Abstract
A full account of synthetic efforts toward a lowly oxidized taxane framework is presented. A non-natural taxane, dubbed “taxadienone”, was synthesized as our first entry into the taxane family of diterpenes. The final synthetic sequence illustrates a seven-step, gram-scale and enantioselective route to this tricyclic compound in 18% overall yield. This product was then modified further to give (+)-taxadiene, the lowest oxidized member of the taxane family of natural products.
Keywords: Total synthesis, Terpene, Taxane, Taxadiene, Cyclase phase
1. Introduction
Taxanes represent a large family of terpenes comprising over 350 natural products, of which many exhibit cytotoxic activity against various types of cancer and also display interesting neurological and antibacterial properties.1 The most celebrated example of these diterpenoids, from both medicinal and structural standpoints, is Taxol® (1; Figure 1).1a,1d,1e Its success as an anti-cancer drug, its densely functionalized and complex structure, and its unique mechanism of action involving the stabilization of microtubules2 have fascinated medicinal chemists, synthetic chemists and biologists alike. While chemical synthesis seems to be no longer needed to solve a supply problem for this particular drug, synthetic chemistry is able to modify biologically active structures in ways that synthetic biology cannot.3 Coupled with the opportunity to invent new methods using a complex framework, this natural product appeared to us as an ideal target for an endeavor in organic synthesis.4–7
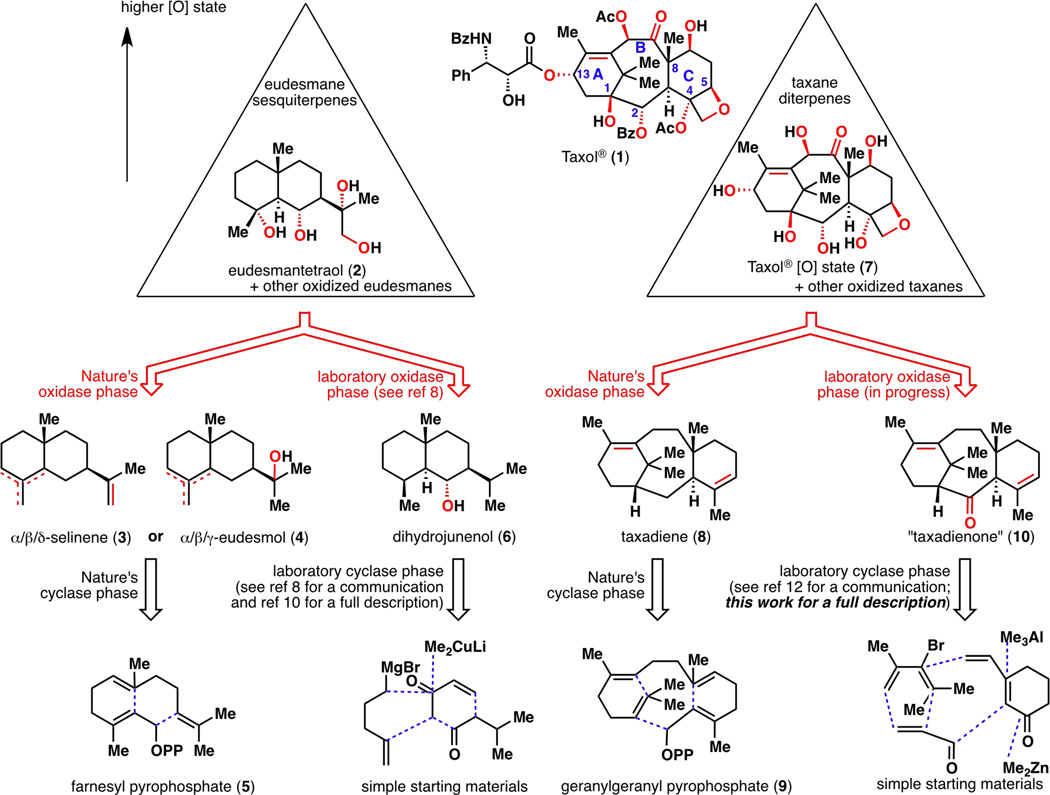
Figure 1.
A two-phase biosynthesis versus terpene synthesis in the eudesmane and taxane families of natural products. [O] = oxidation.
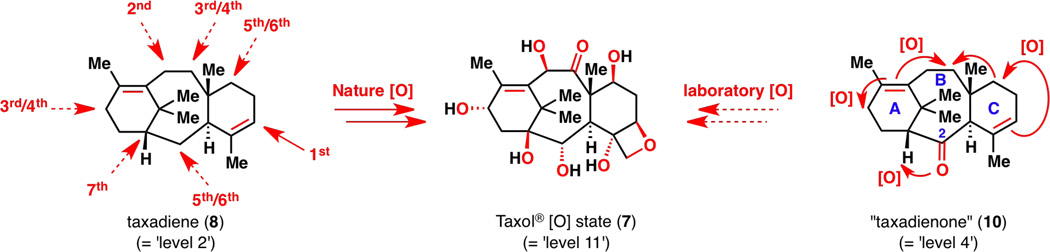
A general two-phase design for the construction of terpenes was recently formulated using eudesmane sesquiterpenes as a proof of concept.8 In Nature, oxidized eudesmanes such as eudesmantetraol (2) most likely arise from C–H oxidation9 of 3 or 4, which in turn arise from farnesyl pyrophosphate (5). In a similar vein, a laboratory two-phase approach allowed for the simplification of target 2 into a lowly oxidized eudesmane framework such as dihydrojunenol (6), followed by retrosynthetic disconnections into simple starting materials such as methyl vinyl ketone and isovaleradehyde.10 Our next objective is to target Taxol® (1) while retaining the same line of logic. Since Taxol® (1) is one of the most highly oxidized taxanes, a two-phase terpene synthesis strategy that targets Taxol® (1) would also generate other taxanes that are lower in oxidation level.11 The ultimate goal is to divergently access as many “pre-Taxol®” compounds as possible (both natural and unnatural) and to learn about the innate reactivity of the taxane framework through various C–H oxidation strategies.
Structurally, Taxol® (1) and other taxanes are highly functionalized diterpenes with a captivating 6-8-6 tricyclic skeleton and a bridgehead olefin. It is adorned with many acetyl and benzoyl groups, as well as a signature side chain at the C13 oxygen atom (see carbon numbering on 1). For retrosynthetic analysis purposes, 1 is treated as if it were devoid of acyl groups and is substituted with oxygenated hydrocarbon 7. Many oxidized taxanes have in common a C2-hydroxyl group and can be envisioned to arise from taxa-4(5),11(12)-dien-2-one, or “taxadienone” (10; quotation marks in the text are removed hereinafter for brevity). This ketone represents a key intermediate for a comprehensive access to the taxane family because it would allow for both the natural C2α-alcohol series and the unnatural C2β-alcohol series. Furthermore, if taxadiene (8), the least oxidized natural product1c in the taxane family, were to be desired, one could simply deoxygenate 10. Thus, taxadienone (10) became the target of our cyclase phase endpoint, which would serve as the diverging starting point toward polyhydroxylated taxanes. The synthesis of both taxadienone (10) and taxadiene (8) has been reported in an earlier communication12 and is described herein as a full account.
2. Results and Discussion
2.1. Initial strategies and failed approaches
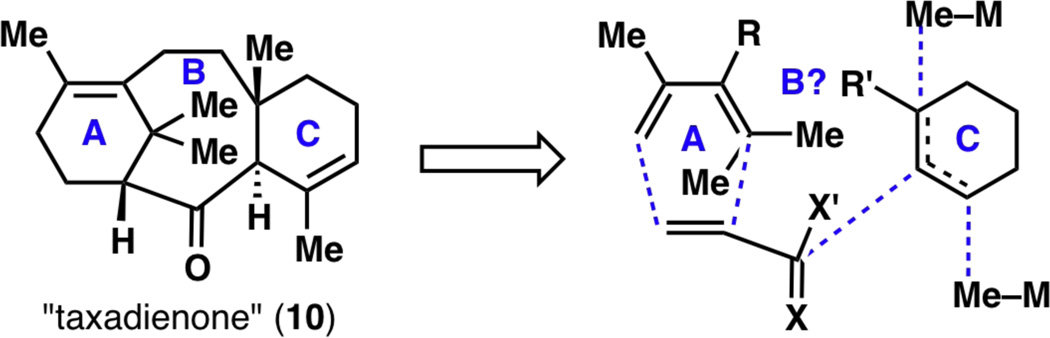
Considering the wealth of chemical knowledge surrounding the synthesis of the taxane framework,4–7 there were so many retrosynthetic routes that could be followed toward the synthesis of taxadienone (10). While each synthesis has its strengths and weaknesses, we were particularly drawn to Nicolaou’s route,4c which involved a Diels–Alder reaction to set the A ring (see ring numbering on 1 in Figure 1). For the C ring, judging from the absence of functional groups in 10, a simple cyclohexane-based starting material was deemed best. Numerous experimental explorations and strategy revisions then came from the synthesis of the B ring (Figure 2).
Figure 2.
Early retrosynthetic disconnections for taxadienone (10).
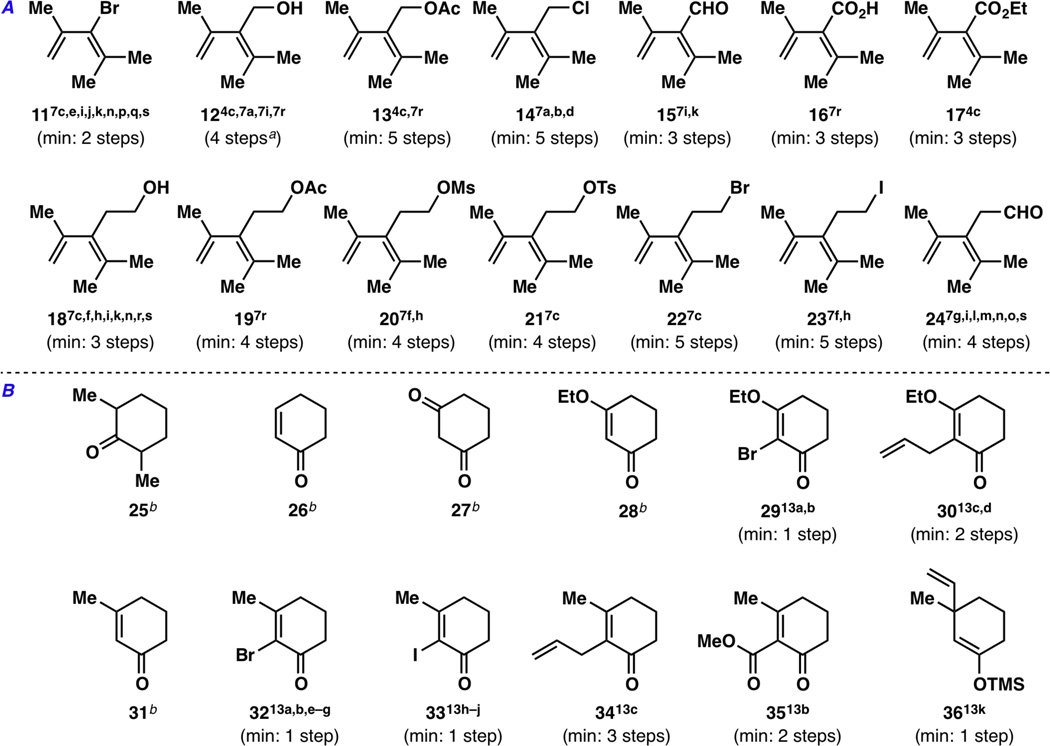
Many other previous attempts at making the taxane skeleton employed a Diels–Alder route for the A ring,6,7 possibly because of its isohypsic and atom-economical nature. These studies, as well as Nicolaou’s A ring synthesis,4c employed a trimethylated butadiene component for their Diels–Alder reactions. The most commonly used diene fragments, along with the number of steps it takes to make them, are shown in Figure 3A. Regarding the taxane C ring, cyclohexane starting materials13 that were deemed useful are listed in Figure 3B.
