Abstract
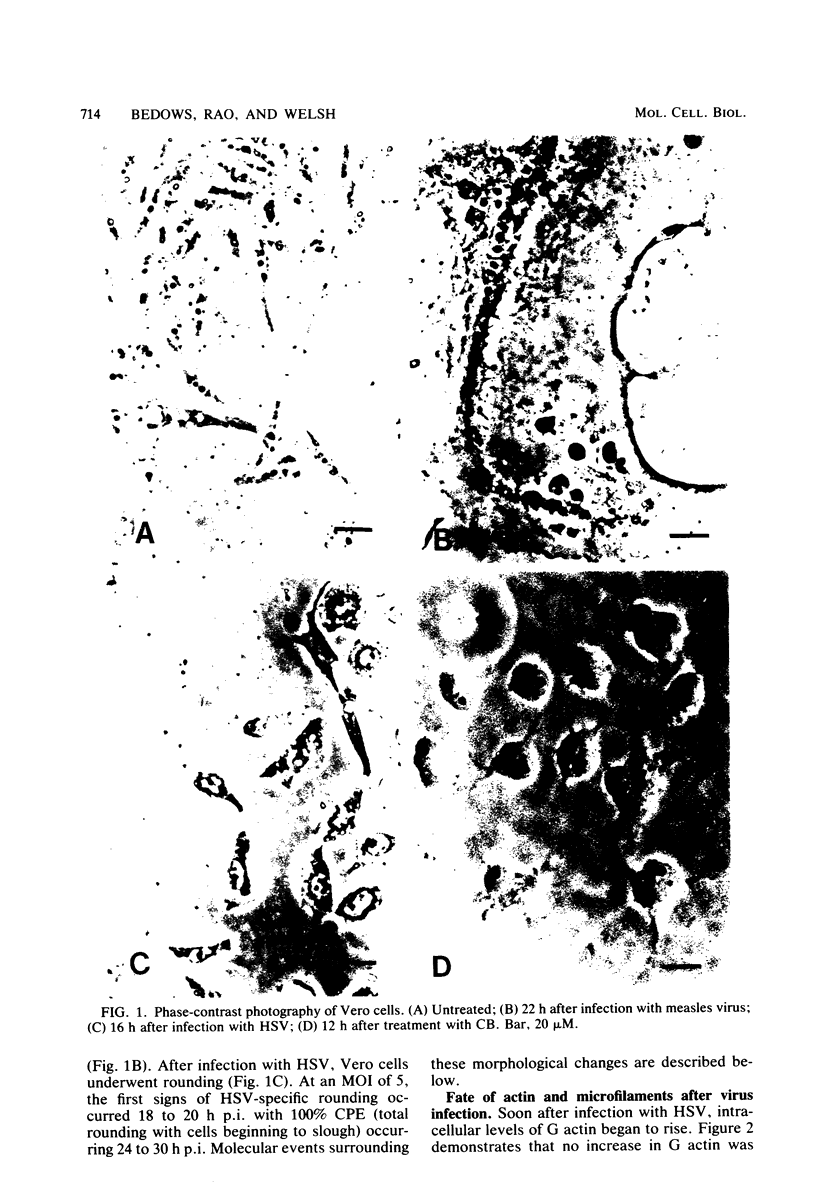
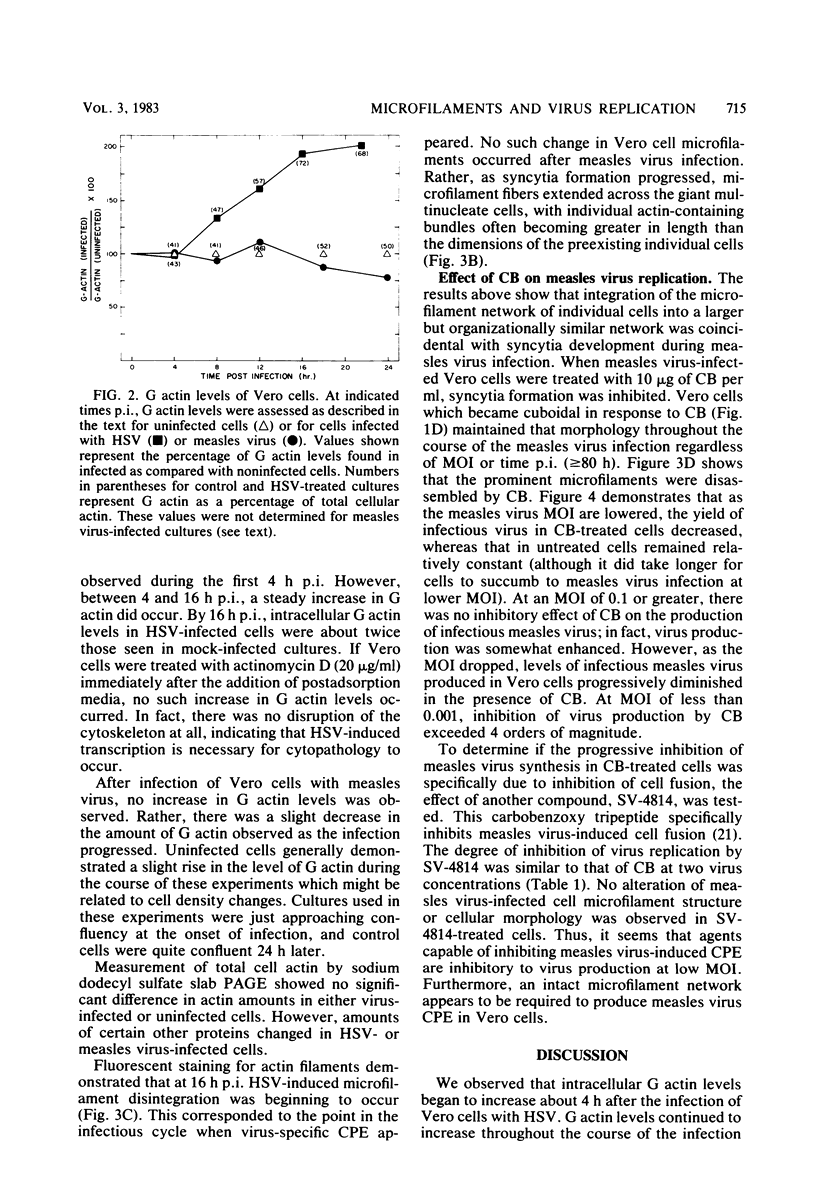
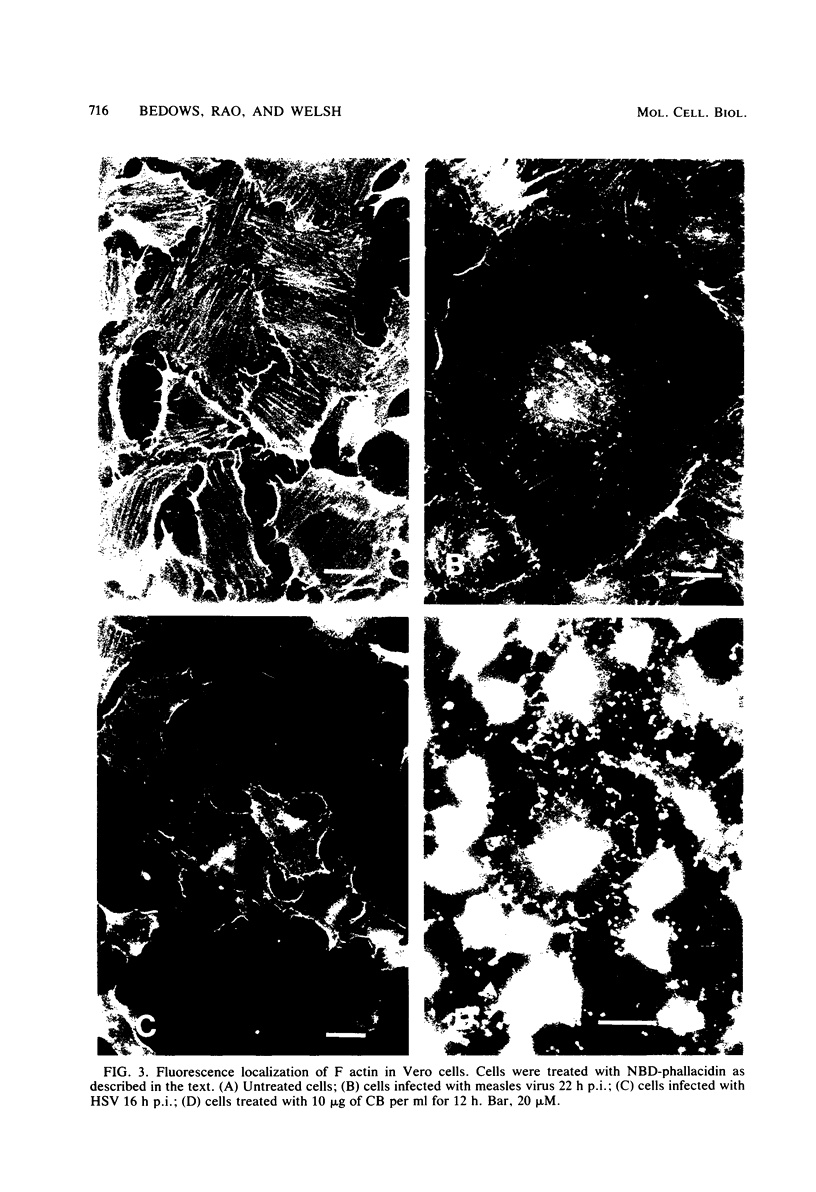
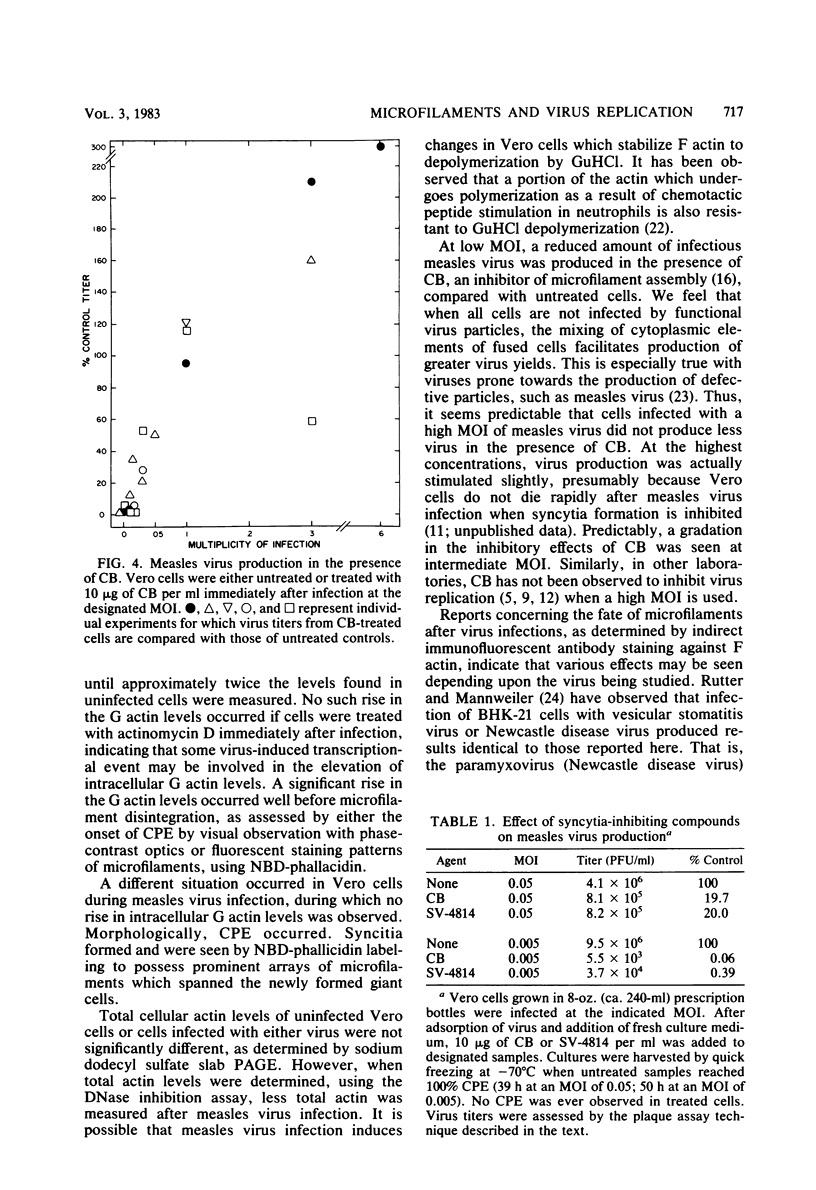
In herpes simplex virus type 1-infected Vero cells, reorganization of microfilaments was observed approximately 4 h postinfection. Conversion of F (filamentous) actin to G (globular) actin, as assessed by a DNase I inhibition assay, was continuous over the next 12 to 16 h, at which time a level of G actin of about twice that observed in uninfected cells was measured. Fluorescent localization of F actin, using 7-nitrobenz-2-oxa-1,3-diazole (NBD)-phallacidin, demonstrated that microfilament fibers began to diminish at about 16 to 18 h postinfection, roughly corresponding to the time that G actin levels peaked and virus-induced cytopathology was first observable. In measles virus-infected cells, no such disassembly of microfilaments occurred. Rather, there was a modest decrease in G actin levels. Fluorescent localization of F actin showed that measles virus-infected Vero cells maintained a complex microfilament network characterized by fibers which spanned the entire length of the newly formed giant cells. Disruption of microfilaments with cytochalasin B, which inhibits measles virus-specific cytopathology, was not inhibitory to measles virus production at high multiplicities of infection (MOI) but was progressively inhibitory as the MOI was lowered. The carbobenzoxy tripeptide SV-4814, which