Abstract
Objectives
Long-term systemic blockade of the renin–angiotensin system (RAS) with either an angiotensin (Ang) II type 1 receptor antagonist or an angiotensin-converting enzyme inhibitor attenuates age-related cardiac remodeling and oxidative damage, and improves myocardial relaxation. However, the role of the brain RAS in mediating the development of diastolic dysfunction during aging is not known. We hypothesized that low brain RAS protects against the development of age-related diastolic dysfunction and left ventricular remodeling.
Methods
Sixty-week-old transgenic male ASrAOGEN rats (n =9), with normal circulating Ang II and functionally low brain Ang II, because of a GFAP promoter-linked angiotensinogen antisense targeted to glia, and age-matched and sex-matched Hannover Sprague–Dawley (SD; n= 9) rats, with normal levels of both circulating and brain Ang II, underwent echocardiograms to evaluate cardiac structure and function. Postmortem hearts were further compared for histological, molecular, and biochemical changes consistent with cardiac aging.
Results
ASrAOGEN rats showed preserved systolic and diastolic function at mid-life and this was associated with a lower, more favorable ratio of the phospholamban–SERCA2 ratio, reduced incidence of histological changes in the left ventricle, and increased cardiac Ang-(1–7) when compared with the in-vivo functional, and ex-vivo structural and biochemical indices from age-matched SD rats. Moreover, ASrAOGEN rats had lower percent body fat and a superior exercise tolerance when compared with SD rats of the same age.
Conclusion
Our data indicate that the central RAS plays a role in the maintenance of diastolic function and exercise tolerance in mid-life and this may be related to effects on body habitus.
Keywords: brain, cardiac aging, diastolic dysfunction, renin–angiotensin system, SERCA2, tissue Doppler
Introduction
Age-related cardiac structural changes lead to reductions in left ventricular (LV) relaxation and compliance, increases LV end-diastolic pressure, exercise intolerance, and frequently, diastolic heart failure. Stimuli for cardiac remodeling associated with aging also include hypertension [1], increases in body weight gain [2], insulin resistance [3], and renal dysfunction [4]. The peripheral and cardiac renin–angiotensin system (RAS), alone or as a consequence of these comorbidities, may further contribute toward the senescent cardiac phenotype by augmenting oxidative pathways and enhancing extracellular matrix formation [5–7]. In hearts from senescent rodents, elevated cardiac angiotensin (Ang) II levels associate with LV fibrosis [8] and late-life treatment with angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin II receptor (AT1R) blockers attenuates age-related collagen deposition and oxidative damage [9]. Long-term, low-dose treatment with ACE-I improves cardiac sarcoplasmic reticulum (SR) calcium-regulatory protein expression in the same aged Brown Norway × Fisher 344 rodent model [9]. Although there are reports of increases in angiotensinogen, AT1R, and AT2R expression, in myocardial tissue from senescent rats independent of circulating RAS [10,11], it is not entirely clear whether the benefits of systemically administered RAS blockade represent a local cardiac tissue and/or a centrally mediated mechanism.
Indeed, orally administered ACE-I and angiotensin receptor blockers can cross the blood–brain barrier [12–14], and experimental evidence shows that central application of various RAS inhibitors attenuates the pressor responses to centrally administered Ang II [15,16]. All components of the RAS have also been identified in the brain [17–19]. Various transgenic animal models suggest that long-term regulation of the cardiovascular system, in states of both disease and good health, involves correlated actions of the RAS and the sympathetic nervous system [20–24]. Taken together with recent clinical data showing that sympathetic overactivity and baroreflex impairment contribute toward hypertension-related diastolic dysfunction [25], it is reasonable to expect that the central RAS may also play an important role in cardiac aging.
In this study, we hypothesized that low brain RAS protects against the development of diastolic dysfunction and LV remodeling at mid-life. Previous reports indicate that the young (ASrAOGEN) rat has normal circulating Ang II and functionally low brain Ang II because of a GFAP promoter-linked angiotensinogen antisense targeted to astrocytes relative to the Hannover Sprague–Dawley (SD). The control SD rats show normal levels of both circulating and brain Ang II [24,26,27] and undergo age-related elevations of arterial pressure and decreases in autonomic and metabolic dysfunction, whereas ASrAOGEN rats are protected from such age-related changes [22]. Therefore, we compared the cardiac function, structure, and cardiac angiotensin peptides in a group of middle-aged rats with opposing degrees of brain RAS activation.
Methods
Middle-aged male rats from the two lines [Hannover SD (n=9) or ASrAOGEN (n=9)] were obtained from the colonies maintained in the Hypertension and Vascular Research Center at Wake Forest University. The Hannover SD rats are the parent line for the transgenic ASrAOGEN rat. All animals were bred and exposed to the same paired housing conditions (12 : 12 light : dark cycle) and provided food and water ad libitum. The two strains underwent echocardiograms at 60 weeks of age and postmortem hearts acquired immediately following sacrifice by rapid guillotine decapitation (~62 weeks of age) were used for histological and biochemical analyses. Animal care was provided in accordance with the Laboratory Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 2011), and all experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine. Accordingly, all possible steps were taken to avoid animal suffering at each stage of the experiment.
Echocardiography
For the echocardiogram, rats were anesthetized with an isoflurane (1.5%)/oxygen mixture by a nose cone during spontaneous ventilation. Using a Philips 5500 echocardiograph (Philips Medical Systems, Andover, Massachusetts, USA) and a 12MHz phased array probe, animals were imaged in a shallow left lateral decubitus position while lying on a warmed mat, with electrocardiographic adhesive electrodes applied to the paws [8,28]. The measurements were carried out using an off-line analysis system (Xcelera 3.1; Koninklijke Philips Electronics, Amsterdam, the Netherlands) by an experimentally blinded observer. LV M-mode images were obtained in the two-dimensional short-axis view, close to the papillary muscles. Diastolic posterior wall thickness (PWTed) and LV end-diastolic dimension (LVEDD) and LV end-systolic dimension (LVESD) were measured using the leading-edge method of the American Society for Echocardiography [29]. The percentage of LV fractional shortening (%FS), an index of the global systolic function, was calculated as [(LVEDD−LVESD)/LVEDD] × 100. The relative wall thickness was calculated as: 2 × PWT/LVEDD. LV mass was calculated using a standard cube formula, which assumes a spherical LV geometry to the formula: LV mass = 1.04 × [(LVEDD+PWT+AWT)3 −LVEDD], where 1.04 is the specific gravity of muscle. Transmitral flow measurements of early filling velocities (Emax) and early deceleration time (Edectime) were obtained using pulsed-Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. Because of the relatively high heart rates and fusion of the early and late Doppler profiles, only the early transmitral filling velocity was measured. Pulsed-Doppler tissue imaging to assess septal mitral annular descent (e′) was also obtained from the four-chamber view. The ratio of early transmitral filling-to-mitral annular descent, or E/e′, was used as an index of filling pressure. All measured and calculated systolic and diastolic indices are represented as the average of at least five consecutive cardiac cycles to minimize beat-to-beat variability.
