Abstract
Because lymphocytes were shown to mediate transplant rejection, their depletion has been studied as a mechanism of preventing rejection and perhaps inducing immunologic tolerance. Agents that profoundly deplete lymphocytes have included monoclonal antibodies, cytotoxic drugs, and radiation. We have studied several such agents but focused on antibodies that deplete not only peripheral blood lymphocytes, but also lymph node lymphocytes. Depletion of lymph node T lymphocytes appears to permit peripheral tolerance at least for T cells in animal models. Nevertheless, B-cell responses may be resistant to such approaches, and T memory cells are likewise relatively resistant to depleting antibodies. We review the experimental and clinical approaches to depletion strategies and outline some of the pitfalls of depletion, such as limitations of currently available agents, duration of tolerance, infection, and malignancy. It is notable that most tolerogenic strategies that have been attempted experimentally and clinically include depleting agents even when they are not named as the underlying strategy. Thus, there is an implicitly acknowledged role for reducing the precursor frequency of donor antigen-specific lymphocytes when approaching the daunting goal of transplant tolerance.
Lymphocytes mediate organ transplant rejection. Agents that deplete lymphocytes (e.g., antibodies and cytotoxic drugs) reduce the rate of acute rejection and are beneficial in reducing maintenance immunosuppression.
Intense immunosuppressive treatment at the time of transplantation is categorized as an induction regimen in organ transplantation. The goals of induction therapy in transplantation have evolved from preventing acute rejection to allowing lower doses of conventional immunosuppression and eventually inducing T-cell nonresponsiveness, also known as operational tolerance (Orlando et al. 2010). Induction therapy has been widely used in organ transplantation, involving 83% of renal transplant and 45% of heart transplant recipients in the United States in 2011 (Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients 2011). The use of depletional agents as an induction therapy also has been growing; 59% of adult kidney transplant recipients and 18% of adult heart transplant recipients now receive lymphodepletion. Generally, depleting antibodies activate the classical complement cascade upon binding to the target antigen and induce complement-mediated cell lysis of cells expressing target antigen. Furthermore, phagocytic cells with Fc receptor (FcR) preferentially engulf antibody-coated cells through ACCC (antibody-dependent cell cytotoxicity). However, other modes of action could also induce lymphocytic depletion, such as limiting survival factors of target cells.
Lymphocyte depletion prior to or beginning at the time of transplantation is beneficial in reducing maintenance immunosuppression (Calne et al. 1998, 1999; Swanson et al. 2002; Kirk et al. 2003; Starzl et al. 2003; Torrealba et al. 2003). Many depleting agents have been studied in animal models and clinical trials, and have been proven efficacious in reducing the rate of acute rejection when combined with maintenance regimens. Indeed, near-tolerance states were induced in many animal models. We showed, for example, that lymphodepletion by anti-CD3 immunotoxin (FN18-CRM9) prolongs renal allograft survival in the nonhuman primate renal transplantation model, but when combined with other immunosuppressive regimens it can induce long-term metastable tolerance (Torrealba et al. 2003, 2004). In human patients, toxicities of chronic calcineurin inhibitor use support the clinical need for depletional strategies (Torrealba et al. 2006). Depleting agents applied in conjunction with CNIs have shown fewer incidents of CNI-related side effects with similar outcomes in preventing acute rejection (Alexander et al. 2006).
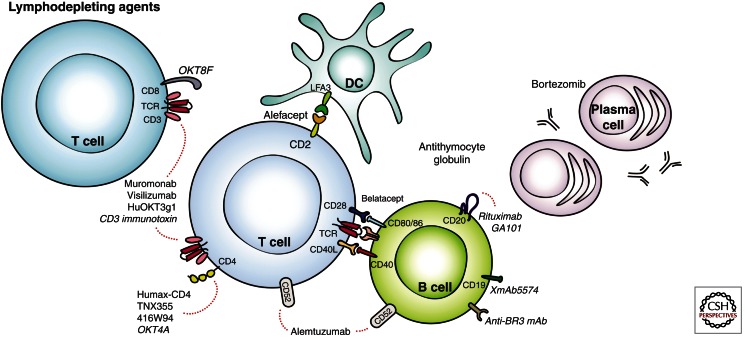
This article provides an overview of lymphodepletion in organ transplantation (Fig. 1). We will discuss small and large animal transplantation models using depletional approaches, agents used in the clinic, and challenges to lymphodepletion, including protective immunity, homeostatic proliferation of recalcitrant memory populations, and humoral responses.
Figure 1.
Lymphodepleting agents. Portrayed in this figure are preclinical and clinical agents found to have depleting properties on T cells, B cells, and plasma cells. The dotted lines indicate target specificities for the agents.
LYMPHODEPLETION IN MOUSE MODELS
The large precursor frequency of allospecific T cells among host T cells poses a significant challenge for transplantation. For that reason, lymphodepletion has become a common immunosuppressive strategy at the time of solid organ transplantation (Kwun et al. 2012a). Although this strategy has helped improve early graft survival, long-term outcomes after depletion are still afflicted with challenges. Animal models have been of great importance in exploring transplant immunology. The rodent model in particular provides essential and critical insight on the basic mechanisms of lymphocyte depletion and homeostasis for field of transplantation, although there are gaps between clinical conditions and animal models (Kwun et al. 2012a). Here we will discuss depletion of T and B cells, plasma cells, and natural killer (NK) T cells in rodent models.
T-Cell Depletion
The first antibodies used since the 1960s, antithymocyte globulin (ATG) induces a rapid and profound lymphodepletion. ATG-induced lymphocyte depletion not only resulted in transplant tolerance in several rodent transplant models but also enhanced regulatory T-cell (Treg) number and function (Feng et al. 2008; Joseph et al. 2012; Shi et al. 2012). Hongo et al. further described that in a combined bone marrow and heart transplant murine model, Treg production of IL-10 and up-regulation of PD-1 was enhanced by IL-4 produced by host natural killer cells (Hongo et al. 2012). Despite the development of newer antibodies, ATG continues to be investigated in murine models today.
The first monoclonal antibody, OKT3, was produced in the late 1970s (Kung et al. 1979), and many researchers have used anti-CD3 mAb for inducing transplant tolerance. The administration of anti-CD3 mAb led to the rapid depletion of T cells from peripheral blood and suppression of heart and islet graft rejection in the rodent model (Nicolls et al. 1993; Tang et al. 2003). However, depletion of T cells from the spleen and lymph nodes was both delayed and incomplete.
As helper CD4 T lymphocytes control humoral and cellular responses, targeting subpopulations using anti-CD4 antibodies appeared to be the best strategy to achieve tolerance to heart allografts in the rodent model (Pearson et al. 1993). Treatment with this monoclonal antibody rapidly depletes the recipient of circulating CD4+ T cells, and repopulation takes about 40–50 days. Their median survival time was over 60 days after transplant (Orosz et al. 1996). Anti-CD4 antibodies depleted Tregs but not as efficiently as CD4+CD25− cells, resulting in an enhanced peripheral CD4+CD25+/CD4+CD25− ratio and thus promoting tolerance (Yi et al. 2008). Cytotoxic CD8 T cells are highly aggressive toward allografts, but unlike CD4 T-cell depletion, the depletion of these cells by the anti-CD8 antibody was not efficient in preventing acute rejection in rodent models.
Mouse T-cell depletion with anti-CD52 antibody alemtuzumab requires the use of human CD52 transgenic mice, in which hCD52 is expressed under the direction of the mouse CD2 promotor and thus only on T cells. Our murine experience with alemtuzumab showed profound peripheral T-cell depletion with the repopulation of CD44hiCD62Llo memory T cells. Despite complete T-cell repopulation by 10 weeks, treated animals showed no evidence of acute rejection, and showed excellent graft survival and function for more than 200 days. Half of the alemtuzumab recipients, however, showed alloantibody production and antibody-mediated chronic rejection (Kwun et al. 2012b). Furthermore, alloantibody-producing animals had increased allospecific B cells found in their spleens, as well as ongoing germinal center reactions represented by CD38-alloreactive B cells.
Other T-cell depleting agents targeting CD2 (Sido et al. 1998) and TCRαβ (Heidecke et al. 1995) have resulted in long-term allograft survival. Sido et al. evaluated three anti-CD2 monoclonal and found that graft survival correlated with dose-dependent down-modulation of CD2, and that humoral responses were preserved. Heidecke et al. observed that, despite incomplete depletion of CD5+ cells, the α/β T-cell receptor blockade was able to induce hyporesponsiveness in a murine heart transplant model after skin graft sensitization.
