meta-analysis. British journal of cancer. 2011;104:882–885. doi: 10.1038/sj.bjc.6606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiseman M. The second world cancer research fund/american institute for cancer research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. The Proceedings of the Nutrition Society. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 12.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of u.S. Adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 13.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 14.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 15.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: A meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, Sinha R, Allison J. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104:1353–1362. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelsey JL, Berkowitz GS. Breast cancer epidemiology. Cancer Res. 1988;48:5615–5623. [PubMed] [Google Scholar]
- 18.The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 20.Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T, Folsom AR. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutrition Research. 1996;16:735–745. [Google Scholar]
- 21.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Association of inflammatory markers with colorectal cancer incidence in the atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev. 2011;20:297–307. doi: 10.1158/1055-9965.EPI-10-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerhan JR, Potter JD, Gilmore JM, Janney CA, Kushi LH, Lazovich D, Anderson KE, Sellers TA, Folsom AR. Adherence to the aicr cancer prevention recommendations and subsequent morbidity and mortality in the iowa women's health study cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1114–1120. [PubMed] [Google Scholar]
- 24.Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective us cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2427–2435. doi: 10.1158/1055-9965.EPI-09-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T. American cancer society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA: a cancer journal for clinicians. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 26.McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, Thun MJ, Gapstur SM. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1089–1097. doi: 10.1158/1055-9965.EPI-10-1173. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29:901–909. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.