Blood pressure
Systolic blood pressure was measured in conscious rats between 55 and 60 weeks of age using an automated tail-cuff system (Narco Bio Systems, Houston, Texas, USA). This noninvasive blood pressure monitoring technique utilizes a methodology similar to the peripheral blood flow occlusion technique used in humans. In brief, an electric pulse transducer located distally from the cuff converts force applied to the active surface of the transducer from the tail blood pressure pulse into an electrical signal. The tail cuff inflates to a pressure greater than the systolic arterial pressure, at which time the blood pressure pulse is nonexistent. As the cuff pressure is released and tail blood pressure exceeds cuff pressure, blood begins to flow through the artery and the returning pulse is detected by the transducer. This corresponds to the systolic arterial pressure. To assist with arterial vasodilation, rats were placed in a 39°C warming box for 10 min before the determination of blood pressure. At least five determinations were carried out in each animal and averaged for a single determination for the session. Rats were not trained to the tail-cuff procedure but were trained to handling by the same investigators who performed the tail-cuff procedures.
Histopathology
Formalin-fixed, postmortem hearts obtained from a separate 60-week-old cohort following sacrifice were transversely sectioned in the mid-ventricular region and processed to paraffin blocks. One routine section from each block was stained with hematoxylin and eosin and evaluated for histopathologic changes, including interstitial fibrosis, myofiber necrosis, intramural coronary artery degeneration, and mononuclear infiltrates, by an investigator at Experimental Pathology Laboratories (Charlottesville, Virginia, USA) who was masked to rat strain. Incidence rates (%) were used to describe representative age-related degenerative lesions in each strain.
Immunoblotting
SR membranes and LV tissue homogenates were prepared as described previously [28]. Briefly, samples were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes. Immunoblots were probed for the key calcium-regulatory proteins, anti-sarcoplasmic endoplasmic reticulum calcium ATPase 2 (SERCA2) (1 : 1000 dilution; Abcam, Cambridge, Massachusetts, USA), and anti-phospholamban (PLB) (1 : 5000 dilution; Abcam). To normalize the variability of protein loading, the antibody to glyceraldehyde-3-phosphate dehydrogenase (1 : 5000 dilution; Cell Signaling Technology, Danvers, Massachusetts, USA) or β-actin (1 : 2000; Cell Signaling Technology) was probed onto the stripped membranes corresponding to the aforementioned proteins (Western Blot Recycling Kit; Alpha Diagnostic International Inc., San Antonio, Texas, USA). The bands were scanned and digitized (MCDI Image Analysis Software; Imaging Research Inc., St Catherines, Ontario, Canada). Each band was normalized to its own loading control protein and expressed in arbitrary units. The PLB-to-SERCA2 ratio was used as a measure of SERCA2 inhibition.
Biochemical analysis
At sacrifice, LV tissues were rapidly collected and snap-frozen in liquid N2 and stored at − 80°C for later assay. Angiotensin peptides were extracted from the tissue samples using C18 Sep-Pak Columns (Waters, Milford, Massachusetts, USA), and the eluate was analyzed by radioimmunoassay for Ang I, Ang II, and Ang-(1–7) as described previously [30].
Body composition analysis
In a separate group of 55-week-old rats representing each group (n=3/group), global body composition was measured with Hologic Discovery (Delphi A; Hologic Inc., Waltham, Massachusetts, USA), a whole-body dual-energy X-ray absorptiometry (DXA) scanner, with software adaptions (software version 3.3 APEX, Delphi A; Hologic Inc.) for small animal scanning [31]. The densitometer was calibrated before rodent scans using a small animal step phantom supplied by the manufacturer. Rats were anesthetized before measurements with an intramuscular injection of a solution containing a mixture of ketamine (60 mg/kg) and xylazine (5 mg/kg). After anesthesia, rats were positioned ventrally on a platform. Forelimbs were positioned about 75° from the base of the neck and hind limbs were externally rotated, with hip, knee, and ankle articulations in 90° flexion. Rat whole-body measurements required 2–3 min for completion. The percent coefficients of variation at our center are 0.54% for bone mineral content, 0.58% for bone mineral density, 0.40% for fat-free mass, and 1.66% for fat mass [31].
Exercise tolerance test
Exercise tolerance tests were conducted in another group of 55-week-old SD and ASrAOGEN rats (n=5/group) to further differentiate functional disparities between strains. Indeed, exercise intolerance is often the first clinical manifestation of diastolic dysfunction [32]. Before testing, animals were acclimated to the one-lane rodent treadmill (Scientific Instruments, Stoelting, Woodale, Illinois, USA) by walking at a speed of 20 cm/s, 10 min/day, for 2 weeks. After acclimatization, 55-week-old rats underwent exercise tolerance testing that involved walking at 20 cm/s for 3min, followed by 2 cm/s increases in speed every 2 min until exhaustion. Time to exhaustion (s) was determined when the rat sat at the lower end of the treadmill, near a shock bar, for more than 5 s.
Statistical analyses
Data shown are expressed as mean ± SEM. Unpaired t-tests were used to compare each genotype at their respective ages. All analyses were carried out using GraphPad Prism (version 5; Graphpad Inc., La Jolla, California, USA). P value less than 0.05 was used for significance.
Results
Physical characteristics
Age, systolic blood pressure, and body weights are shown in Table 1. As reported previously [33,34], the ASrAOGEN had a 37–45% lower body weight and a 10% lower systolic blood pressure when compared with SD rats of the same age. Moreover, % fat, the total fat-to-lean body mass ratio, and the % bone mineral density were lower in 55-week-old ASrAOGENs when compared with their age-matched controls (Table 1).
Table 1.
Physical characteristics
| ASrAOGEN (n=9) | SD (n=9) | |
|---|---|---|
| Age (weeks) | 61 ± 2 | 62 ± 1 |
| Body weight (gm) | 339 ± 9* | 617 ± 14 |
| Systolic BP (mmHg) | 106 ± 2† | 117 ± 3 |
| Lean body massa (gm) | 353 ± 10* | 654 ± 32 |
| Body fata (%) | 14.8 ± 2.2* | 27.3 ± 0.3 |
| Fat : lean ratioa | 0.18 ± 0.03* | 0.38 ± 0.1 |
| Bone mineral densitya (%) | 0.196 ± 0.002 | 0.234 ± 0.004 |
Values represent mean ± SEM.