B-Cell Depletion
Agents that deplete B cells in human recipients, such as alemtuzumab and ATG, have limited efficacy at B-cell depletion in rodents. CD20 is expressed early in B-cell development, down-regulates through maturation, and is then absent on mature plasma cells. Anti-CD20 mAB depletes mature, germinal center, and memory B cells but does not affect plasma cell or serum IgG levels (DiLillo et al. 2008). In rodent models, mature B-cell depletion using anti-CD20 mAb had no effect on acute skin allograft rejection and chronic renal rejection but inhibited the development of allospecific IgG (DiLillo et al. 2011). CD19 is expressed on memory B cells and short-lived plasma cells. Anti-CD19 antibody treatment has the effect of depleting B-cell subsets and some plasma cells resulted in enhanced allograft survival and a reduction in circulating allo-specific IgG in human CD19 transgenic mouse models (DiLillo et al. 2011).
Plasma Cell Depletion
Bortezomib is a proteasome inhibitor that selectively targets the 26s proteasome complex. Plasma cells produce large quantities of protein, a proportion of which will be misfolded and will require degradation and disposal by the proteasome. Inhibition of proteasome activity results in the accumulation of misfolded proteins and consequent apotosis of the plasma cell. In rodent transplant models, proteasome inhibition suppresses ongoing rejection and increases mean survival time after heterotropic heart transplantation, which suggests possible efficacy in transplantation (Luo et al. 2001).
Recent clinical data have shown acute cellular rejection after B-cell depletion with rituximab, suggesting positive or negative effects on graft rejection, depending on the nature of the allograft and response (Clatworthy et al. 2009). Because mature B-cell depletion with CD20 mAb will not be sufficient to prevent acute and chronic allograft rejection, new therapies such as CD19 mAb and the next generation of proteasome inhibitors may offer a new approach for depleting both B cells and plasma cells. For testing these agents to investigate their mechanisms, proper animal models will be needed due to constraints in strain and species specifics (Kwun et al. 2012b).
NK Depletion
NK cells are a subset of lymphocytes with cytokine-secreting and cytotoxic effector functions that respond to viral infection, cell stress, and tumors (Vivier et al. 2008). Defining the role of NK cells in transplantation is less clear because of evidence suggesting their involvement both in rejection and in tolerance. During acute rejection, graft infiltrating NK cells secrete chemokines that amplify local inflammation and IFNγ, which exacerbates allograft rejection and enhances T-cell-mediated immunity. Also, in murine heart transplant models, NK cell depletion has been shown to reduce alloantibody-mediated chronic allograft vasculopathy (Hirohashi et al. 2012). Others have observed that NK cell-depleted recipients showed monocyte and macrophages infiltration in grafts (van der Touw et al. 2012).
LYMPHODEPLETION IN NONHUMAN PRIMATES
En route to clinical translation, lymphodepleting agents have been widely explored in nonhuman primate models of transplantation since the 1980s. Some depleting agents were used as primary induction agents whereas others were incorporated into a combination of therapeutic modalities for transplant tolerance induction. This section will focus on lymphodepletion by pharmacologic and biologic agents and not by virus- or retrovirus-mediated methods.
Polyclonal Antibody Preparations
Polyclonal antilymphocyte globulins, initially prepared from horses immunized with human spleens, thymus, and lymph nodes, have been used for immunosuppression in renal transplantation since the 1960s (Starzl et al. 1963, 1967; Najarian and Simmons 1971). A technology discovered in the 1890s by Mechnikov (Gaber et al. 2010), the mechanisms of action of the modern preparations—rabbit and equine ATG—are vastly diverse due to target antigens that range from surface immune response antigens, costimulation markers, adhesion and trafficking molecules, and many more (Bonnefoy-Berard et al. 1991; Rebellato et al. 1994; Bourdage and Hamlin 1995; Mohty 2007). Preville et al. found that rabbit ATG-administered cynomolgus macaques showed a dose-dependent lymphodepletion in peripheral blood, axillary lymph nodes, and spleens but not in thymuses (Preville et al. 2001). At very high doses (20 mg/kg), peripheral blood T counts dropped to zero within 2 h of the first ATG infusion.
Early nonhuman primate studies showed that combining ATG with other modalities could significantly increase allograft survival and immunologic unresponsiveness. Thomas et al. observed that kidney transplanted rhesus macaques receiving 50 mg/kg of rATG for 5 days followed by a single infusion of unfractionated donor bone marrow on day 12 experienced long-term survival past 248 days without evidence of graft versus host disease, infection, or rejection (compared to 35.8 days for rATG alone, p < 0.001); a second donor bone marrow infusion on day 24 reversed the benefit, however, as the animals rejected with a mean graft survival time of 76.6 days (Thomas et al. 1983). In revisiting the ATG + dBM model, the group observed a reduced median survival of 70 days, still a considerable feat in the absence of chronic immunosuppression (Thomas et al. 1989).
Several groups have observed long-term graft acceptance when combining ATG with other modalities. Pearl et al. studied thymectomized cynomologus macaques depleted with rATG (20 mg/kg × 7 days), and found that anti-CD154, sirolimus, and donor specific whole blood transfusion successfully inhibit the homeostatically proliferating memory T-cell response in the depleted animals to yield rejection-free survivals of >300 days (Pearl et al. 2007). Kawai et al. observed renal allograft tolerance in cynomolgus macaques achieving mixed chimerism after treatment with equine ATG (50 mg/kg × 3 days), nonmyeloablative total-body irradiation, thymic irradiation, and donor bone marrow infusion (Kawai et al. 1995). When anti-CD154 mAb was added to this regimen (+CsA × 28 days), donor bone marrow engraftment was enhanced, leading to long-term renal allograft survival >688 days (Kawai et al. 2004). The further addition of humanized anti-CD8 mAb (cM-T807) depleted remaining CD8 memory T cells and allowed for animals to achieve renal allograft tolerance (Koyama et al. 2007). Other groups have combined rATG with anti-CD20 mAb rituximab (Liu et al. 2007) or with sirolimus (Hirshberg et al. 2003) to prolong islet allograft survival in nonhuman primates.
CD3 Immunotoxin
In contrast to the wide heterogeneity of ATG’s target specificities, the single antigen-specific antirhesus CD3 immunotoxin developed by Neville et al. (Neville et al. 1992) was pursued with much interest by our group and others (Knechtle 2001). The antirhesus monoclonal antibody FN18 conjugated with mutated diphtheria toxin CRM9 was able to deplete CD3 T cells by 2–3 logs in peripheral blood and by 2 logs in lymph nodes, when we administered a three-day course to rhesus macaques (Knechtle et al. 1998). We observed long-term renal allograft acceptance (mean survival time, 614 days) with donor-specific tolerance confirmed by skin grafting in our long-term survivors; however, a high incidence of donor-specific alloantibodies (73%) and chronic rejection (100%) was also observed (Knechtle et al. 1997, 1998; Torrealba et al. 2003).
We ventured to investigate the activation of the humoral response and eventual chronic rejection in the FN19-CRM9 immunotoxin-treated animals, but the drug was replaced with a new recombinant immunotoxin (A-dmDT390-scfbDb[C207]) because of production yield difficulties and potential immunogenicity of the former chemically conjugated construct (Ma et al. 1997; Kim et al. 2007; Wang et al. 2011). Although the use of the new immunotoxin provided profound peripheral T-cell depletion, the rapid proliferation of memory T cells prevented sustained graft survival. Tacrolimus and alefacept were added to target the memory T cells, which resulted in early and consistent alloantibody production and antibody-mediated graft injury in all treated macaques (Page et al. 2012). Nishimura et al. doubled the immunotoxin dose to 50 µg/kg × 8 doses and combined it with an anti-CD2 antibody in a GalT-KO pig-to-baboon thymokidney model, which led to the preservation of graft function but death due to pneumonia or heart failure at 15 and 23 days (Nishimura et al. 2011).
Alemtuzumab
Alemtuzumab (Campath-1H), a humanized IgG1 monoclonal antibody, targets the membrane glycoprotein CD52, resulting in profound and sustained T-cell depletion as well as transient depletion of B cells, macrophages, and natural killer cells (Hale 2001; Magliocca and Knechtle 2006). Its use in nonhuman primates has been limited as most Old World monkeys express CD52 also on red blood cells, potentially leading to lethal hemolytic anemia if administered the drug. Recently, van der Windt et al. evaluated an agglutination assay and identified cynomolgus macaques of Indonesian but not Chinese origin to be RBC-CD52 negative (van der Windt et al. 2010). As they achieved greater than 99.5% depletion of T and B cells with 20 mg/kg of alemtuzumab, this model provides opportunities to further investigate the drug in preclinical studies.