inhibits the ability of Vero cells to fuse after measles virus infection, like cytochalasin B, inhibited measles virus production at low MOI but not at high MOI. Thus, it appears that agents which affect the ability of Vero cells to fuse after measles virus infection may be inhibitory to virus production and that the actin network is essential to this process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedows E., Payne F. E. Host cell factors involved in the production of slowly sedimenting nucleocapsids in measles virus-infected cells. J Virol. 1981 Jan;37(1):103–108. doi: 10.1128/jvi.37.1.103-108.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin Y., Roger M., Robin Y., Thoai N. V. A competitive radioimmunoassay on a magnetic phase for actin detection. FEBS Lett. 1980 Feb 11;110(2):327–329. doi: 10.1016/0014-5793(80)80103-7. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Coombs K., Mann E., Edwards J., Brown D. T. Effects of chloroquine and cytochalasin B on the infection of cells by Sindbis virus and vesicular stomatitis virus. J Virol. 1981 Mar;37(3):1060–1065. doi: 10.1128/jvi.37.3.1060-1065.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnst A., Sundqvist K. G. The mechanisms of appearance of viral glycoproteins at the cell surface membrane. Exp Cell Biol. 1976;44(3-6):198–225. doi: 10.1159/000163112. [DOI] [PubMed] [Google Scholar]