SD, Sprague–Dawley, BP, blood pressure (conscious).
Three 60-week-old animals/group for body composition analyses by DXA (dualenergy X-ray absorptiometry).
P < 0.001;
P < 0.05 vs. SD.
Left ventricular structure and function
Echocardiographic analysis showed that LV chambers and posterior wall dimensions were smaller in ASrAOGENs when compared with SD rats of the same age (Table 2). Although the relative wall thicknesses were not overtly different between groups, calculation of LV mass using echocardiographic parameters showed decreased LV mass in ASrAOGEN rats. Systolic function, as measured by FS, was not significantly different between groups (P=0.06). As reported previously [22,27,35], the mean heart rate of ASrAOGENs, obtained during echocardiograms performed under anesthesia, was almost 10% higher than the SDs heart rate.
Table 2.
Echocardiographic indices of cardiac structure and function
| ASrAOGEN (n=9) | SD (n=9) | |
|---|---|---|
| Heart rate (beats/min) | 359 ± 3* | 324 ± 11 |
| LVESD (cm) | 0.511 ± 0.02* | 0.637 ± 0.03 |
| LVEDD (cm) | 0.799 ± 0.03* | 0.931 ± 0.02 |
| FS (%) | 37 ± 1 | 32 ± 2 |
| PWT (cm) | 0.180 ± 0.004* | 0.207 ± 0007 |
| RWT (cm) | 0.456 ± 0023 | 0.454 ± 0021 |
| LV mass (gm) | 1.01 ± 004† | 1.60 ± 008 |
Values are mean ± SEM.
FS, fractional shortening; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; PWT, posterior wall thickness at end diastole; RWT, relative wall thickness; SD, Sprague–Dawley.
P < 0.01;
P < 0.001 vs. SD.
Assessments of diastolic function using transmitral and tissue Doppler measures are shown in Fig. 1. Early transmitral flow velocities were lower (ASrAOGEN: 74 ± 4 cm/s vs. SD: 90 ± 5 cm/s; P=0.01) and deceleration times were longer in ASrAOGENs (ASrAOGEN: 0.042 ± 0.001 s vs. SD: 0.034 ± 0.002 s; P<0.01). Corresponding to the favorable early deceleration time in ASrAOGEN rats, the echocardiographic index of the LV filling pressure, or E/e′, was 42% lower when compared with age-matched SD rats (P<0.05). Mitral annular velocity, or the tissue Doppler index of myocardial relaxation, was also 17% higher in the transgenic rats with low brain angiotensinogen vs. SD rats, but this did not achieve statistical significance (P=0.118).
Fig. 1.
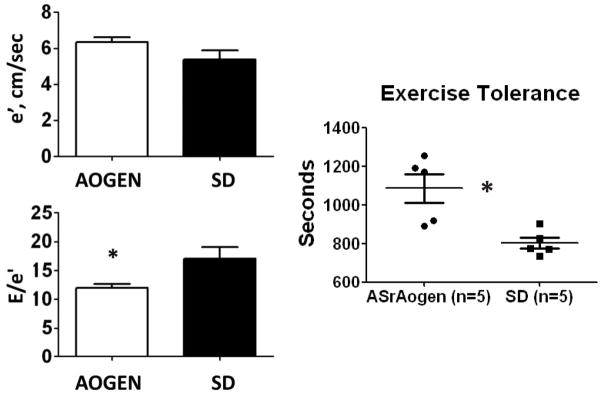
The tissue Doppler measure of diastolic function, mitral annular velocity (e′), was not different between groups whereas the Doppler surrogate to left ventricular filling pressure, early transmitral filling-to-mitral annular velocity (E/e′), was significantly lower in ASrAOGENs (n=9) when compared with Sprague–Dawley (SD) rats (n=9). Time to exhaustion by treadmill exercise capacity testing was longer in ASrAOGENs (n=5) vs. SDs (n=5). *P<0.05 vs. SD.
Immunoblotting
Consistent with the modestly improved functional profile of the middle-aged ASrAOGEN, the hearts from these rats showed a lower PLB-to-SERCA2 ratio compared with the control SD rats. Given that PLB inhibits the action of the SR calcium-regulatory pump [36], or SERCA2, our data indicate that intracellular calcium reuptake is superior in the ASrAOGEN strain (Fig. 2).
Fig. 2.
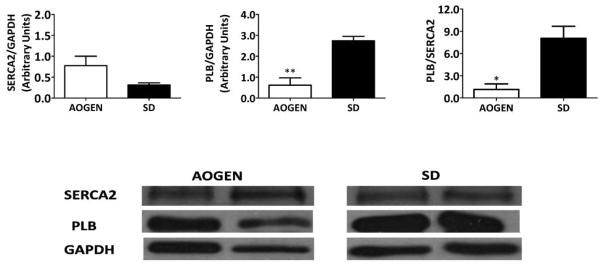
Protein concentrations of (a) sarcoendoplasmic Ca2+ adenosine triphosphatase [anti-sarcoplasmic endoplasmic reticulum calcium ATPase 2 (SERCA2)] and (b) dephosphorylated phospholamban (PLB) normalized to the respective glyceraldehyde-3-phosphate dehydrogenase (GAPDH) loading controls. (c) The PLB/SERCA2 ratio was lower in the ASrAOGEN rats (n=5) when compared with age-matched Sprague–Dawley (SD) rats (n=5). Given that dephosphorylated PLB blocks SERCA2, the lower PLB/SERCA2 ratio of the ASrAOGEN rats indicates enhanced SERCA2 functioning. Values are means ± SEM. *P<0.05; **P<0.01 vs. SD. (d) Representative immunoblots of SERCA2, PLB, and GAPDH from each group are shown.
Left ventricular histology
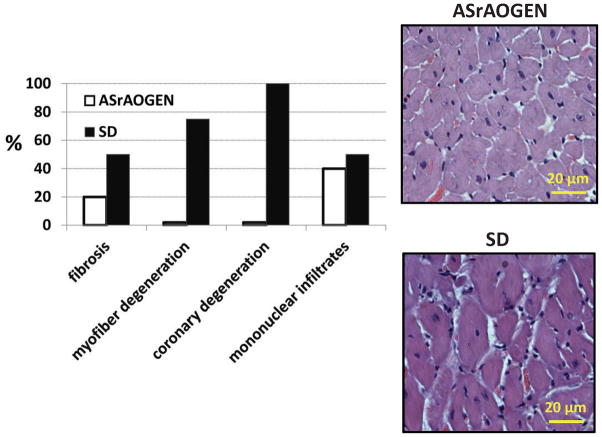
LV histopathology of postmortem hearts from ~60-week-old animals was assessed by hematoxylin and eosin staining (Fig. 3). Although the severity of interstitial fibrosis was considered modest for both groups, it was present in 30% more of the SD than ASrAOGEN specimens at this age. Myofiber necrosis and coronary artery degeneration were identified in almost all of the specimens from SD hearts, but none from ASrAOGEN hearts. A low grade of mononuclear infiltrates was also identified and this occurred in ASrAOGEN and SD hearts at an incidence rate of 40 and 50%, respectively.