Other T-Cell Depleting Agents
The advent of monoclonal antibodies provoked much interest in the transplant community, with several studies applying depleting antibodies to T-cell targets (OKT3, OKT4, OKT8, and OKT11 to CD3, CD4, CD8, and E rosettes, respectively) in nonhuman primate models (Jonker et al. 1983; Letvin et al. 1985; Cosimi et al. 1990; Rao et al. 1991). These early monoclonal antibodies, namely OKT3, although effective at treating acute rejection, were associated with cytokine release syndrome, increased risk of posttransplant lymphoproliferative disease, and xeno-sensitization to its murine component (Chatenoud et al. 1990; Schroeder et al. 1990; Cherikh et al. 2003). To diminish the antimurine response, humanized chimeric preparations of several of the early monoclonal antibodies were developed and are still used in nonhuman primate studies today (Woodle et al. 1992; Delmonico et al. 1993; Engram et al. 2010).
Other depleting agents no longer used in human organ transplantation include alefacept (LFA3-Ig) and fingolimod (FTY720). The CD2-specific fusion protein alefacept (LFA3-Ig), which selectively depletes memory T cells, has been used as adjunct immunosuppression in nonhuman primate models of cardiac, renal, and xenoislet transplantation (Kaplon et al. 1996; Weaver et al. 2009; Page et al. 2012; Thompson et al. 2012). Fingolimod, a sphingoside-1-phosphate receptor agonist administered orally, was found to effectively deplete T and B cells in baboons and cynomolgus macaques (Quesniaux et al. 1999, 2000), and prevent acute rejection in nonhuman primate renal, allo-islet, and xeno-islet transplantation (Schuurman et al. 2002; Wijkstrom et al. 2004; Hering et al. 2006).
B-Cell Depletion
Several B-cell-targeting therapeutics have emerged over the last decade, as our understanding of the impact of B cells on allograft rejection as well as transplant tolerance has increased (Redfield et al. 2011; Kwun et al. 2012a). These strategies may be of particular interest in the setting of T-cell depletion in which humoral immunity is often up-regulated. Their applications may also be extended to sensitized patients pre- and posttransplant.
The following agents targeting the B-cell surface molecules CD19 and CD20, costimulatory target CD40, and B-cell survival factors in the BAFF pathway have resulted in effective B-cell depletion. Rituximab (IDEC-C2B8), originally developed for B-cell lymphomas, has been used in several preclinical models, including islet cell transplantation (in combination with rATG), renal transplantation (with rATG, sirolimus, and donor bone marrow infusion), cardiac transplantation (with cyclosporine A), and pig-to-baboon cardiac xenotransplantation (with rATG, splenectomy, and maintenance immunosuppression) (McGregor et al. 2005; Liu et al. 2007; Maki et al. 2008; Kelishadi et al. 2010). GA101, a humanized IgG1 to CD20, induced further B-cell depletion particularly in lymphoid tissues in cynomolgus macaques (Mossner et al. 2010). XmAb5574, an Fc-engineered antibody to CD19, showed sustained B-cell depletion to <10% of baseline as well as transient NK cell depletion in cynomolguc macaques (Zalevsky et al. 2009). Although some anti-CD40 antibodies are nondepleting, Chi220 administered to rhesus macaques showed near complete peripheral B-cell depletion (Pearson et al. 2002). BR3 is one of three receptors for the B-cell activating factor BAFF; humanized anti-BR3 antibodies reduced B-cell populations in peripheral blood as well as lymphoid tissues, but not as profoundly as anti-CD20 treatment (Lin et al. 2007).
CURRENT LYMPHOCYTIC DEPLETION IN CLINIC
Currently used antibodies to deplete immune cells in solid organ transplant patients include thymoglobulin (rabbit ATG, Genzyme), ATGAM (equine ATG, Pfizer), and alemtuzumab (Campath-1H). Another rabbit ATG is marketed in Europe by Fresenius. Alemtuzumab was bought by Sanofi in 2011 and is being remarketed at a higher price under the name Lemtrada. Thymoglobulin is approved by the U.S. Federal Drug Administration for treatment of acute renal allograft rejection but not as an induction agent nor for other organs, although the drug is commonly used for these other purposes. Alemtuzumab is approved for treatment of lymphoma but not for organ transplantation, and its ownership by more than four drug companies over the past 10 years and the economics of its development make it unlikely to receive regulatory approval in the near future for organ transplantation. Nevertheless, these two agents are commonly used, and there is considerable experience, especially in kidney transplantation, with both. The published data on both agents inform our use and practice, but they leave unclear the ideal dose, duration, and indications for depletional therapy despite extensive experience. According to the 2011 Annual Report of the Scientific Registry of Transplant Recipients (SRTR/OPTN), about 60% of renal transplant patients in the United States received a depleting agent at the time of transplantation in 2010.
As discussed with regard to nonhuman primate (NHP) depletional therapy, CD3-immunotoxin (CD3-IT), developed by Neville for NHP use, potently depletes not only peripheral blood CD3+ T cells, but also lymph node cells, within days. This agent (FN18−CRM9) was in development by Novartis for human use with the intention of developing a genetically derived recombinant fusion protein, thus avoiding chemical conjugation of CD3 mAb and mutated diphtheria toxin (CRM9) and inevitable unconjugated products that would contaminate such a compound. However, this development project was finally aborted after the new constructs, such as a monovalent antibody fused to toxin, failed to have similar potency, binding properties, or stability compared to the NHP original construct, as discussed above. However, taking a cue from the remarkable ability of the CD3-IT depletion to promote tolerance, Calne revisited clinical use of Campath-1H, a potent anti-CD52 mAb produced by Herman Waldmann in the Cambridge, England pathology department (hence the name, with H for humanized). Calne wisely combined Campath-1H with low dose cyclosporine, aiming for near or “prope” tolerance in humans rather than a completely drug-free state. The data from the Cambridge kidney transplant experience with Campath-1H have been published with both early and long-term follow up, and indicate the safety of profound depletion from an infection and malignancy perspective and the ability of depletion to replace some degree of maintenance immunosuppression (Calne et al. 1998; Watson et al. 2005).
Studies by our group supported by the Immune Tolerance Network (ITN) in human kidney transplantation evaluated the ability of high dose alemtuzumab to achieve tolerance in 10 haploidentical living donors or other well-matched renal transplant recipients. Although four of 10 patients achieved stable renal function for >3 years on rapamycin 1 mg daily with levels <4 ng/ml, none of these patients totally withdrew immunosuppression. Another observation of this study was that most other patients (5/10) developed detectable donor-specific alloantibodies after the withdrawal of tacrolimus that occurred at 60 days in the protocol. This high incidence of alloantibodies was shown by our group in other studies to be driven by tacrolimus withdrawal and to correlate with high BAFF levels (Shapiro et al. 2008; Bloom et al. 2009; Ruhil et al. 2012). Nevertheless, this study suggests that well-matched kidney transplant patients treated with profound depletion in the setting of low dose tacrolimus, later weaned from tacrolimus, and then weaned to very low dose rapamycin may become unresponsive. Mechanistic assays are in the process of confirming the nature of the donor-specific unresponsiveness of these selected patients.
An ongoing study by Kirk reflects a modification of the ITN study above and substitutes belatacept for tacrolimus, perhaps an ideal combination that avoids the high alloantibody incidence associated with tacrolimus withdrawal and takes advantage of the known benefit of belatacept to prevent alloantibody. This study has been reported in abstract form (Kirk et al. 2011) and follow-up studies are in progress. Although it is too early to compare such a study to conventional therapy, results have been excellent; however, once again they show that complete drug withdrawal may not be advisable. The longer term outcomes of this study remain of high interest.
Perhaps the best and largest prospective, randomized study of alemtuzumab induction in renal transplantation was by Hanaway et al. (2011). This study compared alemtuzumab to anti-CD25 (Simulect) in low risk recipients and to thymoglobulin in high-risk recipients. All groups received maintenance immunosuppression with tacrolimus, mycophenolate, and steroids. The main findings were that alemtuzumab was associated with significantly less acute rejection compared to Simulect in low risk patients, but that there was no significant difference in acute rejection between the two high-risk groups (alemtuzumab vs. thymoglobulin). There were no significant differences in infection or side effects among the four groups.
Thymoglobulin induction in kidney transplantation continues to be used particularly for patients considered to be at higher than average risk of rejection. According to the SRTR 2011 annual report on kidney transplantation, abuse was driven by a publication from Brennan et al. (2006) comparing anti-CD25 induction to thymoglobulin, with slightly better results seen with depletion. Nevertheless, a recent trend toward less depletion and more reliance on tacrolimus and anti-CD25 reflects the relatively thin layer of objective data that show superiority of depletion for even the higher risk patients. Higher risk refers to retransplants, African-American recipients, and sensitized or previously sensitized but current cross-match negative recipients of renal allografts.