Figure 3.
A) Dienes that have been used in various taxane core syntheses.4c,7a–7s B) Potentially useful cyclohexane starting materials to serve as the taxane C ring.13 The reported minimum number of steps to make these dienes and cyclohexanes are listed for comparison. Note: aDiene 12 can be made in 1 step from tetramethylallene and formaldehyde using a thermal ene reaction,7a but tetramethylallene is prohibitively expensive at >$200/gram (Sigma–Aldrich, April 2013). bCommercially available as of April 2013.
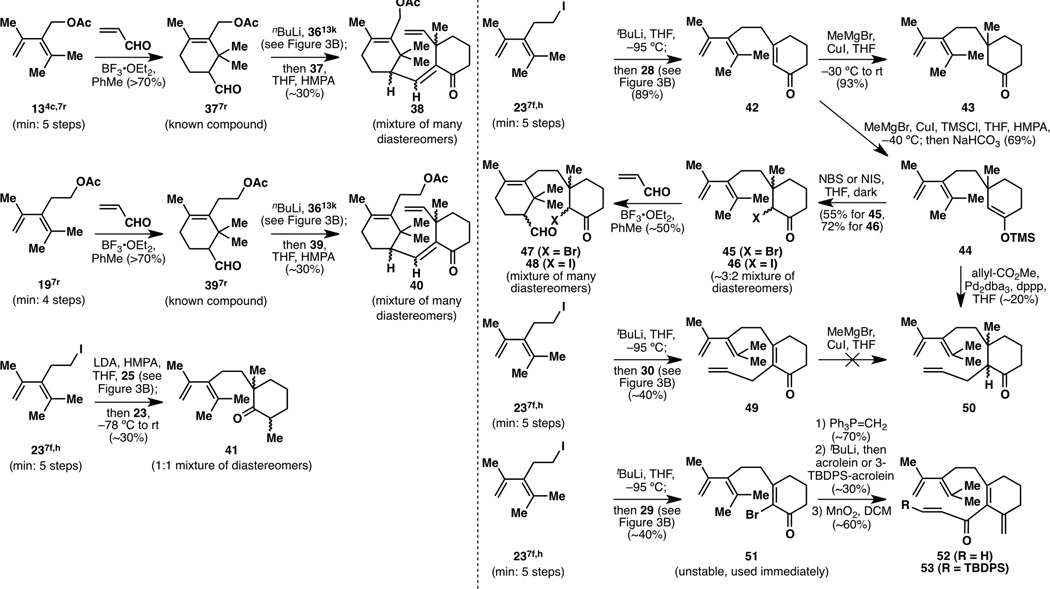
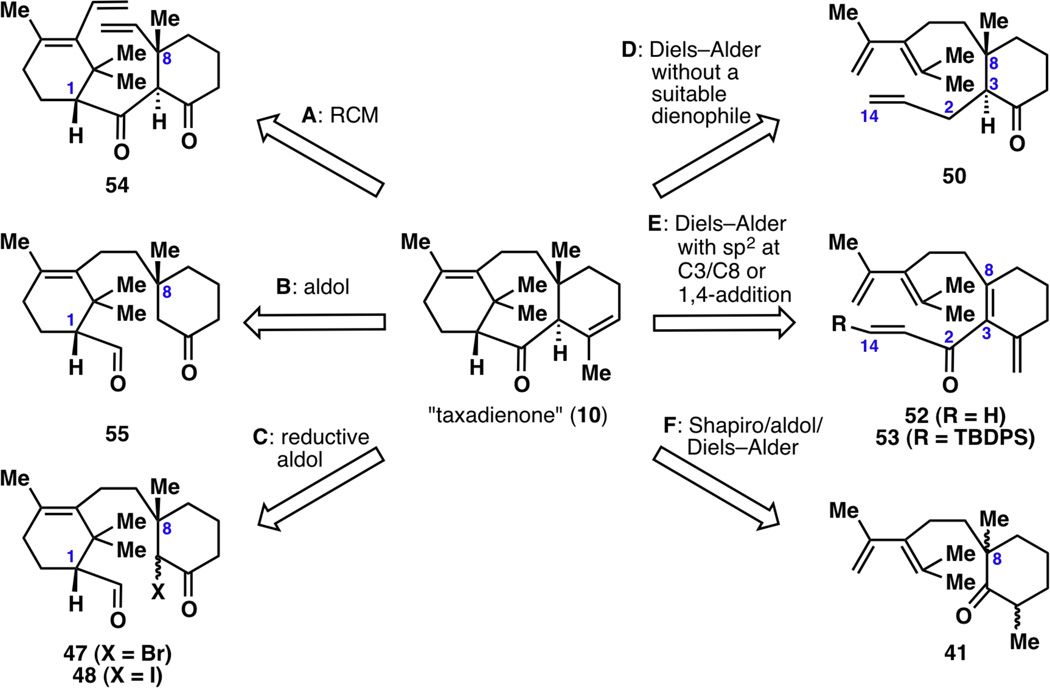
With a collection of A-ring precursors and C-ring frameworks ready to use, potentially useful A-ring/C-ring coupled compounds were synthesized (Figure 4). Many of these were generated as a mixture of inseparable diastereomers (38, 40, 41, 45–48, 50), presenting early problems in the designed routes. Furthermore, many of these steps were only feasible in low yields and were not scalable.
Figure 4.
Selected examples of A-ring/C-ring coupled compounds in initial studies.
Despite the drawbacks in yield and diastereoselectivity in forming many of the compounds presented in Figure 4, these intermediates were used in a number of approaches toward the B ring, and a snapshot of many of the evaluated strategies toward taxadienone (10) is illustrated in Figure 5. For example, the known difficulties in forming the taxane skeleton4 led us to consider a ring-closing metathesis (RCM) strategy to forge the central 8-membered ring since olefin metathesis is a robust method to synthesize medium-sized rings (Figure 5, disconnection A). However, the fact that the required substrate 54 would take many steps to build and that the stereocenters at C1 and C8 would have to be formed with two separate enantioselective reactions dissuaded us from this route. An aldol route was then conceived, partly because the required diketone 55 can be easily synthesized from ketone 43 (disconnection B). However, despite a plethora of attempted experiments using various Lewis and Brønsted acids and bases, the desired cyclization from 55 did not proceed. Similar failures were met when attempting Reformatsky-type cyclizations from 47 or 48 using reducing agents such as Zn,14a,14b SmI2,14c CrCl2,14d Co(PPh3)414e or Et3B14f (disconnection C). Thereafter, closure of the AB ring by a Diels–Alder reaction was envisioned. While allylated ketone 50 does not contain a suitable dienophile for a normal-demand Diels–Alder reaction, it was hoped that a proximity-induced Diels–Alder reaction15 of an electronically neutral dienophile would take place (disconnection D). However, thermal conditions or radical cation conditions using triarylamine hexachloroantimonate16 did not allow closure of the B ring. In a similar vein, Diels–Alder reaction of substrates 52 and 53 was attempted under thermal or radical-based conditions (disconnection E). These intermediates did not undergo [4+2] cyclization, likely due to the conformation engendered by the sp2 carbons at C3 and C8. Furthermore, a methyl 1,4-addition at C8 was not possible, since reaction first occurred at the less hindered C14, even with a large tert-butyldiphenylsilyl group appended at C14. Lastly, ketone 41 was considered a viable intermediate toward the formation of taxadienone (10), since a Shapiro reaction with acrolein trap, oxidation, and Diels–Alder reaction could potentially form an isomer of 10 (disconnection F). However, ketone 41 already required 6 steps to construct (see Figure 4), and the stereocenter at C8 was thought to be challenging to control despite existing methods in asymmetric enolate alkylation.17
Figure 5.
Initial synthetic investigations toward the synthesis of taxadienone (10). Disconnection A: a ring-closing metathesis (RCM) approach would require many steps to even reach the key intermediate 54. Disconnection B: the required aldol closure from 55 simply did not proceed. Disconnection C: the required reductive aldol closure from 47 or 48 did not proceed. Disconnection D: without a suitable dienophile (i.e., using an electronically neutral olefin), the Diels–Alder reaction did not proceed under thermal or radical cation conditions. Disconnection E: with sp2 carbons at C3 and C8, the Diels–Alder reaction did not proceed even under radical cation conditions, and conjugate addition at C8 to install the methyl unit did not proceed because only the undesired conjugate addition onto C14 occurred. Disconnection F: a Shapiro reaction with an acrolein trap, followed by oxidation and Diels–Alder reaction, would not lead to an enantioselective synthesis of 10 because the stereochemistry at C8 could not be set selectively.
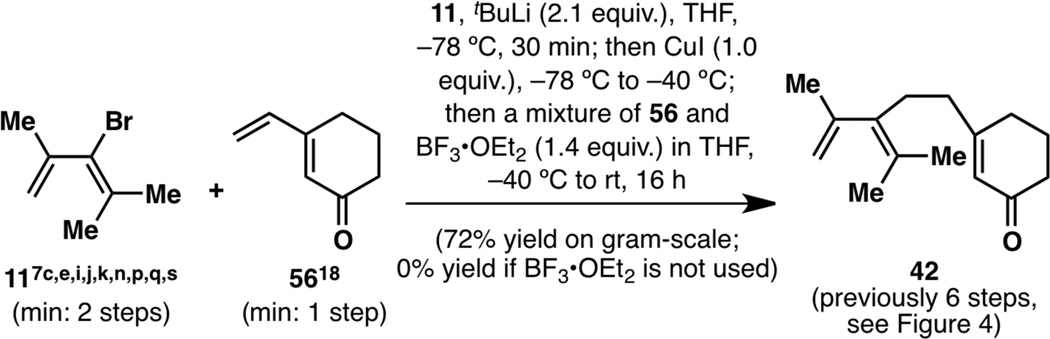
During these initial studies, a convenient 1,6-addition of diene 11 onto known vinylcyclohexenone 5618 was found to take place in good (72%) yield on gram scale (resulting in 1.7 grams of product in one run), resulting in a diene-cyclohexane coupled compound (42) that was previously only feasible in 6 steps (Figure 6). This reaction was initially optimized using BF3•OEt2, but was later optimized with TMSCl (vide infra). As the objective of this research project was a scalable and enantioselective synthesis of the taxane skeleton, this reaction was highly suitable unlike many of the reactions shown in Figure 4. Efforts hereafter were therefore focused around enone 42.
Figure 6.
A scalable 1,6-addition reaction resulting in a compound bearing both an A-ring precursor and a C ring, which then became the focal point of this research project.