Fig. 3.
(a) Incidence rates (%) of histological findings, which include fibrosis, myofiber degeneration, coronary degeneration, and mononuclear infiltrates, were the highest among Sprague–Dawley (SD) rats (n =6) vs. age-matched ASrAOGENs (n=6). (b) Representative hematoxylin and eosin staining of hearts from each group.
Biochemical analyses
The results indicating the potential contribution of the cardiac angiotensins to the subtle differences in diastolic function and LV structure between groups (ASrAOGEN hearts, n=5; SD hearts, n=6) show that cardiac levels of the antiproliferative peptide Ang-(1–7) were significantly higher in ASrAOGEN rats (17.7 ± 3.0 pg/mg tissue; P<0.02) when compared with SD rats (11.8 ± 2.5 pg/mg tissue), and this was associated with a significant increase in Ang-(1–7)/Ang I, or the peptide ratio representing neprilysin (ASrAOGEN: 6.3 ± 1.2 vs. SD: 1.5 ± 0.2; P< 0.01) as opposed to Ang-(1–7)/Ang II, or the peptide ratio representing ACE2 (ASrAOGEN: 4.9 ± 0.2 vs. SD: 4.5 ± 0.5). Cardiac levels of Ang I (ASrAOGEN: 17.7 ± 3.0 pg/mg vs. SD: 11.8 ± 2.5 pg/mg protein; P=0.160) and Ang II (ASrAO GEN: 6.3 ± 1.3 pg/mg vs. SD: 3.8 ± 0.7 pg/mg tissue; P= 0.098) tended to be higher in ASrAOGENs when compared with age-matched SDs.
Exercise tolerance
To further differentiate the functional differences between SD and ASrAOGEN rats, exercise capacity testing was performed in a group of age-matched rats at 55 weeks of age (n=5/group). Time to exhaustion was 26% shorter in SD rats compared with ASrAOGEN rats (Fig. 1).
Discussion
Our study shows that ~60-week-old ASrAOGENs rats, with low brain levels of angiotensinogen, and Ang I and evidence of functional loss of Ang II actions (relative to the control SD parent strain [26,27,37], are protected against early cardiac aging when compared with age-matched SD controls. Specifically, ASrAOGENs showed preserved systolic and diastolic function, and this was further associated with a reduced incidence of cardiac fibrosis, a more favorable SR cardiac calcium-regulatory protein profile, and a higher level of cardiac Ang-(1–7) when compared with the in-vivo functional, and ex-vivo structural, and biochemical indices from SD rats obtained at parallel time points. In conjunction with the conserved cardiac phenotype of the mid-aged ASrAOGENs, these rats also showed a healthier body weight, or lower body weight and % body fat, and superior exercise tolerance when compared with age-matched SDs. Although the bone density of ASrAOGENs was lower than that observed in SDs, the clinical relevance of this observation is not clear, given that both positive and negative relationships between body mass and bone mineral density have been reported in epidemiologic and clinical studies [38]. Nonetheless, the potential role of a low glial RAS contributing toward the low bone mass phenotype of the ASrAOGEN is doubtful as bone remodeling and, specifically, osteoclastogenesis is related to an activated RAS or SNS [39] and in conscious ASrAOGENs, resting sympathetic activity is presumed to be reduced as evidenced by a slightly lower mean arterial pressure (MAP) and heart rate, with higher baroreceptor sensitivity when compared with age-matched SD rats [21,22,33].
In recent years, the RAS has been proposed as a potential target to limit diastolic dysfunction and cardiac remodeling because of aging. We, and others, have shown previously, in senescent rodent models, that increases in cardiac Ang II are associated with cardiac collagen deposition and impairments in diastolic function [8]. In addition, we found that ACE inhibition or AT1R blockade limits the oxidative damage that contributes toward age-associated LV remodeling and lusitropic dysfunction [9,40]. Studies using aged mice also show that enalapril administered from weaning until 24 months of age limits the development of myocardial sclerosis, attenuates age-related reductions in myocardial mitochondrial number, and increases survival [41]. Clinically, and in support of a RAS-mediated mechanism of age-related LVEDD, ACE-I or AT1R blockers are considered first-line therapy for the management of preclinical heart failure (stages A and B) because of hypertension or diabetes [42], two disease processes known for accelerated cardiovascular aging [43–45]. Moreover, in a small randomized, double-blind study involving elderly hypertensive patients with mild diastolic dysfunction, Little et al. [46] showed that 6 months of AT1R blockade led to reductions in exercise-induced increases in systolic blood pressure and improvements in exercise tolerance and quality of life, whereas treatment with the non-RAS-regulated antihypertensive, hydrochlorothiazide, affected exercise-induced increases in blood pressure alone. Taken together, these studies hint at a common causal mechanism in the pathogenesis of diastolic dysfunction, namely, an activated tissue RAS.
Our data in the ~60-week-old ASrAOGENs are consistent with findings from younger animal models of ischemic heart disease that show that inactivation, or low brain RAS, may also protect against LV remodeling and cardiac dysfunction. Specifically, blockade of AT1Rs in the brain of rats subjected to coronary ligation reversed the sympathetic hyperactivity associated with heart failure [47]. Chronic AT1R blockade or attenuation of brain ouabain-like activity (which also contributes toward sympathetic hyperactivity associated with heart failure) retarded the development of LV dilation and dysfunction in rats after myocardial infarction [48,49]. Similarly, transgenic rats with low glial angiotensinogen (ASrAOGEN) are protected from sympathetically induced LV dysfunction and remodeling after myocardial infarction [24] and have an increased lifespan compared with SD rats [21]. Similarly, Araujo et al. [50] reported that intracerebral injections of the ACE inhibitor captopril in rats after an experimental myocardial infarction reduced LV dilatation and improved LV filling. In contrast, selective overexpression of brain ACE2, the counter-regulatory RAS enzyme that breaks down Ang II into its antagonistic product Ang-(1–7), attenuated sympathetic activity and improved baroreflex function in mice with congestive heart failure [51]. Overall, these data imply that the brain RAS, through the modulation of peripheral sympathetic nerve activity, plays a major role in ventricular remodeling and myocardial dysfunction associated with heart failure [21], and this might also explain the subtle cardiac differences between ASrAOGENs and SDs observed in our study.