- Fagraeus A., Tyrrell D. L., Norberg R., Norrby E. Actin filaments in paramyxovirus-infected human fibroblasts studied by indirect immunofluorescence. Arch Virol. 1978;57(4):291–296. doi: 10.1007/BF01320068. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Dockter M. E., Phillips D. R. An improved method for determining the actin filament content of nonmuscle cells by the DNase I inhibition assay. Anal Biochem. 1981 Oct;117(1):170–177. doi: 10.1016/0003-2697(81)90707-7. [DOI] [PubMed] [Google Scholar]
- Genty N., Bussereau F. Is cytoskeleton involved in vesicular stomatitis virus reproduction? J Virol. 1980 Jun;34(3):777–781. doi: 10.1128/jvi.34.3.777-781.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C., Silver S. M., Choppin P. W. Measles virus polypeptides synthesis in infected cells. Virology. 1978 May 1;86(1):254–263. doi: 10.1016/0042-6822(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Griffin J. A., Compans R. W. Effect of cytochalasin B on the maturation of enveloped viruses. J Exp Med. 1979 Aug 1;150(2):379–391. doi: 10.1084/jem.150.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laub F., Kaplan M., Gitler C. Actin polymerization accompanies Thy-1-capping on mouse thymocytes. FEBS Lett. 1981 Feb 9;124(1):35–38. doi: 10.1016/0014-5793(81)80048-8. [DOI] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Madansky C. H., Bratt M. A. Noncytopathic mutants of Newcastle disease virus. J Virol. 1978 Jun;26(3):724–729. doi: 10.1128/jvi.26.3.724-729.