Abstract
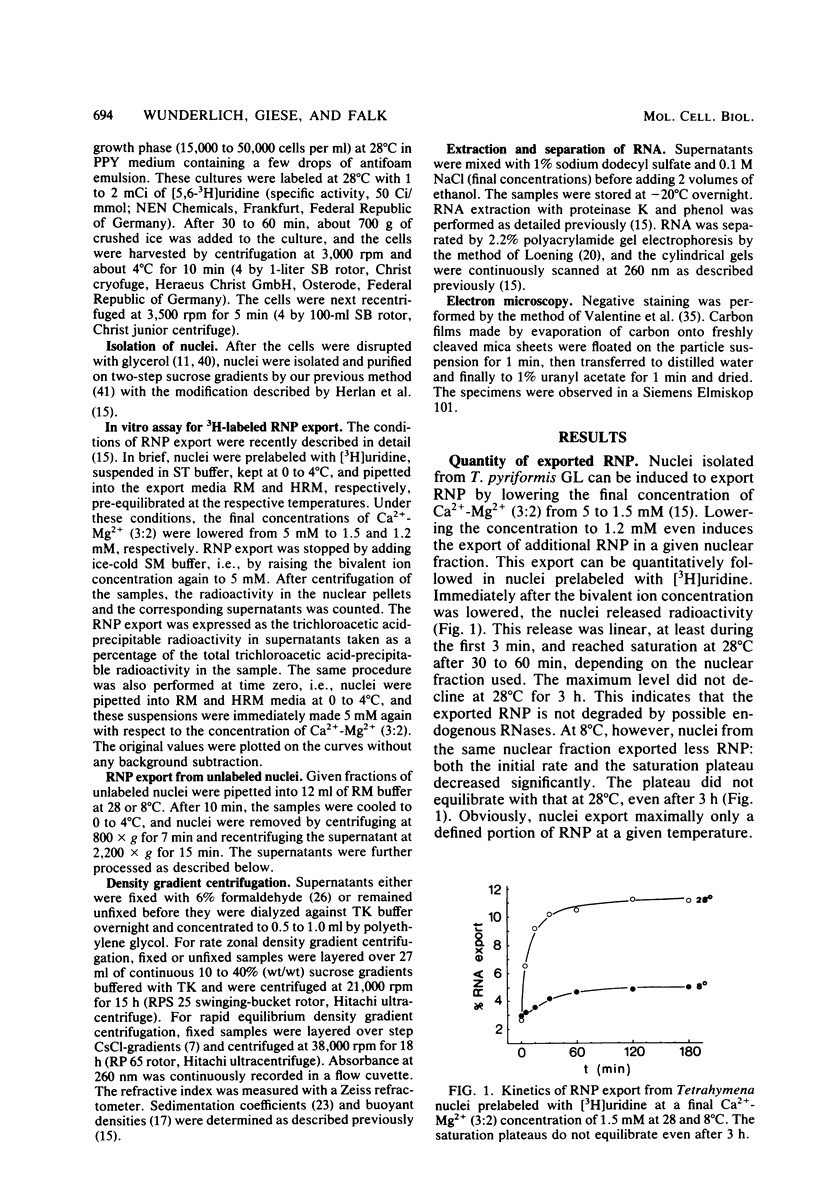
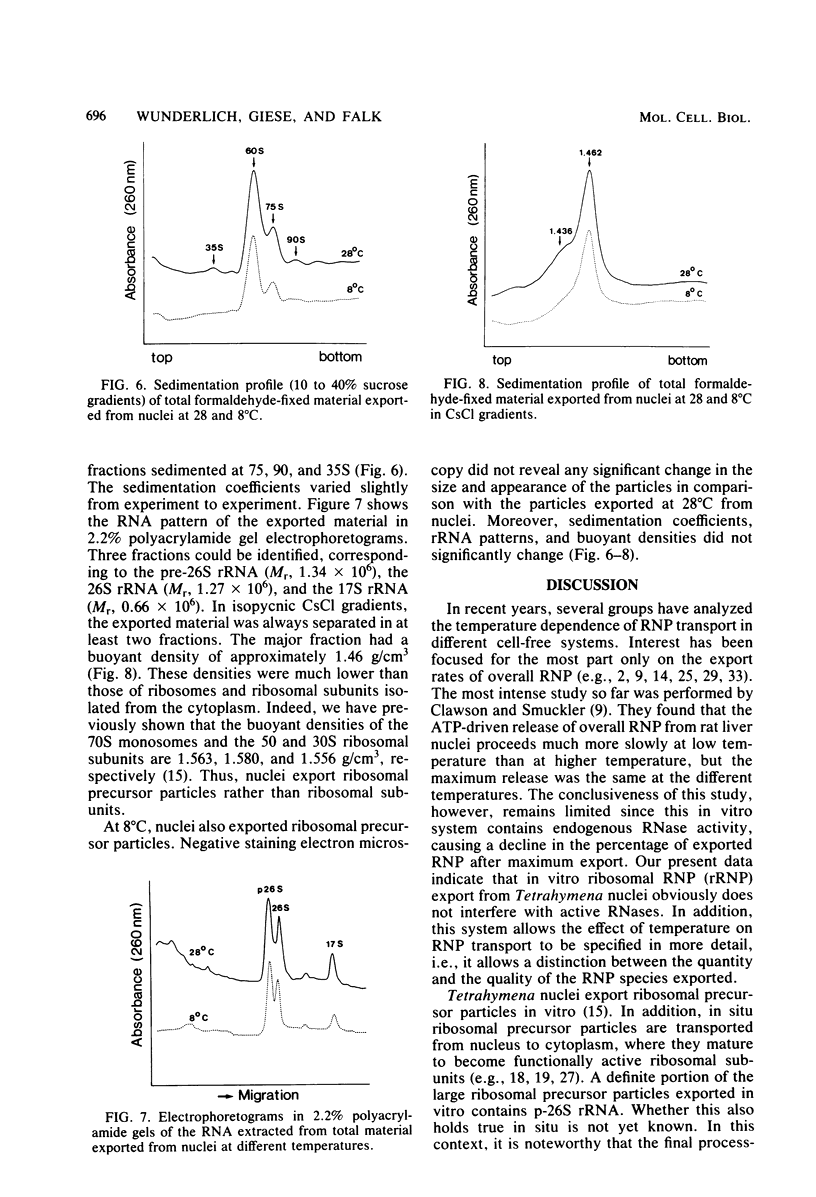
The in vitro export of ribosomal ribonucleoprotein (rRNP) from Tetrahymena nuclei was investigated at the optimal growth temperature of 28 degrees C and at the nonlethal temperature of 8 degrees C. At both temperatures, nuclei exported ribosomal precursor particles that revealed the same physical qualities of size, appearance in negative-staining electron microscopy, sedimentation coefficient, buoyant density, and rRNA pattern. Surprisingly, fewer rRNP particles were exported at 8 than at 28 degrees C, as was revealed by a lower saturation plateau in the export kinetics from nuclei prelabeled with [3H]uridine. Upon a temperature increase from 8 to 28 degrees C, additional rRNP particles were exported. We conclude that nuclei export only a defined portion of rRNP particles at a given temperature, although enough potentially transportable rRNP particles are present in nuclei. Obviously, the reactivity of at least one of the reactants involved directly or indirectly in rRNP export changes with temperature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agutter P. S., McArdle H. J., McCaldin B. Evidence for involvement of nuclear envelope nucleoside triphosphatase in nucleocytoplasmic translocation of ribonucleoprotein. Nature. 1976 Sep 9;263(5573):165–167. doi: 10.1038/263165a0. [DOI] [PubMed] [Google Scholar]
- Agutter P. S., McCaldin B., McArdle H. J. Importance of mammalian nuclear-envelope nucleoside triphosphatase in nucleo-cytoplasmic transport of ribonucleoproteins. Biochem J. 1979 Sep 15;182(3):811–819. doi: 10.1042/bj1820811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S., Richardson J. C. Nuclear non-chromatin proteinaceous structures: their role in the organization and function of the interphase nucleus. J Cell Sci. 1980 Aug;44:395–435. doi: 10.1242/jcs.44.1.395. [DOI] [PubMed] [Google Scholar]