In the present study, ASrAOGENs showed a more favorable diastolic functional and structural cardiac phenotype than age-matched rats from the parent SD strain. Specifically, the increased early deceleration time and reduced E/e′, or filling pressure, in the ASrAOGENs vs. SDs was associated with a lower PLB-to-SERCA2 ratio and a reduced prevalence of cardiac fibrosis and coronary artery degeneration. Although it is not yet clear how a low brain RAS, independent of circulating angiotensin peptides [24,26,37,52], defends against a presumed age-related reduction in SERCA2 expression, maintenance of calcium uptake into the SR could explain the improved Doppler profile of the diastolic function in this strain. As FS was not overtly different between groups, it means that systolic function, per se, did not play a major role in the relative enhancement in diastolic function in ASrAOGENs. Indeed, evidence from various experimental and human models shows that SERCA2 downregulation or an increase in the PLB-to-SERCA2 ratio might contribute toward diastolic heart failure [53,54]. We have also reported a similar relationship between SERCA2 expression and the tissue Doppler index of myocardial relaxation, or e′, in various rodent models mimicking normal cardiac aging [8,28] and that chronic ACE inhibition improves cardiac SR calcium-regulatory protein expression and myocardial relaxation [9]. In addition to a low glial angiotensinogen–cardiac calcium-regulatory connection that could contribute toward the maintenance of diastolic function among ASrAOGENs, further studies will be required to determine the mechanistic link between low brain angiotensinogen and attenuated age-related myocardial remodeling, ventricular stiffness, and elevated filling pressures observed in middle-aged transgenic rats.
Alternatively, or in addition to the beneficial effects of a low brain RAS, enhanced local cardiac Ang-(1–7) production could contribute directly toward a delay in cardiac aging. Although cardiac levels of Ang I and Ang II were not significantly different between groups, there were clear trends for increases in ASrAOGENs, providing the substrate for a relative increase in the production of Ang-(1–7) in ASrAOGENs. Ang-(1–7), together with its receptor mas, and the enzyme ACE2 represent a cardioprotective axis within the RAS system that balance the ACE/Ang II/and AT1R’s adverse effects [52]. Experimental studies show that increases in Ang-(1–7) or ACE2, through overexpression or exogenous administration, prevent the cardiac fibrosis associated with Ang II [55] and improve cardiac function after myocardial infarction [56]. Moreover, recently, we found that chronic inhibition of ACE2 increases LV remodeling in transgenic hypertensive rats, independent of circulating RAS [30]. Nonetheless, the current study suggests that neprilysin, as opposed to ACE2, might be the preferential enzyme responsible for the elevation in Ang-(1–7) at this age in the ASrAOGENs hearts as the ratio representing neprilysin, or Ang-(1–7)/Ang I, was increased but not the ratio representing ACE2, or Ang-(1–7)/Ang II. Indeed, studies involving enzyme metabolism would be required to confirm a neprilysin vs. ACE2 enzyme-regulatory preference, as well as to exclude other potential sources [e.g. increased substrate availability such as (i.e. more cardiac angiotensiogen or Ang I), less Ang-(1–7) metabolism, or less chymase), leading to an increase in cardiac Ang-(1–7). In fact, in Wistar and SD rats, lower levels of neprilysin mRNA or activity have been reported in other tissues [57,58]. Interestingly, neprilysin has been shown to be an Ang-(1–7)-forming enzyme in both the circulation and the cardiovascular tissue [59]. In the aged Brown Norway×Fisher 344 rat, increases in cardiac Ang-(1–7) and the ratio Ang-(1–7)/Ang I are believed to represent a compensatory mechanism regulating ‘healthy’ cardiac aging [28]. Although it is not known how low brain angiotensinogen and/or alterations in central sympathetic activity could influence the local cardiac RAS milieu, targeting therapy to increase Ang-(1–7) could slow cardiac aging and the development of diastolic dysfunction. In fact, long-term treatment with an AT1R antagonist in Fischer 344 rats was associated with an increase in neprilysin and ACE2 mRNA in brain medulla, suggesting a shift to the Ang-(1–7) axis [60]. Ang-(1–7), when administered systemically, protects against cardiac injury in severe forms of hypertension and some of the cardioprotective effects of ACE inhibitors may involve Ang-(1–7) [61,62]. In Lewis rats, decreases in arterial pressure because of the continuous administration of either an ACE-I (lisinopril) or an AT1R antagonist (losartan) increased cardiac ACE2 gene expression and cardiac ACE2 activity, whereas none of the treatments had an effect on cardiac neprilysin mRNA. Losartan but not lisinopril administration was accompanied by increases in cardiac Ang II and Ang-(1–7) content [63]. These data indicate that the regulation of factors influencing the expression and activity of Ang-(1–7) is tissue dependent.
Progressive weight gain is one of the hallmarks of the aging process and obesity has been linked to the development of diastolic dysfunction. Obese individuals [64–67] and experimental animal models of human obesity [68,69] show increases in LV mass that are coupled to reductions in LV filling dynamics. Moreover, the LV hypertrophy that accompanies obesity often develops independent of hemodynamic load [70,71], pointing to a metabolic cause for these cardiac consequences [72,73]. Undoubtedly, the increase in body weight and percent body fat of the ~60-week-old SDs relative to the ASrAOGENs may be a factor in the increased heart size and the decreased LV compliance of this group, as indicated by the lower deceleration time and the higher filling pressure, or E/e′. Both overweight and obesity have been independently associated with impaired diastolic function in individuals at a high cardiovascular risk [74]. Furthermore, given that Wong et al. [65] showed that BMI was a significant predictor of diastolic dysfunction among community dwellers, even after adjusting for age, MAP, and LV mass index, the differences in diastolic function observed between rat strains in the present study might also be because of dissimilarities in body habitus rather than blood pressure and/or heart size. Indeed, accumulation of abnormal metabolites such as triglycerides and extracellular matrix cross-linking by advanced glycation end products could also lead to stiffening of the aged heart and to obesity-related diastolic dysfunction [45,75], particularly given clinical reports of a relative independence of diastolic impairment from LV geometry in obesity [71].
Furthermore, and in support of a metabolic hypothesis to accelerated cardiac aging, the insulin resistance component of the metabolic syndrome has been linked to LV remodeling and diastolic functional stiffness [73]. Although we did not directly measure intramyocardial lipids [68,69] or metabolic parameters as a cause for the alterations in cardiac structure and function in the present study, our previous data show that ASrAOGENs remain lean and insulin sensitive during the aging process, whereas SD rats gain excess weight and develop insulin and leptin resistance during their lifespan [34] similar to mice treated long term with RAS blockers [76]. Future studies will determine whether consumption of a hypercaloric diet offsets the benefits that a low glial angiotensinogen has on maintaining a lean body habitus, favorable fitness level, cardiometabolic profile, and cardiovascular phenotype to middle age and beyond. Similarly, the administration of a high-fat diet to ASrAOGENs could provide additional insights into the complex relationships between fat mass, the mechanical stresses imposed on bone, and subsequent bone density and composition [77].