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. K., Burger M. M., Tschannen R., Schäfer R. Actin filament bundles in vaccinia virus infected fibroblasts. Arch Virol. 1981;67(1):11–18. doi: 10.1007/BF01314597. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Rapp F. Measles virus and its associated diseases. Bacteriol Rev. 1977 Sep;41(3):636–666. doi: 10.1128/br.41.3.636-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E. The effect of a carbobenzoxy tripeptide on the biological activities of measles virus. Virology. 1971 Jun;44(3):599–608. doi: 10.1016/0042-6822(71)90374-6. [DOI] [PubMed] [Google Scholar]
- Rao K. M., Varani J. Actin polymerization induced by chemotactic peptide and concanavalin A in rat neutrophils. J Immunol. 1982 Oct;129(4):1605–1607. [PubMed] [Google Scholar]
- Rutter G., Mannweiler K. Alterations of actin-containing structures in BHK21 cells infected with Newcastle disease virus and vesicular stomatitis virus. J Gen Virol. 1977 Nov;37(2):233–242. doi: 10.1099/0022-1317-37-2-233. [DOI] [PubMed] [Google Scholar]
- Snabes M. C., Boyd A. E., 3rd, Pardue R. L., Bryan J. A DNase I binding/immunoprecipitation assay for actin. J Biol Chem. 1981 Jun 25;256(12):6291–6295. [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. doi: 10.1146/annurev.bi.45.070176.003411. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Ehrnst A. Transmembrane communication in cells chronically infected with measles virus. J Cell Biol. 1979 May;81(2):396–402. doi: 10.1083/jcb.81.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]