One of the lessons learned in the past 15 years about depletion induction therapy in renal transplantation is that whereas it clearly protects from acute rejection in the early posttransplant period, maintenance therapy is necessary during cell repopulation to particularly prevent T memory cells (Tmem) from driving rejection and alloantibody formation. As Pearl nicely showed, Tmem are particularly resistant to depletion and perhaps best controlled by calcineurin inhibitors (CNI) (Pearl et al. 2005). Thus, depletional strategies that have been most successful, such as the alemtuzumab/tacrolimus protocol described by Shapiro et al. (2007), rely on at least a low dose of CNI maintenance therapy. Similarly, thymoglobulin protocols typically use triple drug maintenance therapy with tacrolimus, mycophenolate, and steroids for higher risk patients (Gurk-Turner et al. 2008) with good results.
The long-term safety of depletion with respect to opportunistic infection (cytomegalovirus [CMV], other viral and fungal infections) and malignancy (posttransplantation lymphoproliferative disease [PTLD]) depends on the agent used and the intensity and duration of therapy. Given the age-related senescence of the lymphocyte compartment, it would stand to reason that older patients would tolerate depletion less well than younger patients. Kirk et al. (2007) reported that in contrast to thymoglobulin and OKT3 that are associated with increased risk of PTLD, the cumulative experience with alemtuzumab did not suggest an increased risk of PTLD. However, repeated use of alemtuzumab in transplant patients to maintain a low white blood cell (WBC) count is not recommended and was associated with unacceptable side effects in a Minnesota experience with the drug (Nath et al. 2005).
Use of depleting antibodies in liver transplantation continues at some centers although the majority of U.S. centers do not use any routine induction and only about 15% of recipients receive any induction antibody. Many centers add anti-CD25 induction for patients with renal dysfunction with the notion of delaying CNI introduction until renal function improves. Data from the University of Pittsburgh suggest that using alemtuzumab induction in hepatitis C-positive liver transplant recipients is associated with unacceptable outcomes and is ill-advised (Marcos et al. 2004). Other experiences with thymoglobulin induction in liver transplantation suggest that it does not reduce acute rejection incidence but increases the incidence of leukopenia (Boillot et al. 2009). A recent randomized controlled trial suggests that rather than depleting antibodies, lower dose tacrolimus in combination with everolimus spares renal function and effectively prevents rejection (De Simone et al. 2012). This strategy may be more effective than increasing the potency of induction immunosuppression by depletion in liver transplant patients who are often immunocompromised already from their illness.
CHALLENGES IN LYMPHODEPLETION
Although depletion is highly effective and increasingly used as an induction regimen, lymphodepletional agents are associated with several problems. As mentioned above, polyclonal preparations bring undesirable complications of transient cytokine release, viral reactivation, and antianimal antibody production. Although monoclonal OKT-3 solved some of these issues with polyclonal preparations, it was still subject to a significant antimouse response, as OKT3 is an entirely mouse-derived antibody (Webster et al. 2006). These xenogeneic antibodies can dramatically reduce the efficacy of these agents and extended/repeated use may lead to anaphylaxis and serum sickness (Prin Mathieu et al. 1997; Regan et al. 1997, 2001). The first dose of these antibodies can be followed by fevers, chills, and gastointestinal, respiratory, and cardiac complications, mostly due to the release of pyrogenic cytokines such as IL-6 and TNF-α from target cell lysis (Debets et al. 1989; Chatenoud et al. 1990; Vallhonrat et al. 1999). Alternatively, these cytokines could be released by a transient activation of T cells before they undergo cell lysis (Hardinger 2006; Webster et al. 2006). This cytokine release syndrome can be limited by methylprednisolone. These adverse effects were much improved with the development of monoclonal antibodies and fusion proteins. To avoid xenogeneic antibody production, the gene fragment encoding xenogenic epitopes in the mAb may be exchanged with equivalent portions of human antibodies (Boulianne et al. 1984; Morrison et al. 1984). Other early complications, including cardiovascular and infectious deaths, correlate with lymphodepletion, but the interpretation of this relationship is confounded by the preferential use of antibodies in high-risk patients (Opelz 1995).
Infectious Complications
Patients with prolonged lymphopenia are more likely to develop serious infections, especially when depletion is combined with other immunosuppressants before or after transplantation. Polyclonal antibodies have been shown to increase reactivation and development of primary viral disease with cytomegalovirus, herpes simplex virus, Epstein–Barr virus (EBV), and varicella, which more likely represent intensive immunosuppression (Abbott et al. 2002; Gourishankar et al. 2004). More recent reports have shown no increased risk of cytomegalovirus (CMV) infection, possibly due to the proper usage of antiviral prophylaxis (Webster et al. 2006). Although higher infection rates were observed in patients receiving thymoglobulin compared to nondepleting anti-CD25 mAb treated patients (85.8% vs. 75.2%, P = 0.03), a lower incidence of cytomegalovirus disease was documented from thymoglobulin treated patients (7.8% vs. 17.5%, P = 0.02) (Brennan and Schnitzler 2008). Alemtuzumab did not show a significant risk of infection compared to thymoglobulin or basiliximab (Hanaway et al. 2011). Owing to its intensity on immunosuppression in general, lymphodepletion should be accompanied by broad prophylaxis to prevent opportunistic viral (cytomegalovirus and BK virus) and bacterial infections (urinary tract infection, pneumonia, and bacteremia) (Hibberd et al. 1992; Batiuk et al. 2002).
Malignant Complications
Early depletion approaches, such as polyclonal antibodies and OKT3, have been linked with the risk of developing posttransplantation lymphoproliferative disease (PTLD) when combined with maintenance immunosuppressants (Penn 1990; Malatack et al. 1991; Meier-Kriesche et al. 2002; Cherikh et al. 2003). In an exemplary analysis of 157 consecutive cardiac transplant recipients, a ninefold higher incidence of PTLD was observed in OKT-3 treated cardiac allograft patients. Furthermore, a dose-related risk was also observed, with more frequent PTLD observed in patients receiving a cumulative dose of OKT3 exceeding 75 mg than in those who received a lower total dose (35.7% vs. 6.2%; p < 0.001) (Swinnen et al. 1990). On the other hand, analysis of 207 patients with thymoglobulin did not reveal an association with an additional risk of malignancies (El-Hamamsy et al. 2005). Interestingly, alemtuzumab induction has also shown a low PTLD incidence that approximates that of the nondepletional CD25-specific mAbs (Kirk et al. 2007). This could relate to B-cell depleting properties of alemtuzumab because B cells are the dominant reservoir of EBV. The impact of induction therapy on all-cause mortality remains unclear, especially when combined with newer immunosuppressants for maintenance, as does the risk for increased rejection and malignancy.
Homeostatic Repopulation after Lymphodepletion
Despite its efficacy in controlling T-cell-mediated acute rejection with low dose maintenance immunosuppression, lymphodepletion alone does not prevent acute rejection in the human patient (Trzonkowski et al. 2006). Turka’s group elegantly showed that repopulating T cells and their phenotypic switching to memory type under homeostatic pressure is a great barrier to promoting tolerance (Wu et al. 2004; Neujahr et al. 2006). Lymphocyte reconstitution involves a dynamic balance between the peripheral expansion of residual lymphocytes and the generation of new lymphocytes from progenitors. Following lymphocytic depletion, homeostatic mechanisms drive the immediate reconstitution of lymphocyte populations whereas lymphopoiesis may not fully impact reconstitution for 1–2 years. Two major components for homeostatic maintenance of lymphocyte numbers and diversity include cytokines/chemokines and survival factors (Marrack et al. 2000; Ploix et al. 2001; Jameson 2002). Continuous engagement of T-cell receptors with self-peptide/MHC-complex is also required for the T-cell homeostatic proliferation (Viret et al. 1999).
T-cell subsets have been increasingly studied in the postdepletion setting, particularly after observing increased Treg populations with alemtuzumab or ATG induction (Ciancio et al. 2005; Bloom et al. 2008; Morelon et al. 2010). The exact mechanism associated with relative or absolute expansion of CD4+CD25+ T cells remains unclear; however, it is suggested that the increase of Treg is not completely dependent on the sparing of this subset after depletion, but by active conversion of non-Treg to Treg cells (Bloom et al. 2008). This relative increase in Tregs may counter the postdepletional increase in memory-type T cells and their function. Th17 cells also play a role in transplant rejection, as IL-17 has been found in biopsies of rejecting kidney, lung, and liver allografts (Loong et al. 2002; Vanaudenaerde et al. 2008; Fabrega et al. 2009). One study documented an increased number of Th17 with sirolimus monotherapy after alemtuzumab induction (Hester et al. 2011), although its role in rejection after lymphodepletion is unclear. These observations suggest that T-cell subsets have different susceptibilities to depletion and repopulation dynamics.