Limitations
The present study has a few limitations that deserve further discussion. Echocardiograms were carried out under anesthesia, which could have had differential effects on autonomic tone and cardiac function between strains [78]. Indeed, the effects of anesthesia on resting heart rate have reported lower, similar, or higher values in anesthetized ASrAOGEN rats when compared with the parent strain Sprague–Dawley rat depending on the type and the dose of anesthetics [27,35,79–81]. However, the inclusion of tissue Doppler facilitates the assessment of diastolic function as it is less affected by loading conditions and heart rate than conventional Doppler [82–84]. Second, neither a weight-matched nor a low blood pressure control group was included, which does not negate an indirect effect of a RAS–body habitus interaction or life-time lower MAP, either of which could influence the cardiac phenotype. Third, without the inclusion of a ‘young’ group, it is not entirely clear whether the echocardiographic differences observed between groups are a reflection of aging. Importantly, isolated heart preparations from 5-month-old male ASrAOGENs and Hanover SDs showed similar heart-weight- to-body-weight ratios and basal hemodynamics, including LV pressure, +dP/dtmax, and −dP/dtmax [85].
Clinical perspective.
Cardiac aging is a complex multifactorial process unlikely to be because of a singular cause but rather a combination of alterations in neuroendocrine and inflammatory signaling that contribute toward functional decline and damage to cardiac macromolecules. Although normal cardiovascular aging cannot be prevented, individualized interventions in middle-aged individuals that are focused on counteracting obesity, sedentarism, insulin resistance, and high blood pressure can help slow down ‘pathological aging’. Certainly, higher cardiac Ang-(1–7) in the ASrAOGEN rats relative to age-matched SD rats is consistent with the beneficial cardiac effects observed with the long-term administration of Ang-(1–7) in a number of studies. Herein, using transgenic rats with a targeted disruption of glial angiotensinogen, we conclude that the protection against mid-life increases in body weight gain, blood pressure, LV stiffness, and reductions in exercise tolerance directly or indirectly follows from this disruption. In this respect, the aging ASrAOGEN rat provides a valuable experimental tool for elucidating the potential role of manipulating components of the central RAS in attenuating cardiac aging and, specifically, diastolic dysfunction. A final point not addressed in this discussion and actually shown by the measures of blood pressure is that ASrAOGEN rats do not show the characteristic increase in systolic blood pressure associated with the aging process [33]. This may be a critical factor in the expression of the changes found in the heart as the increased blood pressure amplitude with age (higher systolic blood pressure and lower diastolic blood pressure) is now linked to changes in vascular distensibility or stiffness (e.g. aortic input impedance and LV performance) or what is commonly described as ventricular–vascular coupling [86].
Acknowledgments
The authors thank Ellen Tommasi and Marina Lin for their excellent technical assistance.
The work described here was supported in part by grants from the National Institute of Health R01-AG033727-03 (L.G.), KO8-AG026764-05 Paul Beeson award (L.G.), HL51952 (C.M.F., D.I.Z.), P30 AG021332 (S.B.K.), and the American Heart Association (0715249U to A.J.T.).
Footnotes
This work was presented, in part, as a poster discussion and abstract at the 11th Annual Scientific Meeting Heart Failure Society of America, Washington, DC, 16–29 September 2007.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wachtell K, Bella JN, Rokkedal J, Palmieri V, Papademetriou V, Dahlöf B, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Circulation. 2002;105:1071–1076. doi: 10.1161/hc0902.104599. [DOI] [PubMed] [Google Scholar]
- 2.Gates PE, Gentile CL, Seals DR, Christou DD. Adiposity contributes to differences in left ventricular structure and diastolic function with age in healthy men. J Clin Endocrinol Metab. 2003;88:4884–4890. doi: 10.1210/jc.2003-030673. [DOI] [PubMed] [Google Scholar]
- 3.Sundström J, Lind L, Nyström N, Zethelius B, Andrén B, Hales CN, Lithell HO. Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation. 2000;101:2595–2600. doi: 10.1161/01.cir.101.22.2595. [DOI] [PubMed] [Google Scholar]
- 4.Patel PC, Ayers CR, Murphy SA, Peshock R, Khera A, de Lemos JA, et al. Association of cystatin C with left ventricular structure and function: the Dallas Heart Study. Circ Heart Fail. 2009;2:98–104. doi: 10.1161/CIRCHEARTFAILURE.108.807271. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Carretero OA, Liao TD, Peng H, Shesely EG, Xu J, et al. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1328–H1338. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 8.Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 9.Groban L, Lindsey S, Wang H, Lin MS, Kassik KA, Machado FS, Carter CS. Differential effects of late-life initiation of low-dose enalapril and losartan on diastolic function in senescent Fischer 344 × Brown Norway male rats. Age (Dordr) 2012;34:831–843. doi: 10.1007/s11357-011-9283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heymes C, Swynghedauw B, Chevalier B. Activation of angiotensinogen and angiotensin-converting enzyme gene expression in the left ventricle of senescent rats. Circulation. 1994;90:1328–1333. doi: 10.1161/01.cir.90.3.1328. [DOI] [PubMed] [Google Scholar]
- 11.Heymes C, Silvestre JS, Llorens-Cortes C, Chevalier B, Marotte F, Levy BI, et al. Cardiac senescence is associated with enhanced expression of angiotensin II receptor subtypes. Endocrinology. 1998;139:2579–2587. doi: 10.1210/endo.139.5.6023. [DOI] [PubMed] [Google Scholar]
- 12.Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyss JM, Kadish I, van Groen T. Age-related decline in spatial learning and memory: attenuation by captopril. Clin Exp Hypertens. 2003;25:455–474. doi: 10.1081/ceh-120024988. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Bains JS, Ferguson AV. Functional evidence that the angiotensin antagonist losartan crosses the blood-brain barrier in the rat. Brain Res Bull. 1993;30:33–39. doi: 10.1016/0361-9230(93)90036-b. [DOI] [PubMed] [Google Scholar]
- 15.Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, et al. AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther. 2001;298:62–70. [PubMed] [Google Scholar]