2.2. Revised strategy and further failures, followed by completion of racemic taxadienone (10)
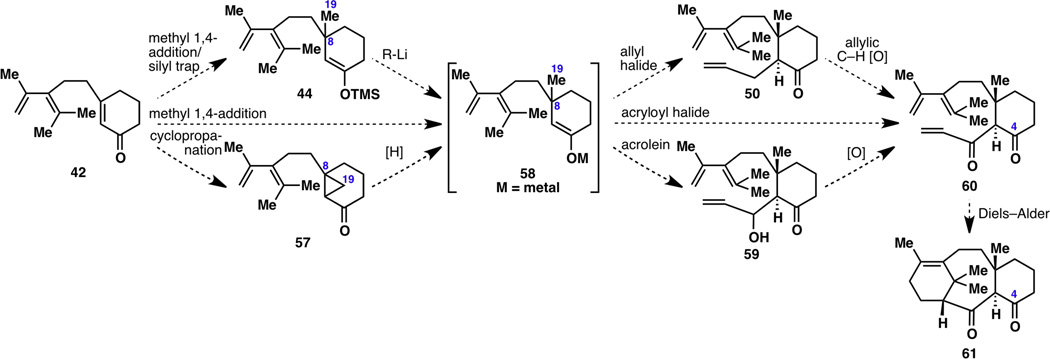
With many grams of enone 42 in hand, the synthetic route was then revised and centered around a single strategy: methylation of the cyclohexenone, followed by a three-carbon appendage and a key Diels–Alder reaction (Figure 7). The first methylation step was restricted to two methods, a methyl 1,4-addition19 or a cyclopropanation,20 because these reactions were the most likely to be amenable to enantioselective synthesis. From transient intermediate 58, a three-carbon appendage could then take place via an SN2 onto an allyl halide or via a 1,2-addition onto acrolein or an acryloyl halide. This three-carbon unit would then have to be oxidized accordingly, by C–H activation or otherwise, to furnish the ketone oxidation state in 60. Finally, the key Diels–Alder reaction from 60 to 61 had good literature precedent through the work of Jenkins7u and Williams6,21 whose intermediates only differed from 60 at the C4 position. The advantage of this sequence over many other possible routes was that the asymmetric construction of the 6-8-6 tricyclic skeleton would only rely on one enantioselective reaction, after which the resulting stereochemical information could be propagated to set all the other stereocenters diastereoselectively.
Figure 7.
Revised strategy for the synthesis of the taxane tricyclic framework. [O] = oxidation, [H] = reduction.
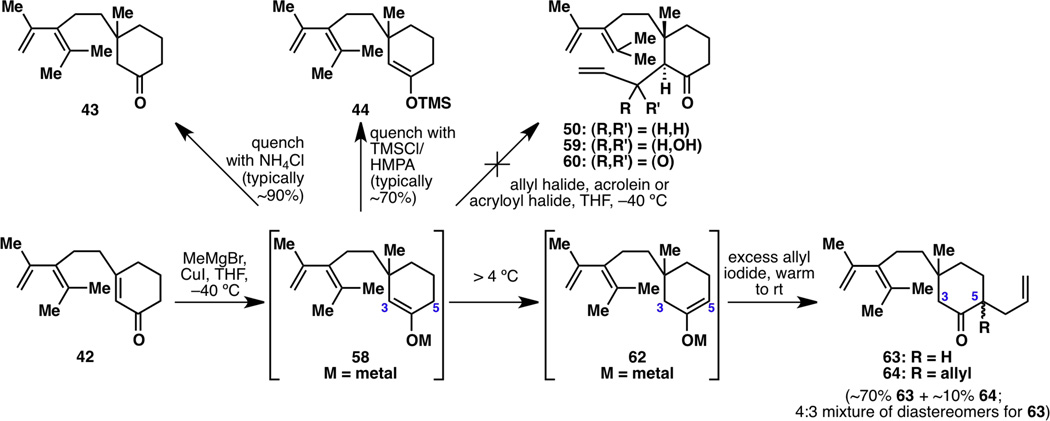
Based on this plan, the most efficient synthesis of 60 from 42 would be a one-step reaction: a methyl conjugate addition followed by trapping with an acryloyl halide. While the methyl 1,4-addition step proceeded smoothly and was quenched with acid to give 43 or trapped with TMSCl to give 44, it could not be trapped with a three-carbon unit to give 50, 59 or 60; only ketone 43 would result (Figure 8). In fact, intermediate 58 was completely unreactive at C3 toward carbon-based electrophiles; while it could be trapped with deuterium oxide or halonium ions at C3 (e.g., to form 45 and 46 in Figure 4), it would only give C5-allylated product 63 and a small amount of C5-bis-allylated product 64 when forced to react with allyl iodide. This most likely occurs because kinetic enolate 58 rearranges to thermodynamic enolate 62 at “high” temperatures (>4 °C), and because the C5 position is less hindered than the C3 position, thus reacting more easily.
Figure 8.
Limited utility of kinetic enolate 58, rearrangement to thermodynamic enolate 62, and failure to allylate at C3.
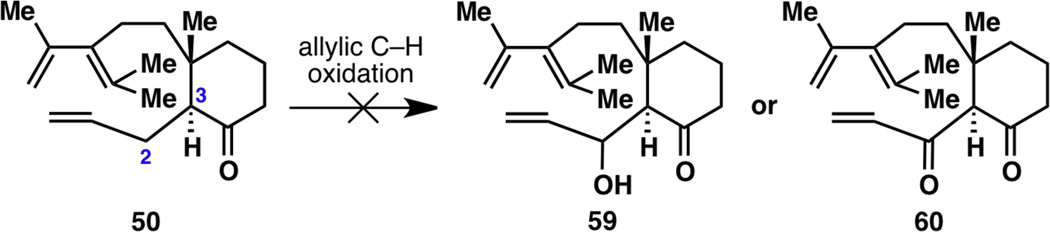
The only successful albeit inefficient and stereochemically non-selective way to place an allyl group onto the C3 position to make 50 was via Pd-catalyzed methods (see Figure 4, 44 to 50). However, not only did the Diels–Alder cyclization of 50 not occur (see Figure 5), but allylic C–H oxidation also did not proceed, only resulting in decomposed material likely due to the lability of the diene moiety (Figure 9). This result demanded that the revised strategy in Figure 7 avoid the formation of 50, and go through 59 or 60 instead.
Figure 9.
Failure to oxidize allylated ketone 50 at the C2 position via allylic C–H oxidation methods.
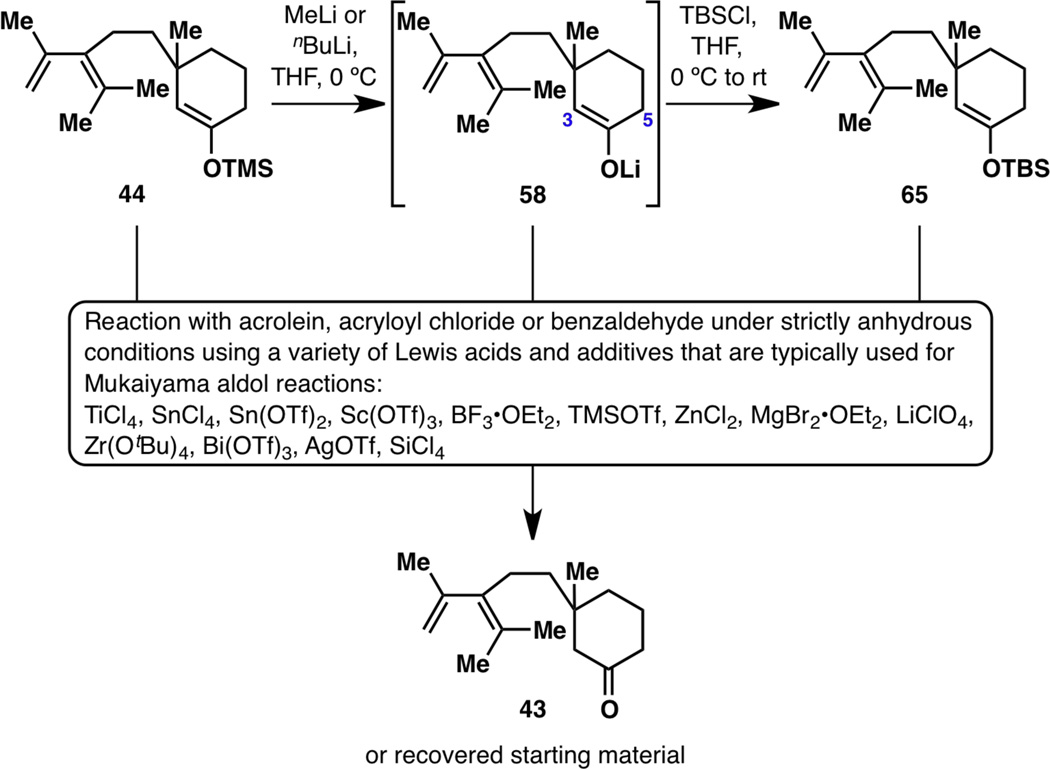
In order to synthesize 59 or 60, much effort was spent on trying to functionalize kinetic enolate 58. Since 58 must be stored in the fridge or freezer and is not stable for much longer than a day, TMS enol ether 44 was stored in large batches to serve as a direct surrogate for 58. Both 44 and 58 were reacted with acrolein, acryloyl chloride and benzaldehyde (as a test substrate) under a variety of Lewis acidic conditions, only to return 44 or result in hydrolyzed ketone 43 (Figure 10). Thinking that perhaps the lability of the trimethylsilyl group in 44 is the root of the problem in the failure of Mukaiyama-type reactions, a more robust TBS enol ether 65 was synthesized and subjected to the same set of Lewis acidic conditions. The only outcome was that the reaction of 65 was much slower than that of 44; when submitted to a larger amount of Lewis acid for a longer period of time, 65 resulted in ketone 43 as well. Believing that trace amounts of water were hydrolyzing 44, 58 and 65 whenever a reaction was run, water was excluded with utmost rigor but 43 would always form.
Figure 10.
Failure to functionalize the C3 position of 44, 58 and 65 using acrolein, acryloyl chloride or benzaldehyde.
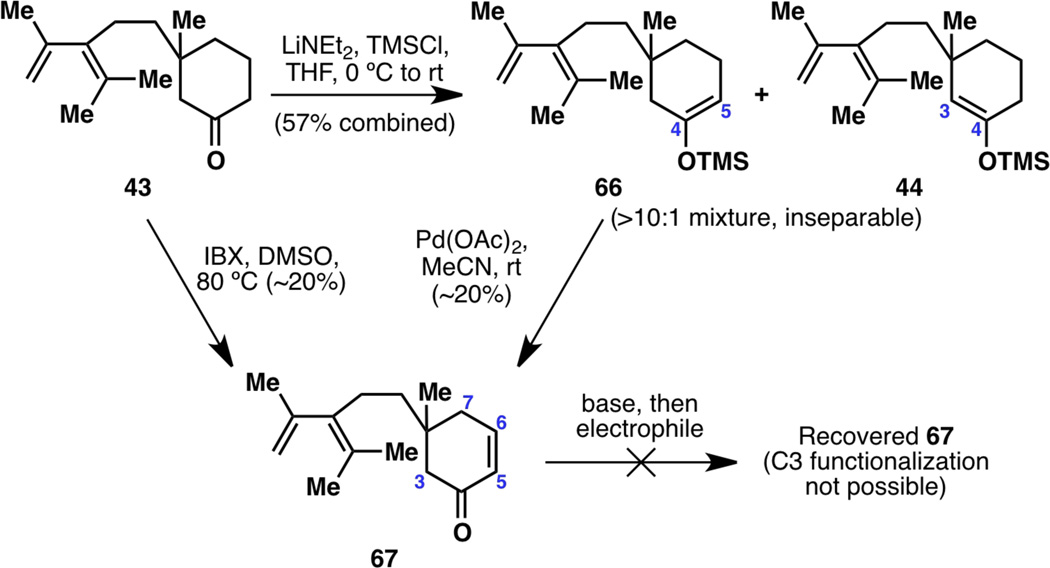
With many failures en route to the taxane framework, the one compound that never failed to form was hydrolyzed ketone 43. The accumulation of this compound in this project prompted an attempt at regenerating the potentially useful TMS enol ether 44. However, ketone 43, while bearing a carbonyl group at C4, would not allow selective functionalization at C3 because it would always deprotonate at C5 first. For example, generation of a TMS enol ether from 43 resulted in Δ4,5 enolate 66, with a diastereoselectivity of greater than 10:1 over the desired Δ3,4 enolate 44: unfortunately, TMS enol ethers 66 and 44 were inseparable and therefore this attempt at material recovery proved to be fruitless (Figure 11). Another try at making use of ketone 43 was the formation of enone 67, to possibly allow for C3-deprotonation. While the formation of 67 was possible through IBX oxidation of 43 or Ito–Saegusa oxidation of 66, the resulting enone could not be deprotonated and functionalized at C3.