- Albrecht C., Van Zyl I. M. A comparative study of the protein components of ribonucleoprotein particles isolated from rat liver and hepatoma nuclei. Exp Cell Res. 1973 Jan;76(1):8–14. doi: 10.1016/0014-4827(73)90412-6. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Chang M. J., Koestner A., Palayoor T., Schumm D. E., Webb T. E. Characteristics of RNA release from rat brain nuclei and effect of neurocarcinogenesis. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1348–1354. doi: 10.1016/0006-291x(80)90434-9. [DOI] [PubMed] [Google Scholar]
- Clawson G. A., Smuckler E. A. Activation energy for RNA transport from isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5400–5404. doi: 10.1073/pnas.75.11.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert W. A., Kaffenberger W., Krohne G., Franke W. W. Introduction of hidden breaks during rRNA maturation and ageing in Tetrahymena pyriformis. Eur J Biochem. 1978 Jul 3;87(3):607–616. doi: 10.1111/j.1432-1033.1978.tb12413.x. [DOI] [PubMed] [Google Scholar]
- Giese G., Fromme I., Wunderlich F. Suppression of thermotropic lipid clustering in Tetrahymena nuclear membranes upon Ca2+/Mg2+-induced membrane contraction. Eur J Biochem. 1979 Apr 2;95(2):275–285. doi: 10.1111/j.1432-1033.1979.tb12963.x. [DOI] [PubMed] [Google Scholar]
- Giese G., Wunderlich F. Increased fluidity and loss of temperature and Ca2+/Mg2+ sensitivity in nuclear membranes upon removal from the membrane-associated nuclear matrix proteins. J Biol Chem. 1980 Feb 25;255(4):1716–1721. [PubMed] [Google Scholar]
- Herlan G., Eckert W. A., Kaffenberger W., Wunderlich F. Isolation and characterization of an RNA-containing nuclear matrix from Tetrahymena macronuclei. Biochemistry. 1979 May 1;18(9):1782–1788. doi: 10.1021/bi00576a023. [DOI] [PubMed] [Google Scholar]
- Herlan G., Giese G., Wunderlich F. In vitro ribosomal ribonucleoprotein transport upon nuclear expansion. Biochemistry. 1980 Aug 19;19(17):3960–3966. doi: 10.1021/bi00558a011. [DOI] [PubMed] [Google Scholar]
- Herlan G., Giese G., Wunderlich F. Influence of nuclear membrane lipid fluidity on nuclear RNA release. Exp Cell Res. 1979 Feb;118(2):305–309. doi: 10.1016/0014-4827(79)90155-1. [DOI] [PubMed] [Google Scholar]
- Herlan G., Wunderlich F., Kreuzer H. P. Central hidden breaks in the cytoplasmic 26S rRNA and its nuclear precursors in Tetrahymena. Cell Biol Int Rep. 1981 Nov;5(11):1033–1038. doi: 10.1016/s0309-1651(81)80030-6. [DOI] [PubMed] [Google Scholar]