Follicular helper T cells (Tfh) play a unique role in helping the B-cell response (Crotty 2011). Although little is known on the direct role of T-cell depletion on Tfh cells, our preclinical and clinical studies have reported increased humoral responses and de novo alloantibody production after T-cell depletion (Barth et al. 2006; Page et al. 2012; Kwun et al. 2012b). Tfh cells are known to express high levels of IL-21, which is required for cognate B-cell help and germinal center (GC) formation (Silver and Hunter 2008). Interestingly, increased serum IL-21 levels have been found in a significant subset of multiple sclerosis patients treated with alemtuzumab, which also correlated to their development of autoimmune thyroiditis (Jones et al. 2009). Incomplete depletion in lymph nodes has been previously reported (Kirk et al. 2007; Page et al. 2012); it is possible that lymph node structures provide more resistance to these depletional agents, which may favor an enhanced Tfh cell and germinal center response. Owing to the increasing interest on antibody-mediated rejection in organ transplantation, follicular helper T-cell subsets in lymphodepletion need further careful evaluation.
CONCLUSION
Lymphodepletion has been an effective strategy for addressing the precursor frequency of alloreactive T cells at the time of organ transplantation and for preventing acute allograft rejection. Several preclinical studies have found that combining lymphodepletion with other modalities may be tolerogenic, although this has yet to be fully achieved in humans. Depletion has allowed, however, for clinicians to decrease maintenance immunosuppression and reduce the side effects of long-term steroids and calcineurin inhibition. For these reasons, it has been an increasingly used method of induction immunosuppression in the United States in recent years. Future studies should evaluate ways to inhibit postdepletional humoral responses and memory T-cell repopulation, while taking care not to over-immunosuppress and subject the patient to infectious and malignant complications.
Footnotes
Editors: Laurence A. Turka and Kathryn J. Wood
Additional Perspectives on Transplantation available at www.perspectivesinmedicine.org
REFERENCES
- Abbott KC, Hypolite IO, Viola R, Poropatich RK, Hshieh P, Cruess D, Hawkes CA, Agodoa LY 2002. Hospitalizations for cytomegalovirus disease after renal transplantation in the United States. Ann Epidemiol 12: 402–409 [DOI] [PubMed] [Google Scholar]
- Alexander JW, Goodman HR, Cardi M, Austin J, Goel S, Safdar S, Huang S, Munda R, Fidler JP, Buell JF, et al. 2006. Simultaneous corticosteroid avoidance and calcineurin inhibitor minimization in renal transplantation. Transpl Int 19: 295–302 [DOI] [PubMed] [Google Scholar]
- Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998–2011 2011. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association, Ann Arbor, MI [Google Scholar]
- Barth RN, Janus CA, Lillesand CA, Radke NA, Pirsch JD, Becker BN, Fernandez LA, Thomas Chin L, Becker YT, Odorico JS, et al. 2006. Transpl Int 19: 885–892 [DOI] [PubMed] [Google Scholar]
- Batiuk TD, Bodziak KA, Goldman M 2002. Infectious disease prophylaxis in renal transplant patients: A survey of U.S. transplant centers. Clin Transplant 16: 1–8 [DOI] [PubMed] [Google Scholar]
- Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, Turka LA, Knechtle SJ 2008. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant 8: 793–802 [DOI] [PubMed] [Google Scholar]
- Bloom D, Chang Z, Pauly K, Kwun J, Fechner J, Hayes C, Samaniego M, Knechtle S 2009. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant 9: 1835–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillot O, Seket B, Dumortier J, Pittau G, Boucaud C, Bouffard Y, Scoazec JY 2009. Thymoglobulin induction in liver transplant recipients with a tacrolimus, mycophenolate mofetil, and steroid immunosuppressive regimen: A five-year randomized prospective study. Liver Transpl 15: 1426–1434 [DOI] [PubMed] [Google Scholar]
- Bonnefoy-Berard N, Vincent C, Revillard JP 1991. Antibodies against functional leukocyte surface molecules in polyclonal antilymphocyte and antithymocyte globulins. Transplantation 51: 669–673 [DOI] [PubMed] [Google Scholar]
- Boulianne GL, Hozumi N, Shulman MJ 1984. Production of functional chimaeric mouse/human antibody. Nature 312: 643–646 [DOI] [PubMed] [Google Scholar]
- Bourdage JS, Hamlin DM 1995. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation 59: 1194–1200 [PubMed] [Google Scholar]
- Brennan DC, Schnitzler MA 2008. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N Engl J Med 359: 1736–1738 [DOI] [PubMed] [Google Scholar]
- Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D 2006. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 355: 1967–1977 [DOI] [PubMed] [Google Scholar]
- Calne R, Friend P, Moffatt S, Bradley A, Hale G, Firth J, Bradley J, Smith K, Waldmann H 1998. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet 351: 1701–1702 [DOI] [PubMed] [Google Scholar]
- Calne R, Moffatt SD, Friend PJ, Jamieson NV, Bradley JA, Hale G, Firth J, Bradley J, Smith KG, Waldmann H 1999. Campath IH allows low-dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation 68: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Ferran C, Legendre C, Thouard I, Merite S, Reuter A, Gevaert Y, Kreis H, Franchimont P, Bach JF 1990. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation 49: 697–702 [DOI] [PubMed] [Google Scholar]
- Cherikh WS, Kauffman HM, McBride MA, Maghirang J, Swinnen LJ, Hanto DW 2003. Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation 76: 1289–1293 [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, Mattiazzi A, Cordovilla T, Roth D, Kupin W, et al. 2005. A randomized trial of three renal transplant induction antibodies: Early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation 80: 457–465 [DOI] [PubMed] [Google Scholar]
- Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, Smith KG 2009. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med 360: 2683–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosimi AB, Delmonico FL, Wright JK, Wee SL, Preffer FI, Jolliffe LK, Colvin RB 1990. Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-CD4 monoclonal antibody. Surgery 108: 406–413; discussion 413–404 [PubMed] [Google Scholar]
- Crotty S 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29: 621–663 [DOI] [PubMed] [Google Scholar]
- Debets JM, Leunissen KM, van Hooff HJ, van der Linden CJ, Buurman WA 1989. Evidence of involvement of tumor necrosis factor in adverse reactions during treatment of kidney allograft rejection with antithymocyte globulin. Transplantation 47: 487–492 [DOI] [PubMed] [Google Scholar]
- Delmonico FL, Cosimi AB, Kawai T, Cavender D, Lee WH, Jolliffe LK, Knowles RW 1993. Nonhuman primate responses to murine and humanized OKT4A. Transplantation 55: 722–728 [DOI] [PubMed] [Google Scholar]
- De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L, et al. 2012. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am J Transplant 12: 3008–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF 2008. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol 180: 361–371 [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Griffiths R, Seshan SV, Magro CM, Ruiz P, Coffman TM, Tedder TF 2011. B lymphocytes differentially influence acute and chronic allograft rejection in mice. J Immunol 186: 2643–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hamamsy I, Stevens LM, Carrier M, Pelletier G, White M, Tremblay F, Perrault LP 2005. Incidence and prognosis of cancer following heart transplantation using RATG induction therapy. Transpl Int 18: 1280–1285 [DOI] [PubMed] [Google Scholar]
- Engram JC, Cervasi B, Borghans JA, Klatt NR, Gordon SN, Chahroudi A, Else JG, Mittler RS, Sodora DL, de Boer RJ, et al. 2010. Lineage-specific T-cell reconstitution following in vivo CD4+ and CD8+ lymphocyte depletion in nonhuman primates. Blood 116: 748–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrega E, Lopez-Hoyos M, San Segundo D, Casafont F, Pons-Romero F 2009. Changes in the serum levels of interleukin-17/interleukin-23 during acute rejection in liver transplantation. Liver Transpl 15: 629–633 [DOI] [PubMed] [Google Scholar]
- Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, Munson PJ, Herndon TM, Chen J, Young NS 2008. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood 111: 3675–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M 2010. Rabbit antithymocyte globulin (thymoglobulin): 25 Years and new frontiers in solid organ transplantation and haematology. Drugs 70: 691–732 [DOI] [PubMed] [Google Scholar]
- Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK 2004. Herpes zoster infection following solid organ transplantation: Incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant 4: 108–115 [DOI] [PubMed] [Google Scholar]
- Gurk-Turner C, Airee R, Philosophe B, Kukuruga D, Drachenberg C, Haririan A 2008. Thymoglobulin dose optimization for induction therapy in high risk kidney transplant recipients. Transplantation 85: 1425–1430 [DOI] [PubMed] [Google Scholar]
- Hale G 2001. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy 3: 137–143 [DOI] [PubMed] [Google Scholar]
- Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, Croy R, Holman J 2011. Alemtuzumab induction in renal transplantation. N Engl J Med 364: 1909–1919 [DOI] [PubMed] [Google Scholar]
- Hardinger KL 2006. Rabbit antithymocyte globulin induction therapy in adult renal transplantation. Pharmacotherapy 26: 1771–1783 [DOI] [PubMed] [Google Scholar]
- Heidecke CD, Hancock WW, Jakobs F, Zantl N, Kurrle R, Westerholt S, Sewczik T, Deusch K, Kupiec-Weglinski J 1995. α/β–T cell receptor-directed therapy in rat cardiac allograft recipients. Treatment before alloantigen exposure prevents sensitization and abrogates accelerated rejection. Transplantation 59: 78–84 [DOI] [PubMed] [Google Scholar]
- Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, et al. 2006. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 12: 301–303 [DOI] [PubMed] [Google Scholar]
- Hester J, Mills N, Shankar S, Carvalho-Gaspar M, Friend P, Wood KJ 2011. Th17 cells in alemtuzumab-treated patients: The effect of long-term maintenance immunosuppressive therapy. Transplantation 91: 744–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd PL, Tolkoff-Rubin NE, Cosimi AB, Schooley RT, Isaacson D, Doran M, Delvecchio A, Delmonico FL, Auchincloss H Jr, Rubin RH 1992. Symptomatic cytomegalovirus disease in the cytomegalovirus antibody seropositive renal transplant recipient treated with OKT3. Transplantation 53: 68–72 [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB 2012. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant 12: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshberg B, Preston EH, Xu H, Tal MG, Neeman Z, Bunnell D, Soleimanpour S, Hale DA, Kirk AD, Harlan DM 2003. Rabbit antithymocyte globulin induction and sirolimus monotherapy supports prolonged islet allograft function in a nonhuman primate islet transplantation model. Transplantation 76: 55–60 [DOI] [PubMed] [Google Scholar]
- Hongo D, Tang X, Dutt S, Nador RG, Strober S 2012. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood 119: 1581–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC 2002. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol 2: 547–556 [DOI] [PubMed] [Google Scholar]
- Jones JL, Phuah CL, Cox AL, Thompson SA, Ban M, Shawcross J, Walton A, Sawcer SJ, Compston A, Coles AJ 2009. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest 119: 2052–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker M, Goldstein G, Balner H 1983. Effects of in vivo administration of monoclonal antibodies specific for human T cell subpopulations on the immune system in a rhesus monkey model. Transplantation 35: 521–526 [DOI] [PubMed] [Google Scholar]
- Joseph A, Neff K, Richard J, Gao L, Bangari D, Joly M, Culm-Merdek K, Garman R, Williams J, Richards S, et al. 2012. Transient low-dose methotrexate induces tolerance to murine anti-thymocyte globulin and together they promote long-term allograft survival. J Immunol 189: 732–743 [DOI] [PubMed] [Google Scholar]
- Kaplon RJ, Hochman PS, Michler RE, Kwiatkowski PA, Edwards NM, Berger CL, Xu H, Meier W, Wallner BP, Chisholm P, et al. 1996. Short course single agent therapy with an LFA-3-IgG1 fusion protein prolongs primate cardiac allograft survival. Transplantation 61: 356–363 [DOI] [PubMed] [Google Scholar]
- Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH 1995. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 59: 256–262 [PubMed] [Google Scholar]
- Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, et al. 2004. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant 4: 1391–1398 [DOI] [PubMed] [Google Scholar]
- Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, Higuchi M, Laaris A, Cheng XF, McMahon C, et al. 2010. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest 120: 1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GB, Wang Z, Liu YY, Stavrou S, Mathias A, Goodwin KJ, Thomas JM, Neville DM 2007. A fold-back single-chain diabody format enhances the bioactivity of an anti-monkey CD3 recombinant diphtheria toxin-based immunotoxin. Protein Eng Des Sel 20: 425–432 [DOI] [PubMed] [Google Scholar]
- Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, Cendales LK, Tadaki DK, Harlan DM, Swanson SJ 2003. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation 76: 120–129 [DOI] [PubMed] [Google Scholar]
- Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, Potdar S, Shapiro R, Dharnidharka VR, Kauffman HM 2007. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant 7: 2619–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk AD, Mead S, Xu H, Mehta A, Guasch A, Cheeseman J, Joseph J, Horan J, Kean L, Larsen CP, et al. 2011. Kidney transplantation using alemtuzumab induction and belatacept/sirolimus maintenance therapy. Am J Transplant 11: 4521199347 [Google Scholar]
- Knechtle SJ 2001. Treatment with immunotoxin. Philos Trans R Soc London B Biol Sci 356: 681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knechtle SJ, Vargo D, Fechner J, Zhai Y, Wang J, Hanaway MJ, Scharff J, Hu H, Knapp L, Watkins D, et al. 1997. FN18-CRM9 immunotoxin promotes tolerance in primate renal allografts. Transplantation 63: 1–6 [DOI] [PubMed] [Google Scholar]
- Knechtle SJ, Fechner JH Jr, Dong Y, Stavrou S, Neville DM Jr, Oberley T, Buckley P, Armstrong N, Rusterholz K, Hong X, et al. 1998. Primate renal transplants using immunotoxin. Surgery 124: 438–446; discussion 446–437 [PubMed] [Google Scholar]
- Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, Sogawa H, Murakami T, Strom TB, Colvin RB, et al. 2007. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant 7: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P, Goldstein G, Reinherz EL, Schlossman SF 1979. Monoclonal antibodies defining distinctive human T cell surface antigens. Science 206: 347–349 [DOI] [PubMed] [Google Scholar]
- Kwun J, Bulut P, Kim E, Dar W, Oh B, Ruhil R, Iwakoshi N, Knechtle SJ 2012a. The role of B cells in solid organ transplantation. Semin Immunol 24: 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwun J, Oh BC, Gibby AC, Ruhil R, Lu VT, Kim DW, Page EK, Bulut OP, Song MQ, Farris AB, et al. 2012b. Patterns of de novo allo B cells and antibody formation in chronic cardiac allograft rejection after alemtuzumab treatment. Am J Transplant 12: 2641–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Ritz J, Guida LJ, Yetz JM, Lambert JM, Reinherz EL, Schlossman SF 1985. In vivo administration of lymphocyte-specific monoclonal antibodies in nonhuman primates: I. Effects of anti-T11 antibodies on the circulating T cell pool. Blood 66: 961–966 [PubMed] [Google Scholar]
- Lin WY, Gong Q, Seshasayee D, Lin Z, Ou Q, Ye S, Suto E, Shu J, Lee WP, Lee CW, et al. 2007. Anti-BR3 antibodies: A new class of B-cell immunotherapy combining cellular depletion and survival blockade. Blood 110: 3959–3967 [DOI] [PubMed] [Google Scholar]
- Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, Green T, Rickels MR, Tomaszewski JE, Koeberlein B, et al. 2007. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med 13: 1295–1298 [DOI] [PubMed] [Google Scholar]
- Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY 2002. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol 197: 322–332 [DOI] [PubMed] [Google Scholar]
- Luo H, Wu Y, Qi S, Wan X, Chen H, Wu J 2001. A proteasome inhibitor effectively prevents mouse heart allograft rejection. Transplantation 72: 196–202 [DOI] [PubMed] [Google Scholar]