Figure 11.
Attempting to make use of hydrolyzed ketone 43: synthesis of a TMS enol ether from 43 resulting primarily in Δ4,5 enol ether 66, as well as formation of enone 67.
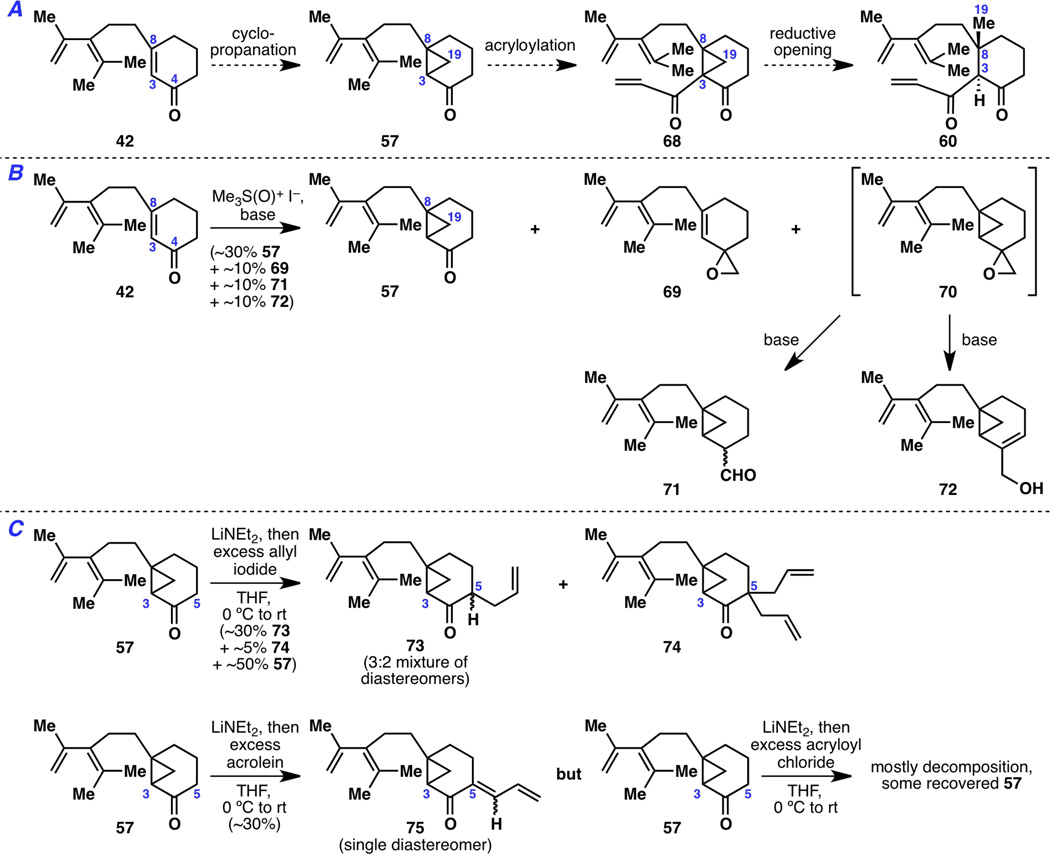
Due to the accumulated failures when using intermediates 44 and 58, a methyl conjugate addition strategy was temporarily suspended and a plan to synthesize cyclopropane 57 was put into action instead (Figure 12A). The goal here was to append a three-carbon unit to the cyclopropane C3 position to make 68 and open the cyclopropane thereafter. After all, this method of introducing the C19 methyl group was featured in Kuwajima’s successful syntheses of taxusin (8)5b,5c and Taxol®(1).4n,4o While Kuwajima performed a Simmons–Smith cyclopropanation on an allylic alcohol substrate, an enone moiety was present in 42 and thus a Corey–Chaykovsky cyclopropanation was carried out (Figure 12B). As a result, cyclopropanated ketone 57 did form in low yield (~30%), but it was accompanied by side products 69, 71 and 72 (in a combined ~30%). Aldehyde 71 and allylic alcohol 72 most likely arise from cyclopropane epoxide 70, which results from over-methylenation; since the less reactive ketone moiety seemingly competes with the enone olefin for reaction with the Corey–Chaykovsky reagent, this suggests that access to the sterically hindered C3–C8 olefin is difficult. As for a three-carbon appendage onto ketone 57, it was hoped that the increased s-character of the C–H bond in a cyclopropane would aid in deprotonation and functionalization.22 However, treatment with an unhindered strong base only led to C5 deprotonation and functionalization, resulting in 73 and 74 upon allylation and 75 upon acrolein addition (Figure 12C).
Figure 12.
A) A cyclopropanation strategy that was B) inefficient during the cyclopropane synthesis step and that C) failed at cyclopropane C3 functionalization.
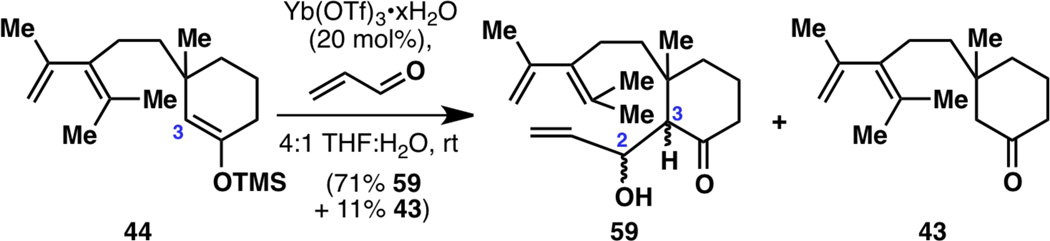
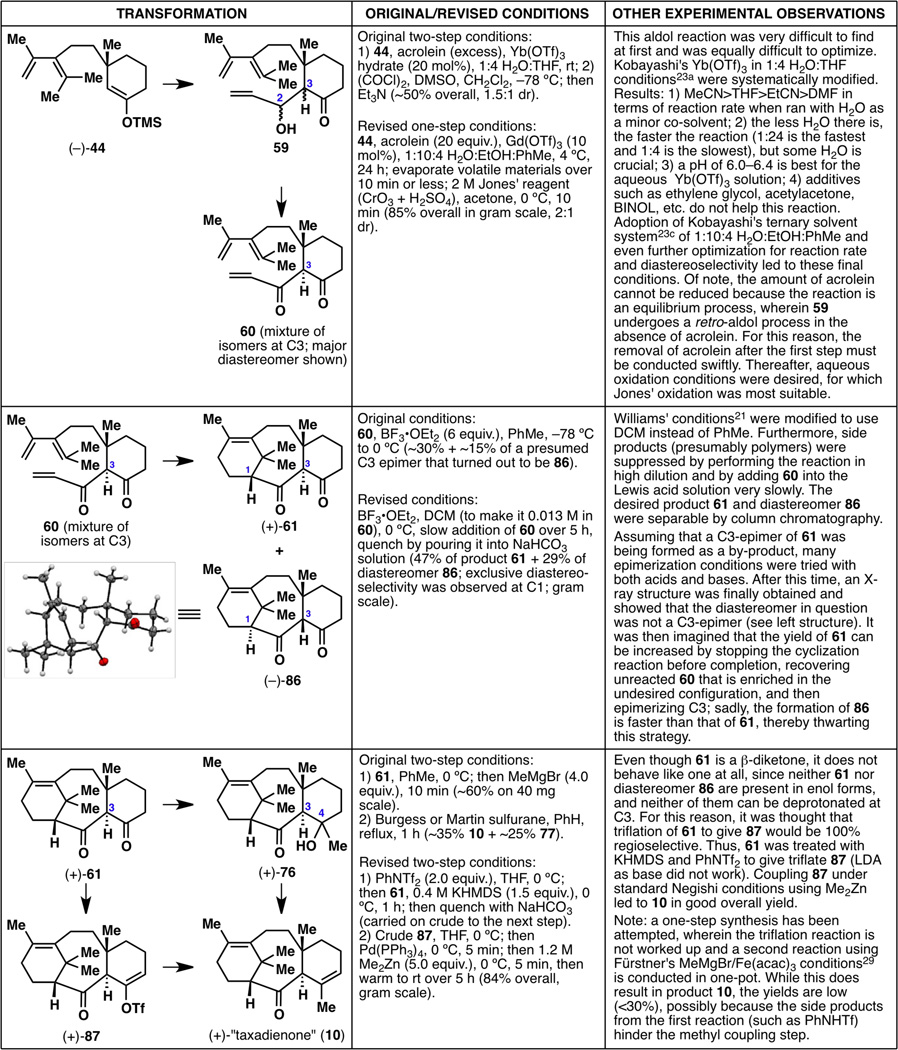
With the cyclopropane strategy now also a dead-end, it was again time to reevaluate all the synthesis routes that had been examined and to revisit failed reactions that seemingly should have worked. Since the synthesis plan laid out in Figure 7 was still very attractive, we analyzed every failed reaction and concluded that the reaction that should have worked is the aldol reaction of 44 with acrolein to give aldol product 59. While we had always assumed that the hydrolysis of TMS enol ether 44 to ketone 43 involved water, this time we asked ourselves the question: what would happen if we deliberately added water? Perhaps 44 actually reacts with acrolein to give 59 but suffers a fast retro-aldol reaction, whose rate can be slowed down by the addition of water? Thus, a bold move was made to include water in the reaction conditions, this time employing aldol conditions that only works well with water23 (Figure 13). This reaction of 44 to 59, to our surprise and delight, resulted in an extension of three carbons at C3 while retaining a functional group at C2. This represented a turning point in this project, as all the “dead ends and detours” seemed to finally give way to a successful total synthesis.24
Figure 13.
The elusive aldol reaction at C3, requiring the unexpected additive: water.