- Leick V., Engberg J., Emmersen J. Nascent subribosomal particles in Tetrahymena pyriformis. Eur J Biochem. 1970 Apr;13(2):238–246. doi: 10.1111/j.1432-1033.1970.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Leick V. Formation of subribosomal particles in the macronuclei of Tetrahymena pyriformis. Eur J Biochem. 1969 Mar;8(2):221–228. doi: 10.1111/j.1432-1033.1969.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londesborough J. The causes of sharply bent or discontinuous Arrhenius plots for enzyme-catalysed reactions. Eur J Biochem. 1980 Apr;105(2):211–215. doi: 10.1111/j.1432-1033.1980.tb04491.x. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Nägel W. C., Wunderlich F. Effect of temperature on nuclear membranes and nucleo-cytoplasmic RNA-transport in Tetrahymena grown at different temperatures. J Membr Biol. 1977 Apr 7;32(1-2):151–164. doi: 10.1007/BF01905214. [DOI] [PubMed] [Google Scholar]
- Patel N. T., Folse D. S., Holoubek V. Release of repetitive nuclear RNA into the cytoplasm in liver of rats fed 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1979 Nov;39(11):4460–4465. [PubMed] [Google Scholar]
- Patterson R. J., Lyerly M., Stuart S. E. RNA metabolism in isolated nuclei: effect of temperature on RNA transport from intact and membrane-denuded nuclei. Cell Biol Int Rep. 1981 Jan;5(1):27–36. doi: 10.1016/0309-1651(81)90154-5. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Buoyant densities of cytoplasmic ribonucleoprotein particles of mammalian cells: distinctive character of ribosome subunits and the rapidly labeled components. J Mol Biol. 1966 Apr;16(2):255–268. doi: 10.1016/s0022-2836(66)80171-7. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Pousada C., Cyrne M. L., Hayes D. Characterization of preribosomal ribonucleoprotein particles from Tetrahymena pyriformis. Eur J Biochem. 1979 Dec 17;102(2):389–397. doi: 10.1111/j.1432-1033.1979.tb04254.x. [DOI] [PubMed] [Google Scholar]