- 16.Gohlke P, Von Kügelgen S, Jürgensen T, Kox T, Rascher W, Culman J, Unger T. Effects of orally applied candesartan cilexetil on central responses to angiotensin II in conscious rats. J Hypertens. 2002;20:909–918. doi: 10.1097/00004872-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Diz DI, Jessup JA, Westwood BM, Bosch SM, Vinsant S, Gallagher PE, Averill DB. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exp Pharmacol Physiol. 2002;29:473–482. doi: 10.1046/j.1440-1681.2002.03659.x. [DOI] [PubMed] [Google Scholar]
- 18.Cuadra AE, Shan Z, Sumners C, Raizada MK. A current view of brain renin–angiotensin system: Is the (pro)renin receptor the missing link? Pharmacol Ther. 2010;125:27–38. doi: 10.1016/j.pharmthera.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 20.Grobe JL, Xu D, Sigmund CD. An intracellular renin–angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diz DI, Kasper SO, Sakima A, Ferrario CM. Aging and the brain renin–angiotensin system: insights from studies in transgenic rats. Cleve Clin J Med. 2007;74 (Suppl 1):S95–S98. doi: 10.3949/ccjm.74.suppl_1.s95. [DOI] [PubMed] [Google Scholar]
- 22.Diz DI, Arnold AC, Nautiyal M, Isa K, Shaltout HA, Tallant EA. Angiotensin peptides and central autonomic regulation. Curr Opin Pharmacol. 2011;11:131–137. doi: 10.1016/j.coph.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lal A, Veinot JP, Ganten D, Leenen FH. Prevention of cardiac remodeling after myocardial infarction in transgenic rats deficient in brain angiotensinogen. J Mol Cell Cardiol. 2005;39:521–529. doi: 10.1016/j.yjmcc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Huang BS, Ganten D, Leenen FH. Prevention of sympathetic and cardiac dysfunction after myocardial infarction in transgenic rats deficient in brain angiotensinogen. Circ Res. 2004;94:843–849. doi: 10.1161/01.res.0000120864.21172.5a. [DOI] [PubMed] [Google Scholar]
- 25.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Arenare F, Spaziani D, Mancia G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension. 2009;53:205–209. doi: 10.1161/HYPERTENSIONAHA.108.121467. [DOI] [PubMed] [Google Scholar]
- 26.Huang BS, Ganten D, Leenen FH. Responses to central Na(+) and ouabain are attenuated in transgenic rats deficient in brain angiotensinogen. Hypertension. 2001;37 (Part 2):683–686. doi: 10.1161/01.hyp.37.2.683. [DOI] [PubMed] [Google Scholar]
- 27.Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, et al. Baroreceptor reflex regulation in anesthetized transgenic rats with low glia-derived angiotensinogen. Am J Physiol Heart Circ Physiol. 2007;292:H1412–H1419. doi: 10.1152/ajpheart.00984.2006. [DOI] [PubMed] [Google Scholar]
- 28.Groban L, Jobe H, Lin M, Houle T, Kitzman DA, Sonntag W. Effects of short-term treadmill exercise training or growth hormone supplementation on diastolic function and exercise tolerance in old rats. J Gerontol A Biol Sci Med Sci. 2008;63:911–920. doi: 10.1093/gerona/63.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 30.Trask AJ, Groban L, Westwood BM, Varagic J, Ganten D, Gallagher PE, et al. Inhibition of angiotensin-converting enzyme 2 exacerbates cardiac hypertrophy and fibrosis in Ren-2 hypertensive rats. Am J Hypertens. 2010;23:687–693. doi: 10.1038/ajh.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, et al. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- 32.Kitzman DW, Groban L. Exercise intolerance. Cardiol Clin. 2011;29:461–477. doi: 10.1016/j.ccl.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasper SO, Carter CS, Ferrario CM, Ganten D, Ferder LF, Sonntag WE, et al. Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin–angiotensin systems. Physiol Genomics. 2005;23:311–317. doi: 10.1152/physiolgenomics.00163.2005. [DOI] [PubMed] [Google Scholar]
- 34.Kasper SO, Ferrario CM, Ganten D, Diz DI. Rats with low brain angiotensinogen do not exhibit insulin resistance during early aging. Endocrine. 2006;30:167–174. doi: 10.1385/ENDO:30:2:167. [DOI] [PubMed] [Google Scholar]
- 35.Arnold AC, Sakima A, Ganten D, Ferrario CM, Diz DI. Modulation of reflex function endogenous angiotensins in older transgenic rats with low glial angiotensinogen. Hypertension. 2008;51:1326–1331. doi: 10.1161/HYPERTENSIONAHA.107.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koss KL, Grupp IL, Kranias EG. The relative phospholamban and SERCA2 ratio: a critical determinant of myocardial contractility. Basic Res Cardiol. 1997;92 (Suppl 1):17–24. doi: 10.1007/BF00794064. [DOI] [PubMed] [Google Scholar]
- 37.Schinke M, Baltatu O, Böhm M, Peters J, Rascher W, Bricca G, et al. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci USA. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23:17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asaba Y, Ito M, Fumoto T, Watanabe K, Fukuhara R, Takeshita S, et al. Activation of renin–angiotensin system induces osteoporosis independently of hypertension. J Bone Miner Res. 2009;24:241–250. doi: 10.1359/jbmr.081006. [DOI] [PubMed] [Google Scholar]
- 40.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 41.Ferder L, Inserra F, Romano L, Ercole L, Pszenny V. Effects of angiotensin-converting enzyme inhibition on mitochondrial number in the aging mouse. Am J Physiol. 1993;265:C15–C18. doi: 10.1152/ajpcell.1993.265.1.C15. [DOI] [PubMed] [Google Scholar]
- 42.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 43.Desai A, Fang JC. Heart failure with preserved ejection fraction: hypertension, diabetes, obesity/sleep apnea, and hypertrophic and infiltrative cardiomyopathy. Heart Fail Clin. 2008;4:87–97. doi: 10.1016/j.hfc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Lakatta EG. Similar myocardial effects of aging and hypertension. Eur Heart J. 1990;11 (Suppl G):29–38. doi: 10.1093/eurheartj/11.suppl_g.29. [DOI] [PubMed] [Google Scholar]
- 45.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Little WC, Zile MR, Klein A, Appleton CP, Kitzman DW, Wesley-Farrington DJ. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383–385. doi: 10.1016/j.amjcard.2006.01.106. [DOI] [PubMed] [Google Scholar]
- 47.Huang BS, Ahmad M, Tan J, Leenen FH. Sympathetic hyperactivity and cardiac dysfunction post-MI: different impact of specific CNS versus general AT1 receptor blockade. J Mol Cell Cardiol. 2007;43:479–486. doi: 10.1016/j.yjmcc.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 48.Leenen FH, Yuan B, Huang BS. Brain ‘ouabain’ and angiotensin II contribute to cardiac dysfunction after myocardial infarction. Am J Physiol. 1999;277:H1786–H1792. doi: 10.1152/ajpheart.1999.277.5.H1786. [DOI] [PubMed] [Google Scholar]
- 49.Westcott KV, Huang BS, Leenen FH. Brain renin–angiotensin–aldosterone system and ventricular remodeling after myocardial infarct: a review. Can J Physiol Pharmacol. 2009;87:979–988. doi: 10.1139/Y09-067. [DOI] [PubMed] [Google Scholar]