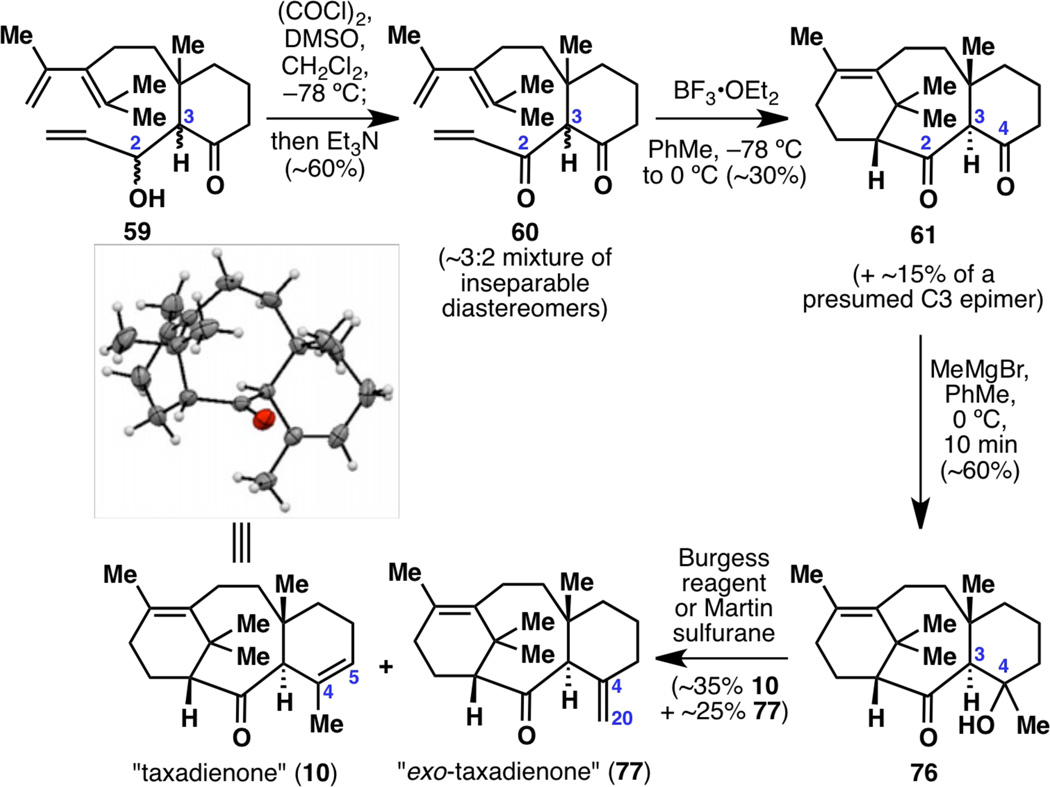
With aldol product 59 in hand, a first-generation synthesis of racemic taxadienone (10) was not far out of reach (Figure 14). Oxidation of the allylic alcohol under Swern conditions gave uncyclized diketone 60, which was an inseparable mixture of diastereomers at C3 (dr~3:2). Hoping that a Lewis acidic reaction would funnel both isomers of this mixture toward the desired diastereomer, the key Diels–Alder cyclization to 61 was accomplished using previously described acidic conditions,6,7f,7u,21 generating a 6-8-6 tricyclic framework for the first time in this project. Although the reaction yield was low and the diastereomeric ratio did not appear to improve during the cyclization, 61 was carried onward, with optimizations performed at a later time (vide infra).
Figure 14.
Completion of racemic taxadienone (10).
Although diketone 61 appears to have two reactive carbonyl groups, the C2 carbonyl is quite hindered due to the nearby gem-dimethyl group, and thus addition of MeMgBr onto 61 occurred selectively at the C4 position to give 76 as a single diastereomer. It is of note that this reaction does not occur when conducted in THF or in Et2O. Finally, keto-alcohol 76 was dehydrated using Burgess reagent or Martin sulfurane to give the desired taxadienone (10) as a major product and its isomer exo-taxadienone (77) as a significant but still a minor product. Most fortunately, taxadienone (10) turned out to be crystalline, and an X-ray structure confirmed the connectivity and relative stereochemistry of the molecule.
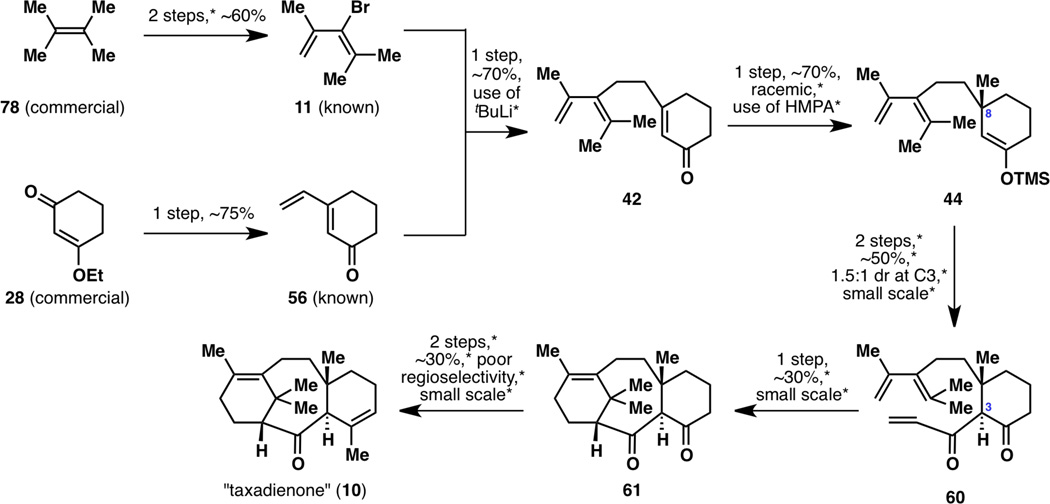
Although each step in this reaction sequence was modest at best, a first-generation synthesis of the taxane framework was now complete (Figure 15). Many more hurdles remained, however, to reach our goal of a scalable and enantioselective synthesis of taxadienone (10). Possible areas of improvement are noted, including the known two-step synthesis from 78 to 11 that could be shortened, the use of pyrophoric tBuLi, the asymmetry-inducing step, the use of toxic HMPA, the two-step synthesis from 44 to 60 that could be shortened and rendered scalable, the low diastereomeric ratio when forming 60, the low yield and scalability of the Diels–Alder cyclization step, and the two-step synthesis from 61 to 10 that could be shortened, rendered scalable, and made regioselective.
Figure 15.
A summary of the nine-step, first-generation racemic synthesis of taxadienone (10), with possible areas of improvement marked with *.
2.3. Enantioselective route to taxadienone (10), total synthesis of taxadiene (8), and reaction optimizations from the vantage point of scalability
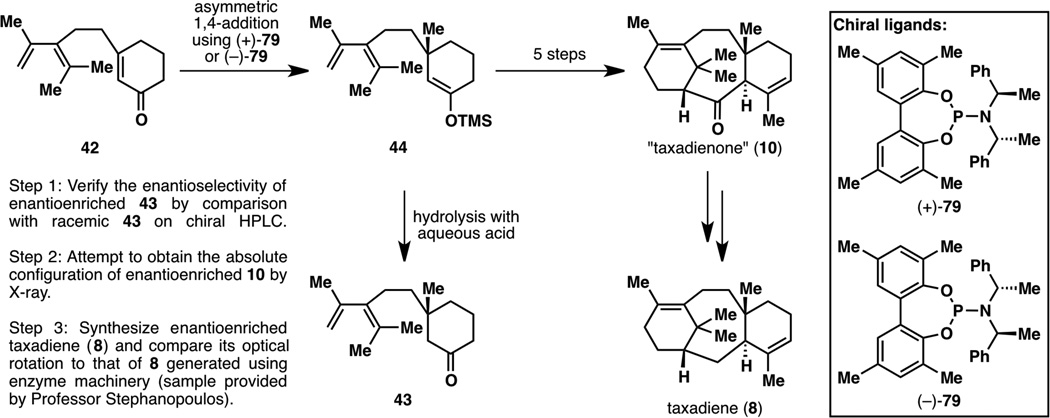
One of the advantages of the first route to taxadienone (10) described in Figure 15 is that only one asymmetric reaction would be needed to generate enantiomerically enriched taxadienone (10); another is that the C8 stereocenter, once formed, is not epimerizable. Although asymmetric conjugate additions using alkyl nucleophiles are well-known,19 early developments only involved the formation of chiral tertiary carbon centers from disubstituted enones.19a–19e Only recently (since 2005) has there been methodology that allows for the construction of chiral quaternary stereocenters, with only two major research groups studying the addition of methyl nucleophiles, those of Alexakis19f–19h and Hoveyda.19i–19k A decision was then made to employ Alexakis’ chemistry simply based on the ease of preparation of both enantiomeric series of the chiral catalyst.19h
The plan was then to test the enantioselectivity and absolute configuration of the asymmetric addition at three stages (Figure 16): 1) after generation of the first achiral intermediate 44, hydrolysis would form an enantioenriched sample of 43 that could be tested against the previously synthesized racemic 43, using chiral HPLC to obtain the enantiomeric ratio; 2) enantioenriched 44 could then be elaborated onto taxadienone (10), for which an attempt could be made at determining the absolute configuration using X-ray crystallography despite the absence of heavy atoms;25 3) enantioenriched taxadiene (8) could be synthesized from 10 and the sign of the optical rotation could be compared to that of the bioengineered sample of 8 (which was found to be of positive optical rotation).26 The absolute configuration of the asymmetric synthesis needed to be verified at two distinct stages because taxadiene (8), while being a natural product,1c was never isolated in large enough amounts for sufficient purification and analysis, and thus its optical rotation had never been recorded.
Figure 16.
Plan of action for testing the enantioselectivity and absolute configuration of the asymmetric addition step. Note: the desired enantiomeric series of products are displayed.
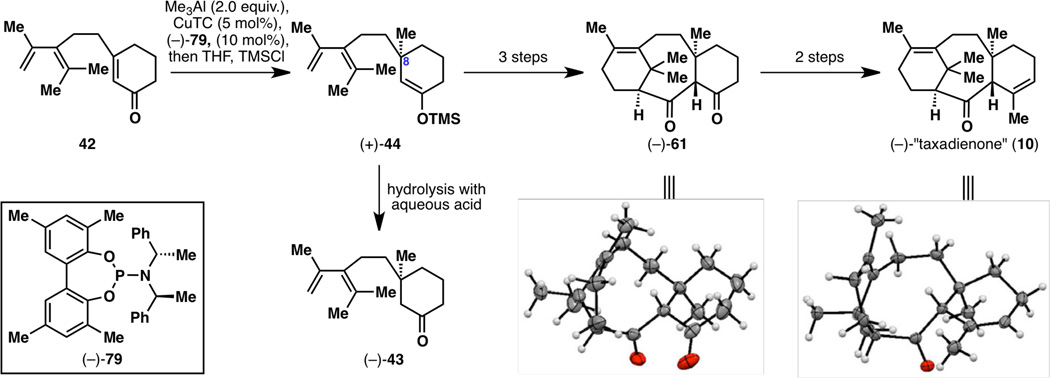
At the outset, one enantiomer of chiral ligand 79 was chosen at random, and it was decided that studies would be conducted on (−)-79. The first asymmetric reaction that was performed using Alexakis’ conditions (2 equiv. Me3Al, 5 mol% CuTC, 10 mol% (−)-79, Et2O, −30 °C, 18 h)19h was successful, resulting in 88% yield and 93% ee of (−)-43 (Figure 17). However, since Alexakis was unsuccessful in trapping the enolate intermediate with a silyl group,19h the synthesis and isolation of 44 indeed proved to be difficult. After some experimentation, it was found that the enolate intermediate after the conjugate addition can be silylated after dilution with THF then adding TMSCl and Et3N, and that (+)-44 can be isolated after high dilution with hexanes and quenching with basic alumina. After three steps from (+)-44, diketone (−)-61 was obtained; unexpectedly, this compound was found to crystallize on one occasion and thus an X-ray structure was obtained. A high-precision X-ray crystallographic analysis resulted in a small enough uncertainty in the Flack parameter to allow for absolute configuration determination;25 however, (−)-61 turned out to have the wrong configuration. For further verification, (−)-61 was elaborated into (−)-10, whereby determination of the absolute configuration by X-ray analysis again established the wrong configuration of this molecule. Having enough confidence after these two assignments, the correct enantiomeric series was then targeted, using chiral catalyst (+)-79.
Figure 17.
Synthesis of (+)-44, (−)-43, (−)-61 and (−)-10, with X-ray structures of the latter two compounds displaying the wrong absolute configuration.