- Ma S, Hu H, Thompson J, Stavrou S, Scharff J, Neville DM Jr 1997. Genetic construction and characterization of an anti-monkey CD3 single-chain immunotoxin with a truncated diphtheria toxin. Bioconjug Chem 8: 695–701 [DOI] [PubMed] [Google Scholar]
- Magliocca JF, Knechtle SJ 2006. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int 19: 705–714 [DOI] [PubMed] [Google Scholar]
- Maki T, Carville A, Stillman IE, Sato K, Kodaka T, Minamimura K, Ogawa N, Kanamoto A, Gottschalk R, Monaco AP, et al. 2008. SV40 infection associated with rituximab treatment after kidney transplantation in nonhuman primates. Transplantation 85: 893–902 [DOI] [PubMed] [Google Scholar]
- Malatack JF, Gartner JC Jr, Urbach AH, Zitelli BJ 1991. Orthotopic liver transplantation, Epstein-Barr virus, cyclosporine, and lymphoproliferative disease: A growing concern. J Pediatr 118: 667–675 [DOI] [PubMed] [Google Scholar]
- Marcos A, Eghtesad B, Fung JJ, Fontes P, Patel K, Devera M, Marsh W, Gayowski T, Demetris AJ, Gray EA, et al. 2004. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: With particular reference to hepatitis C virus. Transplantation 78: 966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer BC, Swanson B, Kappler J 2000. Homeostasis of αβTCR+ T cells. Nat Immunol 1: 107–111 [DOI] [PubMed] [Google Scholar]
- McGregor CG, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM, Tazelaar HD, Kremers WK, Walker RC, Byrne GW, et al. 2005. Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. J Thoracic Cardio Surgery 130: 844–851 [DOI] [PubMed] [Google Scholar]
- Meier-Kriesche HU, Arndorfer JA, Kaplan B 2002. Association of antibody induction with short- and long-term cause-specific mortality in renal transplant recipients. J Am Soc Nephrol 13: 769–772 [DOI] [PubMed] [Google Scholar]
- Mohty M 2007. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 21: 1387–1394 [DOI] [PubMed] [Google Scholar]
- Morelon E, Lefrancois N, Besson C, Prevautel J, Brunet M, Touraine JL, Badet L, Touraine-Moulin F, Thaunat O, Malcus C 2010. Preferential increase in memory and regulatory subsets during T-lymphocyte immune reconstitution after thymoglobulin induction therapy with maintenance sirolimus vs cyclosporine. Transplant Immunol 23: 53–58 [DOI] [PubMed] [Google Scholar]
- Morrison SL, Johnson MJ, Herzenberg LA, Oi VT 1984. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci 81: 6851–6855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, et al. 2010. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najarian JS, Simmons RL 1971. The clinical use of antilymphocyte globulin. N Engl J Med 285: 158–166 [DOI] [PubMed] [Google Scholar]
- Nath DS, Kandaswamy R, Gruessner R, Sutherland DE, Dunn DL, Humar A 2005. Fungal infections in transplant recipients receiving alemtuzumab. Transplant Proc 37: 934–936 [DOI] [PubMed] [Google Scholar]
- Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA 2006. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol 176: 4632–4639 [DOI] [PubMed] [Google Scholar]
- Neville DM Jr, Scharff J, Srinivasachar K 1992. In vivo T-cell ablation by a holo-immunotoxin directed at human CD3. Proc Natl Acad Sci 89: 2585–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolls MR, Aversa GG, Pearce NW, Spinelli A, Berger MF, Gurley KE, Hall BM 1993. Induction of long-term specific tolerance to allografts in rats by therapy with an anti-CD3-like monoclonal antibody. Transplantation 55: 459–468 [DOI] [PubMed] [Google Scholar]
- Nishimura H, Scalea J, Wang Z, Shimizu A, Moran S, Gillon B, Sachs DH, Yamada K 2011. First experience with the use of a recombinant CD3 immunotoxin as induction therapy in pig-to-primate xenotransplantation: The effect of T-cell depletion on outcome. Transplantation 92: 641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opelz G 1995. Efficacy of rejection prophylaxis with OKT3 in renal transplantation. Collaborative Transplant Study. Transplantation 60: 1220–1224 [PubMed] [Google Scholar]
- Orlando G, Hematti P, Stratta RJ, Burke GW 3rd, Di Cocco P, Pisani F, Soker S, Wood K 2010. Clinical operational tolerance after renal transplantation: Current status and future challenges. Ann Surg 252: 915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz CG, Wakely E, Bergese SD, VanBuskirk AM, Ferguson RM, Mullet D, Apseloff G, Gerber N 1996. Prevention of murine cardiac allograft rejection with gallium nitrate. Comparison with anti-CD4 monoclonal antibody. Transplantation 61: 783–791 [DOI] [PubMed] [Google Scholar]
- Page EK, Page AJ, Kwun J, Gibby AC, Leopardi F, Jenkins JB, Strobert EA, Song M, Hennigar RA, Iwakoshi N, et al. 2012. Enhanced de novo alloantibody and antibody-mediated injury in rhesus macaques. Am J Transplant 12: 2395–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD 2005. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant 5: 465–474 [DOI] [PubMed] [Google Scholar]
- Pearl JP, Xu H, Leopardi F, Preston E, Kirk AD 2007. CD154 blockade, sirolimus, and donor-specific transfusion prevents renal allograft rejection in cynomolgus monkeys despite homeostatic T-cell activation. Transplantation 83: 1219–1225 [DOI] [PubMed] [Google Scholar]
- Pearson TC, Bushell AR, Darby CR, West LJ, Morris PJ, Wood KJ 1993. Lymphocyte changes associated with prolongation of cardiac allograft survival in adult mice using anti-CD4 monoclonal antibody. Clin Exp Immunol 92: 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TC, Trambley J, Odom K, Anderson DC, Cowan S, Bray R, Lin A, Hollenbaugh D, Aruffo A, Siadak AW, et al. 2002. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation 74: 933–940 [DOI] [PubMed] [Google Scholar]
- Penn I 1990. Cancers complicating organ transplantation. N Engl J Med 323: 1767–1769 [DOI] [PubMed] [Google Scholar]
- Ploix C, Lo D, Carson MJ 2001. A ligand for the chemokine receptor CCR7 can influence the homeostatic proliferation of CD4 T cells and progression of autoimmunity. J Immunol 167: 6724–6730 [DOI] [PubMed] [Google Scholar]
- Preville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, Revillard JP 2001. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation 71: 460–468 [DOI] [PubMed] [Google Scholar]
- Prin Mathieu C, Renoult E, Kennel De March A, Bene MC, Kessler M, Faure GC 1997. Serum anti-rabbit and anti-horse IgG, IgA, and IgM in kidney transplant recipients. Nephrol Dial Transplant 12: 2133–2139 [DOI] [PubMed] [Google Scholar]
- Quesniaux V, Fullard L, Arendse H, Davison G, Markgraaff N, Auer R, Ehrhart F, Kraus G, Schuurman HJ 1999. A novel immunosuppressant, FTY720, induces peripheral lymphodepletion of both T and B cells and immunosuppression in baboons. Transplant Immunol 7: 149–157 [DOI] [PubMed] [Google Scholar]
- Quesniaux VF, Menninger K, Kunkler A, Vedrine C, Bernhard M, Hedinger R, Kraus G, Schuurman HJ 2000. The novel immunosuppressant FTY720 induces peripheral lymphodepletion of both T and B cells in cynomolgus monkeys when given alone, with Cyclosporine Neoral or with RAD. Transplant Immunol 8: 177–187 [DOI] [PubMed] [Google Scholar]
- Rao PE, Olini G, Kille J, Muchmore E, Talle MA, Brake G, Rudnick SA 1991. OKT3E, an anti-CD3 antibody that does not elicit side effects or antiidiotype responses in chimpanzees. Transplantation 52: 691–697 [DOI] [PubMed] [Google Scholar]
- Rebellato LM, Gross U, Verbanac KM, Thomas JM 1994. A comprehensive definition of the major antibody specificities in polyclonal rabbit antithymocyte globulin. Transplantation 57: 685–694 [DOI] [PubMed] [Google Scholar]
- Redfield RR II, Rodriguez E, Parsons R, Vivek K, Mustafa MM, Noorchashm H, Naji A 2011. Essential role for B cells in transplantation tolerance. Curr Opin Immunol 23: 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J, Campbell K, van Smith L, Pouletty P, Schroeder TJ, Guttmann RD, Buelow R 1997. Characterization of anti-thymoglobulin, anti-Atgam and anti-OKT3 IgG antibodies in human serum with an 11-min ELISA. Transplant Immunol 5: 49–56 [DOI] [PubMed] [Google Scholar]
- Regan JF, Lyonnais C, Campbell K, Smith LV, Buelow R 2001. Total and active thymoglobulin levels: Effects of dose and sensitization on serum concentrations. Transplant Immunol 9: 29–36 [DOI] [PubMed] [Google Scholar]