- Schumm D. E., Niemann M. A., Palayoor T., Webb T. E. In vivo equivalence of a cell-free system from rat liver for ribosomal RNA processing and transport. J Biol Chem. 1979 Dec 10;254(23):12126–12130. [PubMed] [Google Scholar]
- Shearer R. W. Altered RNA transport without derepression in a rat kidney tumor induced by dimethylnitrosamine. Chem Biol Interact. 1979 Sep;27(1):91–98. doi: 10.1016/0009-2797(79)90152-2. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., Read B. D., McElhaney R. N. Membrane enzymes: artifacts in Arrhenius plots due to temperature dependence of substrate-binding affinity. Science. 1978 Feb 24;199(4331):902–904. doi: 10.1126/science.146257. [DOI] [PubMed] [Google Scholar]
- Smuckler E. A., Koplitz M. Altered nuclear RNA transport associated with carcinogen intoxication in rats. Biochem Biophys Res Commun. 1973 Nov 16;55(2):499–507. doi: 10.1016/0006-291x(73)91114-5. [DOI] [PubMed] [Google Scholar]
- Stuart S. E., Clawson G. A., Rottman F. M., Patterson R. J. RNA transport in isolated myeloma nuclei. Transport from membrane-denuded nuclei. J Cell Biol. 1977 Jan;72(1):57–66. doi: 10.1083/jcb.72.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S. E., Rottman F. M., Patterson R. J. Nuclear restriction of nucleic acids in the presence of ATP. Biochem Biophys Res Commun. 1975 Jan 20;62(2):439–447. doi: 10.1016/s0006-291x(75)80158-6. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Batz W., Speth V., Wallach D. F. Reversible, thermotropic alteration of nuclear membrane stucture and nucleocytoplasmic RNA transport in Tetrahymena. J Cell Biol. 1974 Jun;61(3):633–640. doi: 10.1083/jcb.61.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich F., Giese G., Bucherer C. Expansion and apparent fluidity decrease of nuclear membranes induced by low Ca/Mg. Modulation of nuclear membrane lipid fluidity by the membrane-associated nuclear matrix proteins? J Cell Biol. 1978 Nov;79(2 Pt 1):479–490. doi: 10.1083/jcb.79.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich F., Herlan G. Reversibly contractile nuclear matrix. Its isolation, structure, and composition. J Cell Biol. 1977 May;73(2):271–278. doi: 10.1083/jcb.73.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]