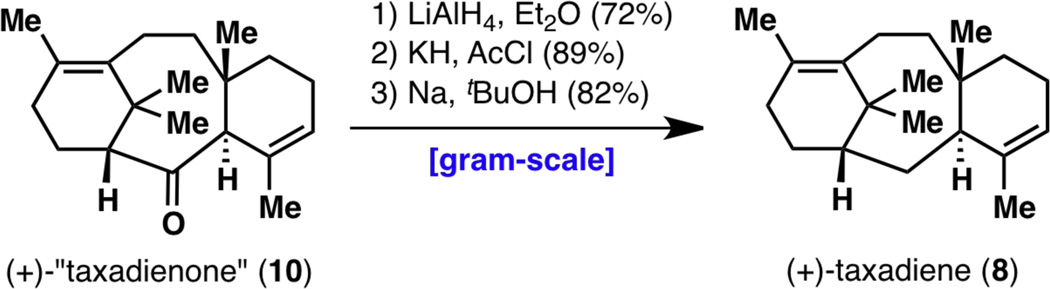
Restarting the synthesis with (+)-79, TMS enol ether (−)-44 was formed, from which (+)-43, (+)-61 and (+)-10 were generated (i.e., Figure 17 but with all the stereocenters inverted). While (+)-61 could not be crystallized on this occasion, (+)-10 reproducibly yielded crystals (from Et2O–MeOH) and the absolute configuration was confirmed to be the desired one by X-ray crystallography (see Figure 18). However, another method of confirmation of absolute configuration was still desired. To this end, deoxygenation studies were conducted on (+)-10 to generate taxadiene (8), which was used to verify that the synthetic route also generated a sample with a positive optical rotation.26
Figure 18.
X-ray structure of enantiomerically enriched (+)-taxadienone (10) with the correct absolute configuration and deoxygenation from (+)-taxadienone (10) to taxadiene (8).
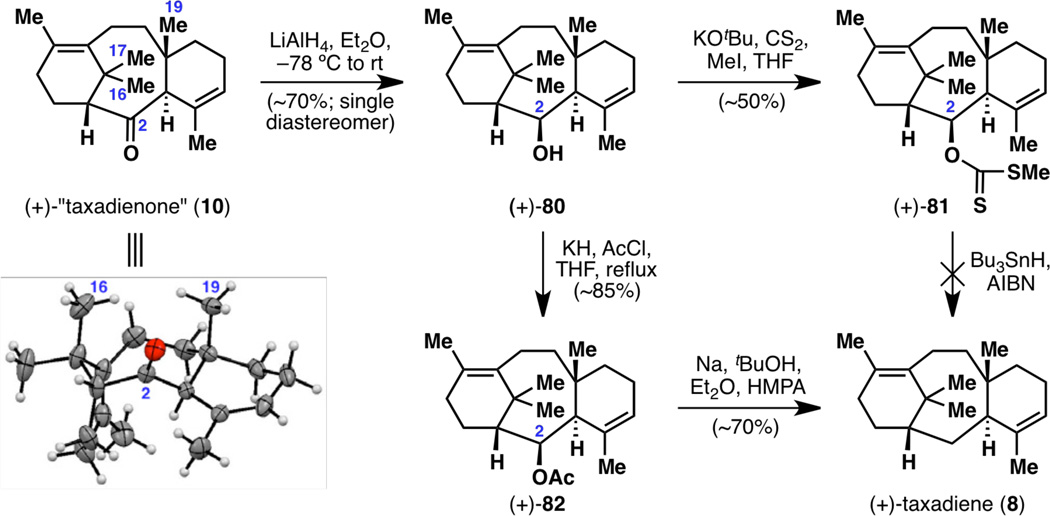
The final stages of the synthesis of a natural taxane, taxadiene (8), were not simple: classic methods of ketone deoxygenation such as Clemmensen and Wolff–Kishner reductions failed (including methodology in recent reports such as Myers’ method27), largely due to the failure of forming the requisite hydrazone (Figure 18). The C2 carbonyl group is quite hindered at the β face due to the C16 and C19 methyl groups, which can be seen in the X-ray structure of (+)-10. This effect had also been observed in the inertness of the C2 carbonyl to undergo attack by a Grignard reagent in diketone 61 (see Figure 14). It is therefore difficult for any functional group to leave from the β face as well: even if hydrazine could attack taxadienone (10) at its α face, a molecule of water needs to depart from the β face, and as this is too energetically prohibited, the hydrazinohydrin intermediate expels hydrazine back from the α face to simply return the starting material 10. Thus, we resorted to a three-step deoxygenation procedure. LiAlH4 reduction of (+)-10 proceeded smoothly to give (+)-80, and xanthate formation, while requiring the use of KOtBu as base, occurred in moderate yield to give xanthate (+)-81. However, standard Barton–McCombie deoxygenation never provided the desired taxadiene (8; for which authentic NMR spectra were available),1c,6 as evidenced by NMR analysis of the crude product. At this juncture, unusual deoxygenation methods were attempted: acetylation followed by Li/EtNH2,28a K/tBuNH2,28a or Na/HMPA;28b SO3/pyridine followed by NaH/LiAlH4;28c and Ph2SiHCl/InCl3.28d Out of these methods, the one that eventually led to taxadiene (8) was the acetylation-Na/tBuOH/HMPA strategy.28b Thus, (+)-80 was treated with KH and AcCl in refluxing THF (reactions using Ac2O did not work, and KH led to a faster reaction than KHMDS or NaH) to give (+)-82, and Na/tBuOH/HMPA in Et2O led to taxadiene (8). While this synthetic sample of taxadiene (8) was found to have an optical rotation of +170°, that of the bioengineered sample26 was +135°. Despite the discrepancy in the absolute value of these optical rotations,12 the sign of the optical rotation of these two samples matched, thereby lending further support for the use of ligand (+)-79 to synthesize the correct enantiomeric series of the taxane skeleton.
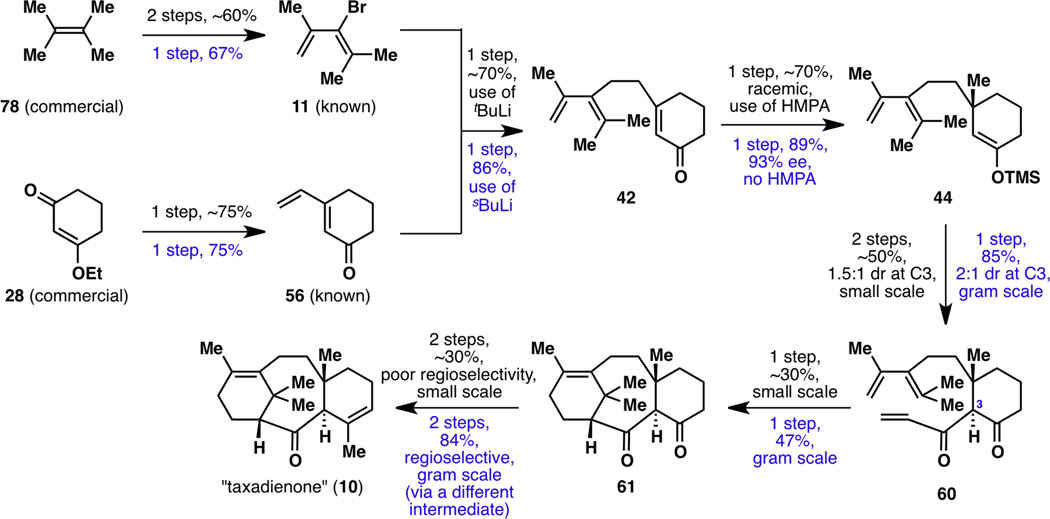
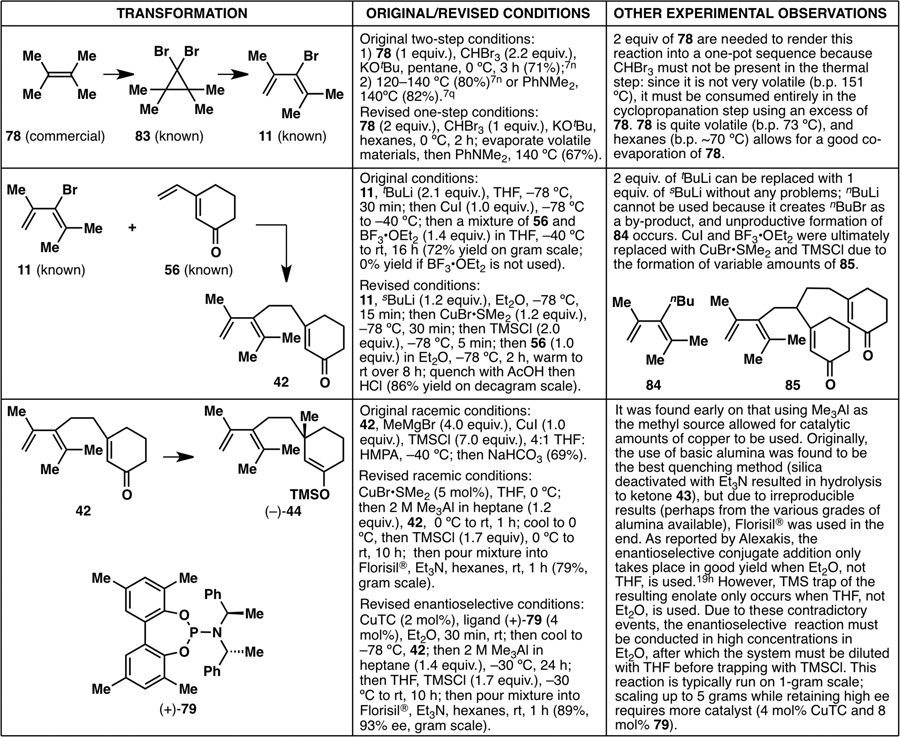
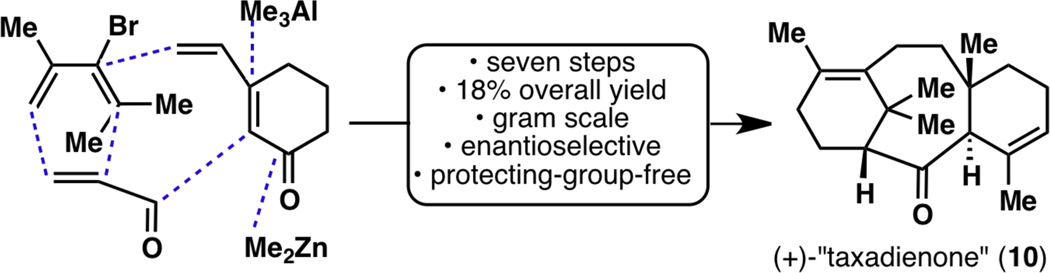
With the enantioselective synthesis of taxadienone (10) and taxadiene (8) now complete, it was time to revisit the entire synthesis from the vantage point of reaction scalability (Figure 19). Almost every single step of this sequence was optimized, on one occasion even resulting in a different synthetic intermediate than what was originally carried out. As a result, the nine-step synthesis of taxadienone (10) was reduced to a seven-step sequence, with the overall yield increasing from 1.5% to 18%. Extensive optimizations of all reactions (except for the reaction from 28 to 5618) from the starting alkene 78 to enantiomerically enriched (+)-taxadienone (10) are shown in Figure 20.
Figure 19.
The nine-step, first-generation racemic synthesis of taxadienone (10; shown in black) as well as its seven-step, second-generation enantioselective synthesis (shown in blue).
Figure 20.
Optimization of most of the steps in the enantioselective synthesis of (+)-taxadienone (10), resulting in more efficient reaction conditions and even going through different synthetic intermediates.