- Ruhil R, Oh B, Lu VT, Bulut P, Kwun J, Talebagha S, Knechtle S, Iwakoshi N 2012. Tacrolimus inhibits alloantibody and plasma cell development but permits germinal center reaction and accumulation of memory B cells: Implications for humoral rejection. Am J Transplant 12: 53 [Google Scholar]
- Schroeder TJ, First MR, Mansour ME, Hurtubise PE, Hariharan S, Ryckman FC, Munda R, Melvin DB, Penn I, Ballistreri WF, et al. 1990. Antimurine antibody formation following OKT3 therapy. Transplantation 49: 48–51 [DOI] [PubMed] [Google Scholar]
- Schuurman HJ, Menninger K, Audet M, Kunkler A, Maurer C, Vedrine C, Bernhard M, Gaschen L, Brinkmann V, Quesniaux V 2002. Oral efficacy of the new immunomodulator FTY720 in cynomolgus monkey kidney allotransplantation, given alone or in combination with cyclosporine or RAD. Transplantation 74: 951–960 [DOI] [PubMed] [Google Scholar]
- Shapiro R, Ellis D, Tan HP, Moritz ML, Basu A, Vats AN, Kayler LK, Erkan E, McFeaters CG, James G, et al. 2007. Alemtuzumab pre-conditioning with tacrolimus monotherapy in pediatric renal transplantation. Am J Transplant 7: 2736–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Zeevi A, Basu A, Tan HP, Kayler LK, Blisard DM, Thai NL, Girnita AL, Randhawa PS, Gray EA, et al. 2008. Alemtuzumab preconditioning with tacrolimus monotherapy—The impact of serial monitoring for donor-specific antibody. Transplantation 85: 1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Lees JR, Scott DW, Farber DL, Bartlett ST 2012. Endogenous expansion of regulatory T cells leads to long-term islet graft survival in diabetic NOD mice. Am J Transplant 12: 1124–1132 [DOI] [PubMed] [Google Scholar]
- Sido B, Dengler TJ, Otto G, Zimmermann R, Muller P, Meuer SC 1998. Differential immunosuppressive activity of monoclonal CD2 antibodies on allograft rejection versus specific antibody production. Eur J Immunol 28: 1347–1357 [DOI] [PubMed] [Google Scholar]
- Silver JS, Hunter CA 2008. With a little help from their friends: interleukin-21, T cells, and B cells. Immunity 29: 7–9 [DOI] [PubMed] [Google Scholar]
- Starzl TE, Marchioro TL, Waddell WR 1963. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet 117: 385–395 [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Marchioro TL, Hutchinson DE, Porter KA, Cerilli GJ, Brettschneider L 1967. The clinical use of antilymphocyte globulin in renal homotransplantation. Transplantation 5: 1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, Corry RJ, Jordan ML, Fontes P, Gayowski T, et al. 2003. Tolerogenic immunosuppression for organ transplantation. Lancet 361: 1502–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SJ, Hale DA, Mannon RB, Kleiner DE, Cendales LC, Chamberlain CE, Polly SM, Harlan DM, Kirk AD 2002. Kidney transplantation with rabbit antithymocyte globulin induction and sirolimus monotherapy. Lancet 360: 1662–1664 [DOI] [PubMed] [Google Scholar]
- Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O’Sullivan EJ, Johnson MR, Heroux AL, Dizikes GJ, Pifarre R, Fisher RI 1990. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med 323: 1723–1728 [DOI] [PubMed] [Google Scholar]
- Tang Q, Smith JA, Szot GL, Zhou P, Alegre ML, Henriksen KJ, Thompson CB, Bluestone JA 2003. CD28/B7 regulation of anti-CD3-mediated immunosuppression in vivo. J Immunol 170: 1510–1516 [DOI] [PubMed] [Google Scholar]
- Thomas FT, Carver FM, Foil MB, Pryor WH, Larkin EW, Hall WR, Haisch CE, Thomas JM 1983. Long-term incompatible kidney survival in outbred higher primates without chronic immunosuppression. Ann Surg 198: 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JM, Carver M, Cunningham P, Sash C, Park K, Thomas F 1989. Promotion of incompatible allograft acceptance in rhesus monkeys given posttransplant antithymocyte globulin and donor bone marrow. II. Effects of adjuvant immunosuppressive drugs. Transplantation 47: 209–215 [DOI] [PubMed] [Google Scholar]
- Thompson P, Badell IR, Lowe M, Turner A, Cano J, Avila J, Azimzadeh A, Cheng X, Pierson RN 3rd, Johnson B, et al. 2012. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am J Transplant 12: 1765–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba JR, Fernandez LA, Kanmaz T, Oberley TD, Schultz JM, Brunner KG, Peters D, Fechner JH Jr, Dong Y, Hu H, et al. 2003. Immunotoxin-treated rhesus monkeys: A model for renal allograft chronic rejection. Transplantation 76: 524–530 [DOI] [PubMed] [Google Scholar]
- Torrealba JR, Katayama M, Fechner JH Jr, Jankowska-Gan E, Kusaka S, Xu Q, Schultz JM, Oberley TD, Hu H, Hamawy MM, et al. 2004. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-β1+ CD4+ T regulatory cell infiltrates. J Immunol 172: 5753–5764 [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Zilvetti M, Friend P, Wood KJ 2006. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation 82: 1342–1351 [DOI] [PubMed] [Google Scholar]
- Vallhonrat H, Williams WW, Cosimi AB, Tolkoff-Rubin N, Ginns LC, Wain JC, Preffer F, Olszak I, Wee S, Delmonico FL, et al. 1999. In vivo generation of C4d, Bb, iC3b, and SC5b-9 after OKT3 administration in kidney and lung transplant recipients. Transplantation 67: 253–258 [DOI] [PubMed] [Google Scholar]
- Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, Wuyts WA, Van Raemdonck DE, Dupont LJ, Verleden GM 2008. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant 8: 1911–1920 [DOI] [PubMed] [Google Scholar]
- van der Touw W, Burrell B, Lal G, Bromberg JS 2012. NK Cells are required for costimulatory blockade induced tolerance to vascularized allografts. Transplantation 94: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt DJ, Smetanka C, Macedo C, He J, Lakomy R, Bottino R, Ekser B, Echeverri GJ, Metes D, Ijzermans JN, et al. 2010. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. Am J Transplant 10: 773–783 [DOI] [PubMed] [Google Scholar]
- Viret C, Wong FS, Janeway CA Jr, 1999. Designing and maintaining the mature TCR repertoire: The continuum of self-peptide:self-MHC complex recognition. Immunity 10: 559–568 [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S 2008. Functions of natural killer cells. Nat Immunol 9: 503–510 [DOI] [PubMed] [Google Scholar]
- Wang Z, Duran-Struuck R, Crepeau R, Matar A, Hanekamp I, Srinivasan S, Neville DM Jr, Sachs DH, Huang CA 2011. Development of a diphtheria toxin based antiporcine CD3 recombinant immunotoxin. Bioconjug Chem 22: 2014–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Bradley JA, Friend PJ, Firth J, Taylor CJ, Bradley JR, Smith KG, Thiru S, Jamieson NV, Hale G, et al. 2005. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation—efficacy and safety at five years. Am J Transplant 5: 1347–1353 [DOI] [PubMed] [Google Scholar]
- Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, Kampen RL, Stempora L, Song M, Larsen CP, et al. 2009. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med 15: 746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster AC, Pankhurst T, Rinaldi F, Chapman JR, Craig JC 2006. Monoclonal and polyclonal antibody therapy for treating acute rejection in kidney transplant recipients: A systematic review of randomized trial data. Transplantation 81: 953–965 [DOI] [PubMed] [Google Scholar]
- Wijkstrom M, Kenyon NS, Kirchhof N, Kenyon NM, Mullon C, Lake P, Cottens S, Ricordi C, Hering BJ 2004. Islet allograft survival in nonhuman primates immunosuppressed with basiliximab, RAD, and FTY720. Transplantation 77: 827–835 [DOI] [PubMed] [Google Scholar]
- Woodle ES, Thistlethwaite JR, Jolliffe LK, Zivin RA, Collins A, Adair JR, Bodmer M, Athwal D, Alegre ML, Bluestone JA 1992. Humanized OKT3 antibodies: Successful transfer of immune modulating properties and idiotype expression. J Immunol 148: 2756–2763 [PubMed] [Google Scholar]
- Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, et al. 2004. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med 10: 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Zhen Y, Zeng C, Zhang L, Zhao Y 2008. Depleting anti-CD4 monoclonal antibody (GK1.5) treatment: Influence on regulatory CD4+CD25+Foxp3+ T cells in mice. Transplantation 85: 1167–1174 [DOI] [PubMed] [Google Scholar]
- Zalevsky J, Leung IW, Karki S, Chu SY, Zhukovsky EA, Desjarlais JR, Carmichael DF, Lawrence CE 2009. The impact of Fc engineering on an anti-CD19 antibody: Increased Fcγ receptor affinity enhances B-cell clearing in nonhuman primates. Blood 113: 3735–3743 [DOI] [PubMed] [Google Scholar]