- 50.Araujo IG, Trindade DC, Mecawi AS, Sonoda-Côrtes R, Werneck-de-Castro JP, Costa-E-Sousa RH, et al. Inhibition of brain renin–angiotensin system improves diastolic cardiac function following myocardial infarction in rats. Clin Exp Pharmacol Physiol. 2009;36:803–809. doi: 10.1111/j.1440-1681.2009.05159.x. [DOI] [PubMed] [Google Scholar]
- 51.Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension. 2011;58:1057–1065. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baltatu O, Silva JA, Jr, Ganten D, Bader M. The brain renin–angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension. 2000;35:409–412. doi: 10.1161/01.hyp.35.1.409. [DOI] [PubMed] [Google Scholar]
- 53.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, et al. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 54.Stüdeli R, Jung S, Mohacsi P, Perruchoud S, Castiglioni P, Wenaweser P, et al. Diastolic dysfunction in human cardiac allografts is related with reduced SERCA2a gene expression. Am J Transplant. 2006;6:775–782. doi: 10.1111/j.1600-6143.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 55.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, et al. Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 56.Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, van Gilst WH. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 57.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 58.Reckelhoff JF, Baylis C. Proximal tubular metalloprotease activity is decreased in the senescent rat kidney. Life Sci. 1992;50:959–963. doi: 10.1016/0024-3205(92)90174-n. [DOI] [PubMed] [Google Scholar]
- 59.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilliam-Davis S, Gallagher PE, Payne VS, Kasper SO, Tommasi EN, Westwood BM, et al. Long-term systemic angiotensin II type 1 receptor blockade regulates mRNA expression of dorsomedial medulla renin–angiotensin system components. Physiol Genomics. 2011;43:829–835. doi: 10.1152/physiolgenomics.00167.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006;290:H684–H691. doi: 10.1152/ajpheart.00632.2005. [DOI] [PubMed] [Google Scholar]
- 62.Benter IF, Yousif MH, Al-Saleh FM, Raghupathy R, Chappell MC, Diz DI. Angiotensin-(1–7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-L-arginine methyl ester. J Cardiovasc Pharmacol. 2011;57:559–567. doi: 10.1097/FJC.0b013e31821324b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 64.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 66.Alpert MA, Lambert CR, Terry BE, Cohen MV, Mukerji V, Massey CV, et al. Influence of left ventricular mass on left ventricular diastolic filling in normotensive morbid obesity. Am Heart J. 1995;130:1068–1073. doi: 10.1016/0002-8703(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 67.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pascual M, Pascual DA, Soria F, Vicente T, Hernández AM, Tébar FJ, Valdés M. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grandi AM, Zanzi P, Piantanida E, Gaudio G, Bertolini A, Guasti L, Venco A. Obesity and left ventricular diastolic function: noninvasive study in normotensives and newly diagnosed never-treated hypertensives. Int J Obes Relat Metab Disord. 2000;24:954–958. doi: 10.1038/sj.ijo.0801261. [DOI] [PubMed] [Google Scholar]
- 72.Bajraktari G, Koltai MS, Ademaj F, Rexhepaj N, Qirko S, Ndrepepa G, Elezi S. Relationship between insulin resistance and left ventricular diastolic dysfunction in patients with impaired glucose tolerance and type 2 diabetes. Int J Cardiol. 2006;110:206. doi: 10.1016/j.ijcard.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 73.Dinh W, Lankisch M, Nickl W, Gies M, Scheyer D, Kramer F, et al. Metabolic syndrome with or without diabetes contributes to left ventricular diastolic dysfunction. Acta Cardiol. 2011;66:167–174. doi: 10.1080/ac.66.2.2071247. [DOI] [PubMed] [Google Scholar]
- 74.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Effect of diabetes and hypertension on left ventricular diastolic function in a high-risk population without evidence of heart disease. Eur J Heart Fail. 2010;12:454–461. doi: 10.1093/eurjhf/hfq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens. 2004;17:23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 76.Gilliam-Davis S, Payne VS, Kasper SO, Tommasi EN, Robbins ME, Diz DI. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer-344 rats. Am J Physiol Heart Circ Physiol. 2007;293:H1327–H1333. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- 77.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21:1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimokawa A, Kunitake T, Takasaki M, Kannan H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst. 1998;72:46–54. doi: 10.1016/s0165-1838(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 79.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension. 2009;54:1001–1008. doi: 10.1161/HYPERTENSIONAHA.109.138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campos LA, Couto AS, Iliescu R, Santos JA, Santos RA, Ganten D, et al. Differential regulation of central vasopressin receptors in transgenic rats with low brain angiotensinogen. Regul Pept. 2004;119:177–182. doi: 10.1016/j.regpep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 81.Gomes da Silva AQ, Xavier CH, Campagnole-Santos MJ, Caligiorne SM, Baltatu OC, Bader M, et al. Cardiovascular responses evoked by activation or blockade of GABA(A) receptors in the hypothalamic PVN are attenuated in transgenic rats with low brain angiotensinogen. Brain Res. 2012;1448:101–110. doi: 10.1016/j.brainres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 82.Dumesnil JG, Paulin C, Pibarot P, Coulombe D, Arsenault M. Mitral annulus velocities by Doppler tissue imaging: practical implications with regard to preload alterations, sample position, and normal values. J Am Soc Echocardiogr. 2002;15:1226–1231. doi: 10.1067/mje.2002.123396. [DOI] [PubMed] [Google Scholar]
- 83.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 84.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quiñones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 85.Campos LA, Iliescu R, Fontes MA, Schlegel WP, Bader M, Baltatu OC. Enhanced isoproterenol-induced cardiac hypertrophy in transgenic rats with low brain angiotensinogen. Am J Physiol Heart Circ Physiol. 2006;291:H2371–H2376. doi: 10.1152/ajpheart.01145.2005. [DOI] [PubMed] [Google Scholar]
- 86.Nichols WW, O’Rourke MF, Avolio AP, Yaginuma T, Murgo JP, Pepine CJ, Conti CR. Effects of age on ventricular-vascular coupling. Am J Cardiol. 1985;55:1179–1184. doi: 10.1016/0002-9149(85)90659-9. [DOI] [PubMed] [Google Scholar]