While the contents of Figure 20 will not be reiterated, it is important to note that these optimization efforts have resulted in an enantioselective and scalable synthesis of a laboratory cyclase phase endpoint that enables future efforts to elaborate the taxane pyramid. In summary, the synthesis of enantiomerically enriched (+)-taxadienone (10) was achieved in a total of eight steps, with a longest linear sequence of seven steps. A few grams of (+)-10 could be synthesized by one chemist over the course of seven days, with an overall yield of 18% from 78 or 20% from 28. Furthermore, the last three steps leading to enantiomerically enriched (+)-taxadiene (8) were also carried out on gram scale, providing a scalable access to Nature’s cyclase phase endpoint1c as well (Figure 21). It is of note that this ten-step synthesis of (+)-taxadiene (8) compares favorably with the only total synthesis of (±)-8 thus far, in 26 steps, back in 1995.6
Figure 21.
Gram-scale synthesis of (+)-taxadiene (8) from (+)-taxadienone (10).
3. Conclusion, Strategic Perspective and Future Outlook
The efficiency with which the synthesis of (+)-taxadienone (10) was completed is partly due to the low oxidation state of the target, which was an intended objective of this study (Figure 22). With only one heteroatom present in target 10, protecting group chemistry was minimized,30 side reactions were reduced, and the use of versatile but harsh organometallic reagents was enabled. The generation of ample quantities of this tricyclic terpene should enable subsequent use as a starting material in a creative exploration of C–H oxidation chemistry.
Figure 22.
Summary of the taxane cyclase phase.
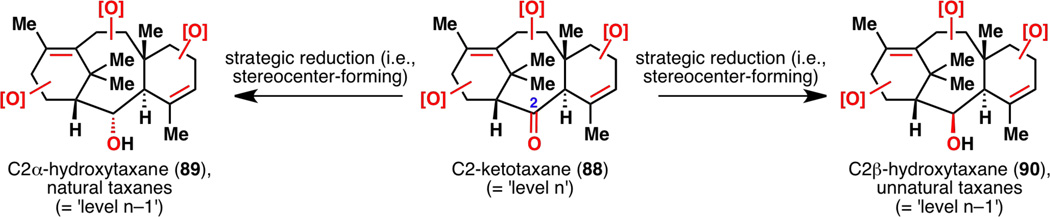
With the completion of a successful cyclase phase in the laboratory, an oxidase phase can then be planned based on oxidations of olefins and C–H bonds. In this project, one functional group has been placed strategically on each of the A, B and C rings of the taxane skeleton in taxadienone (10)—one heteroatom and two olefins—to propagate oxidative information in the most efficient manner (Figure 23). This was designed because unlike in Nature, where enzymes can oxidize substrates virtually anywhere they want (for the assumed order of C–H oxidation on the taxane skeleton in Nature,31 see 8), long-range C–H oxidations on rigid systems are very limited in the laboratory. Furthermore, taxadienone (10) has an oxygen atom at C2, which is strategic because in principle, it would allow for the generation of a series of natural taxanes with a C2α-hydroxyl group (89) and a series of unnatural taxanes with a C2β-hydroxyl group (90) for medicinal chemistry research (Figure 24).
Figure 23.
Nature’s presumed oxidation sequence31 from taxadiene (8) and a planned propagation of oxidative information from the three functional groups of taxadienone (10). [O] = oxidation.
Figure 24.
Strategic advantage of having an over-oxidized functional group at C2: generating a series of natural and unnatural taxanes. [O] = oxidation.
A dauntingly complex, yet intriguing system on which to implement the two-phase strategy is that of the taxanes, and the value of this project lies in building upon the formidable efforts of other researchers that have spearheaded Csp3–H oxidation strategies.11 Ultimately, we hope that future endeavors in pursuing a two-phase terpene total synthesis (on taxanes or on other terpene families) will aid in identifying gaps in current methodology and provide numerous opportunities for invention. Our laboratory is currently proceeding onward with the taxane oxidase phase and these studies will be reported in due course.
4. Experimental section
4.1. General
All reactions were carried out under an inert nitrogen atmosphere with dry solvents under anhydrous conditions unless otherwise stated. Dry acetonitrile (MeCN), dichloromethane (DCM), diethyl ether (Et2O), tetrahydrofuran (THF), toluene (PhMe) and triethylamine (Et3N) were obtained by passing the previously degassed solvents through activated alumina columns. Reagents were purchased at the highest commercial quality and used without further purification, unless otherwise stated. Yields refer to chromatographically and spectroscopically (1H NMR) homogeneous material, unless otherwise stated. Reactions were monitored by thin layer chromatography (TLC) carried out on 0.25 mm E. Merck silica plates (60F-254), using UV light as the visualizing agent and an acidic solution of p-anisaldehyde and heat, ceric ammonium molybdate and heat, or KMnO4 and heat as developing agents. Flash silica gel chromatography was performed using E. Merck silica gel (60, particle size 0.043–0.063 mm), flash alumina chromatography was performed using Brockmann Grade 1 aluminum oxide (activated, basic, 58 Å, 60 mesh powder), and flash Florisil® chromatography was conducted using Acros magnesium silicate (activated, 60–100 mesh). Chiral HPLC was performed using a Hitachi LaChrom Elite HPLC system. NMR spectra were recorded on Bruker DRX-600 and AMX-400 instruments and were calibrated using residual undeuterated solvent as an internal reference (CHCl3 at 7.26 ppm 1H NMR, 77.16 ppm 13C NMR). The following abbreviations were used to explain NMR peak multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. High-resolution mass spectra (HRMS) were recorded on an Agilent LC/MSD TOF mass spectrometer by electrospray ionization time-of-flight (ESI-TOF) reflectron experiments. IR experiments were recorded on a Perkin-Elmer Spectrum 100 FT-IR spectrometer. Optical rotations were obtained on a Perkin-Elmer 341 polarimeter. Melting points were recorded on a Fisher-Johns 12–144 melting point apparatus and are uncorrected.
4.2. Experimental procedures and data of synthetic intermediates
Note: Procedures and data for bromodiene 11, vinylcyclohexenone 56, enone 42, TMS enol ether 44, ketone 43, aldol product 59, uncyclized diketone 60, cyclized diketone 61, diastereomeric diketone 86, enol triflate 87, taxadienone (10), taxadienol 80, acetoxytaxadiene 82, and taxadiene (8) have been reported in the Supplementary Information of reference 12 and will not be reiterated below.
4.2.1. C5-Allylated ketone 63 and bis-allylated ketone 64
To a flame-dried 10 mL microwave vial equipped with a stir bar was added TMS enol ether (±)-44 (31.4 mg, 0.102 mmol, 1.00 equiv.) in THF (500 µL) and cooled to 0 °C. A solution of MeLi in Et2O (1.6 M; 70 µL, 0.112 mmol, 1.10 equiv.) was added dropwise and this mixture was stirred at 0 °C for 1 h. A solution of freshly prepared allyl iodide (40 µL, 0.437 mmol, 4.29 equiv.) in THF (400 µL) was added and this reaction mixture was warmed to room temperature overnight. The reaction was quenched with saturated aqueous NH4Cl (1 mL), diluted with H2O (3 mL) and extracted with EtOAc (3 × 3 mL). The combined organic layers was washed with H2O (5 mL) then brine (5 mL), dried over MgSO4, and then evaporated to dryness in vacuo. Purification by silica gel flash chromatography (gradient of EtOAc/hexanes) yielded three separate compounds (in order of decreasing Rf), (±)-64 (3.0 mg, 9% yield), (±)-63a (8.5 mg, 30% yield) and (±)-63b (11.5 mg, 39% yield). While 63a and 63b are C5-epimers of one another, the relative stereochemistry at C5 for 63a and 63b is unknown.
Data for (±)-63a: Appearance: Slightly yellow oil. TLC: Rf = 0.74–0.77 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.78 (dddd, J = 17.0, 10.1, 7.8, 6.4 Hz, 1 H), 5.06–4.96 (m, 2 H), 4.91 (dq, J = 3.0, 1.5 Hz, 1 H), 4.53 (dq, J = 2.7, 0.8 Hz, 1 H), 2.52 (dddt, J = 14.4, 6.7, 5.4, 1.4 Hz, 1 H), 2.34–2.24 (m, 1 H), 2.22 (d, J = 12.8 Hz, 1 H), 2.12 (dd, J = 12.8, 1.6 Hz, 1 H), 2.08–1.93 (m, 4 H), 1.75 (dd, J = 1.5, 0.9 Hz, 3 H), 1.65 (s, 3 H), 1.65 (s, 3 H), 1.64–1.58 (m, 2 H), 1.48 (m, 1 H), 1.32 (m, 2 H), 0.85 (s, 3 H) ppm. 13C NMR (151 MHz, CDCl3): δ 212.5, 146.5, 136.6, 136.4, 125.1, 116.4, 113.3, 53.5, 49.6, 43.0, 39.7, 36.1, 33.7, 28.9, 25.2, 22.9, 22.8, 21.9, 19.6 ppm. IR (neat): ν = 3075, 2915, 2854, 1710, 1640, 1443, 1380, 1371, 1285, 1201, 1190, 1072, 993, 910, 893 cm−1. HRMS (ESI-TOF): calc’d for C19H30O [M+H+] 275.2369, found 275.2367.
Data for (±)-63b: Appearance: Slightly yellow oil. TLC: Rf = 0.71–0.74 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.76 (dddd, J = 16.8, 10.1, 7.6, 6.5 Hz, 1 H), 5.06–4.96 (m, 2 H), 4.88 (dq, J = 2.9, 1.5 Hz, 1 H), 4.51 (dq, J = 2.7, 0.9 Hz, 1 H), 2.48 (dddt, J = 14.7, 6.7, 5.5, 1.4 Hz, 1 H), 2.32–2.22 (m, 1 H), 2.22 (dd, J = 13.2, 2.0 Hz, 1 H), 2.15 (dd, J = 13.0, 0.8 Hz, 1 H), 2.06–1.86 (m, 4 H), 1.77–1.69 (m, 1 H), 1.73 (dd, J = 1.4, 0.9 Hz, 3 H), 1.63 (s, 3 H), 1.63 (s, 3 H), 1.59–1.41 (m, 2 H), 1.17 (m, 2 H), 0.99 (s, 3 H) ppm. 13C NMR (151 MHz, CDCl3): δ 212.3, 146.5, 136.5, 136.3, 125.2, 116.4, 113.3, 54.0, 49.2, 39.2, 36.3, 35.4, 33.8, 28.3, 27.5, 25.1, 22.9, 21.9, 19.6 ppm. IR (neat): ν = 3075, 2928, 2855, 1709, 1640, 1459, 1444, 1373, 1283, 1228, 1193, 1075, 999, 909, 893 cm−1. HRMS (ESI-TOF): calc’d for C19H30O [M+H+] 275.2369, found 275.2374.
Data for (±)-64: Appearance: Slightly yellow oil. TLC: Rf = 0.77–0.79 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.76–5.56 (m, 2 H), 5.10–5.00 (m, 4 H), 4.90 (dq, J = 2.9, 1.4 Hz, 1 H), 4.53 (dq, J = 2.7, 0.9 Hz, 1 H), 2.38–2.23 (m, 4 H), 2.24 (d, J = 14.0 Hz, 1 H), 2.14 (dd, J = 14.0, 1.2 Hz, 1 H), 2.00 (m, 2 H), 1.74 (dd, J = 1.4, 0.9 Hz, 3 H), 1.73–1.66 (m, 3 H), 1.65 (s, 3 H), 1.64 (s, 3 H), 1.28–1.18 (m, 3 H), 0.90 (s, 3 H) ppm. 13C NMR (151 MHz, CDCl3): δ 214.2, 146.5, 136.3, 133.9, 133.7, 125.2, 118.3, 118.3, 113.3, 51.2, 50.6, 40.0, 39.7, 39.6, 38.7, 31.9, 31.4, 25.1, 25.1, 22.9, 21.9, 19.6 ppm. IR (neat): ν = 3075, 2925, 2857, 1703, 1638, 1444, 1372, 1328, 1286, 1201, 1160, 1082, 993, 910, 893 cm−1. HRMS (ESI-TOF): calc’d for C22H34O [M+H+] 315.2682, found 315.2689.
4.2.2. TBS enol ether 65
To a flame-dried 20 mL microwave vial equipped with a stir bar was added TMS enol ether (±)-44 (65.0 mg, 0.212 mmol, 1.00 equiv.) in THF (3 mL) and cooled to 0 °C. A solution of MeLi in Et2O (1.6 M; 150 µL, 0.24 mmol, 1.13 equiv.) was added dropwise and this mixture was stirred at 0 °C for 1 h. A solution of freshly distilled TBSCl (distilled over CaH2; 200.0 mg, 1.327 mmol, 6.26 equiv.) in THF (3 mL) was added and this reaction mixture was stirred at 0 °C for 1 h. The reaction was quenched at 0 °C with 1:1 MeOH:Et3N (3 mL), diluted with H2O (5 mL) and extracted with EtOAc (3 × 7 mL). The combined organic layers was washed with H2O (10 mL) then brine (10 mL), dried over Na2SO4, and then evaporated to dryness in vacuo. Although TMS enol ether 44 is unstable on silica gel, TBS enol ether 120 is stable and can be purified by silica gel. Thus, purification by silica gel flash chromatography (hexanes) yielded (±)-65 (40.7 mg, 55% yield). Appearance: Colorless oil. TLC: Rf = 0.37–0.40 (silica gel, hexanes). 1H NMR (400 MHz, CDCl3): δ 4.89 (dq, J = 2.9 and 1.4 Hz, 1 H), 4.66 (s, 1 H), 4.53 (dq, J = 2.7, 0.9 Hz, 1 H), 2.02 (m, 2 H), 1.95 (m, 2 H), 1.75 (dd, J = 1.4, 0.9 Hz, 3 H), 1.67 (m, 2 H), 1.66 (s, 6 H), 1.42 (m, 1 H), 1.35–1.20 (m, 3 H), 0.96 (s, 3 H), 0.92 (s, 9 H), 0.13 (s, 3 H), 0.12 (s, 3 H) ppm. 13C NMR (151 MHz, CDCl3): δ 149.7, 146.9, 137.3, 124.5, 114.4, 112.9, 42.0, 34.7 (two carbons), 30.1, 28.0, 26.2, 25.9, 23.0, 21.9, 19.8, 19.6, 18.2, −4.1, −4.3 ppm. IR (neat): ν = 3074, 2933, 2866, 1660, 1444, 1366, 1263, 1250, 1203, 1137, 963, 938, 894, 840, 752 cm−1. HRMS (ESI-TOF): calc’d for C22H40OSi [M+H+] 349.2921, found 349.2924.
4.2.3. Δ4, 5 -TMS enol ether 66 (>90% pure, contains some 44)
To a flame-dried 10 mL microwave vial equipped with a stir bar was added lithium diethylamide (2.0 M, synthesized by adding nBuLi to diethylamine; 250 µL, 0.500 mmol, 1.52 equiv.) and this was cooled to 0 °C. Freshly distilled TMSCl (distilled over CaH2; 200 µL, 1.58 mmol, 4.80 equiv.) was added, followed by a solution of ketone (±)-43 (77.0 mg, 0.329 mmol, 1.0 equiv.) in THF (1 mL). The reaction mixture was stirred at 0 °C for 30 min and then warmed to room temperature over 1 h. It was then cooled to 0 °C, 1:1 MeOH:Et3N (1 mL) was added, and this reaction mixture was warmed to room temperature over 1 h. The reaction was diluted with H2O (3 mL) and extracted with EtOAc (3 × 2 mL). The combined organic layers was washed with H2O (4 mL) then brine (4 mL), dried over Na2SO4, and then evaporated to dryness in vacuo. Purification by Et3N-treated silica gel chromatography (2% Et3N in hexanes) yielded >90% pure (±)-66 with <10% of (±)-44 (combined: 69.0 mg, 57% yield), along with recovered (±)-43 (27.2 mg, 35% yield). Appearance: Colorless oil. TLC: Rf = 0.00–0.20 (silica gel, hexanes, streaks due to decomposition when without Et3N). 1H NMR (400 MHz, CDCl3): δ 4.89 (dq, J = 2.9, 1.5 Hz, 1 H), 4.82 (tt, J = 3.7, 1.3 Hz, 1 H), 4.53 (dq, J = 2.7, 1.0 Hz, 1 H), 2.08–1.98 (m, 4 H), 1.86 (m, 1 H), 1.75 (dd, J = 1.5, 1.0 Hz, 3 H), 1.70 (m, 1 H), 1.66 (s, 3 H), 1.65 (s, 3 H), 1.27 (m, 4 H), 0.91 (s, 3 H), 0.17 (s, 9 H) ppm. 13C NMR (151 MHz, CDCl3): δ 149.3, 146.8, 137.0, 124.6, 113.0, 103.2, 42.5, 40.4, 33.0, 32.9, 25.3, 24.0, 23.0, 21.9, 21.2, 19.6, 0.5 ppm. IR (neat): ν = 3074, 2933, 2866, 1660, 1444, 1366, 1263, 1250, 1203, 1137, 963, 938, 894, 840, 752 cm−1. HRMS (ESI-TOF): calc’d for C22H40OSi [M+H+] 349.2921, found 349.2924.
4.2.4. Methylated enone 67
This compound can be prepared in ~20% yield using one of two ways: from ketone 43 or TMS enol ether 66. From ketone 43: To a flame-dried 20 mL microwave vial was added IBX (611.0 mg, 2.182 mmol, 4.87 equiv.), followed by a solution of ketone 43 (105.0 mg, 0.4480 mmol, 1.00 equiv.) in DMSO (4 mL). This mixture was heated to 80 °C for 8 h. The reaction was cooled to room temperature, upon which it was partitioned using saturated aqueous NaHCO3 (5 mL) and EtOAc (5 mL). The aqueous layer was extracted with EtOAc (2 × 3 mL). The combined organic layers was washed with H2O (5 mL) then brine (5 mL), dried over MgSO4, and then evaporated to dryness in vacuo. Purification by flash silica gel chromatography afforded 67 (21.0 mg, 20% yield). From TMS enol ether 66: To a flame-dried 5 mL microwave vial were added TMS enol ether 66 (42.0 mg, 0.115 mmol, 1.00 equiv.), MeCN (1 mL) and Pd(OAc)2 (30.0 mg, 0.134 mmol, 1.17 equiv.), in that order, and this dark reaction mixture was stirred at room temperature for 16 h. This mixture was directly filtered on Celite (eluting with EtOAc). The obtained solution was evaporated in vacuo and purified by flash silica gel chromatography to afford 67 (5.4 mg, 20% yield) and ketone 43 (9.9 mg, 37%). Appearance: Yellow oil. TLC: Rf = 0.57–0.59 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 6.86 (ddd, J = 10.1, 4.6, 3.7 Hz, 1 H), 6.03 (dt, J = 10.1, 1.9 Hz, 1 H), 4.90 (dq, J = 3.0, 1.5 Hz, 1 H), 4.52 (dq, J = 2.7, 0.9 Hz, 1 H), 2.33 (dt, J = 17.8, 3.0 Hz, 1 H), 2.30 (AB quartet, 2 H), 2.18 (dddd, J = 18.8, 4.6, 1.8, 1.1 Hz, 1 H), 2.03 (m, 2 H), 1.74 (dd, J = 1.5, 0.9 Hz, 3 H), 1.65 (s, 3 H), 1.63 (s, 3 H), 1.37 (m, 2 H), 1.02 (s, 3 H) ppm. 13C NMR (151 MHz, CDCl3): δ 200.1, 148.4, 146.4, 136.1, 129.2, 125.4, 113.4, 50.3, 40.1, 38.2, 36.6, 25.3, 24.7, 22.8, 21.9, 19.6 ppm. IR (neat): ν = 3074, 2960, 2915, 2871, 1679, 1631, 1444, 1386, 1343, 1284, 1246, 1168, 1124, 893, 733 cm−1. HRMS (ESI-TOF): calc’d for C16H24O [M+H+] 233.1900, found 233.1902.
4.2.5. Corey–Chaykovsky products 57, 69, 71 and 72
To a flame-dried 20 mL microwave vial were added trimethylsulfoxonium iodide (58.0 mg, 0.264 mmol, 1.00 equiv.), DMSO (200 µL) and NaH (60% dispersion in mineral oil, ~11 mg, ~0.28 mmol, ~1.0 equiv.). After stirring for 30 min at room temperature, enone 42 (57.5 mg, 0.263 mmol, 1.00 equiv.) was added and the reaction mixture was stirred overnight. H2O (1 mL) was added and then this reaction mixture was extracted with Et2O (3 × 1 mL). The combined organic layers was washed with H2O (2 mL) then brine (2 mL), dried over MgSO4, and then evaporated to dryness in vacuo. Purification by flash silica gel chromatography afforded 57 (18.3 mg, 30% yield), 69 (6.2 mg, 10% yield), 71 (6.1 mg, 10% yield), 72 (6.2 mg, 10% yield).
Data for (±)-57: Appearance: Slightly yellow oil. TLC: Rf = 0.56–0.60 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 4.89 (dq, J = 2.9, 1.5 Hz, 1 H), 4.50 (dq, J = 2.7, 0.9 Hz, 1 H), 2.27 (ddd, J = 18.4, 5.6, 3.1 Hz, 1 H), 2.14 (t, J = 8.5 Hz, 2 H), 2.08–1.92 (m, 2 H), 1.82–1.67 (m, 2 H), 1.73 (dd, J = 1.5, 0.9 Hz, 3 H), 1.64 (s, 6 H), 1.62 (m, 1 H), 1.59 (dd, J = 10.0, 4.5 Hz, 1 H), 1.49–1.30 (m, 3 H), 0.92 (dd, J = 10.0, 5.2 Hz, 1 H) ppm. 13C NMR (151 MHz, CDCl3): δ 209.7, 146.3, 135.9, 125.4, 113.4, 37.8, 36.3, 33.8, 28.6, 27.9, 25.6, 22.8, 21.8, 19.6, 18.4, 17.4 ppm. IR (neat): ν = 3074, 2914, 2855, 1688, 1631, 1444, 1372, 1323, 1243, 1216, 1170, 1076, 935, 891, 871 cm−1. HRMS (ESI-TOF): calc’d for C16H24O [M+H+] 233.1900, found 233.1904.
Data for (±)-69: Appearance: Slightly yellow oil. TLC: Rf = 0.74–0.77 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.50 (bs, 1 H), 4.91 (dq, J = 3.0, 1.5 Hz, 1 H), 4.54 (dq, J = 2.7, 0.9 Hz, 1 H), 2.69 (s, 2 H), 2.30 (m, 1 H), 2.23–2.13 (m, 4 H), 2.11–1.92 (m, 3 H), 1.75 (dd, J = 1.5, 0.9 Hz, 3 H), 1.68–1.58 (m, 2 H), 1.66 (s, 6 H) ppm. 13C NMR (151 MHz, CDCl3): δ 146.6, 136.7, 136.3, 125.4, 120.5, 113.3, 57.7, 54.4, 36.3, 36.1, 29.6, 29.5, 24.2, 22.9, 21.9, 19.7 ppm. IR (neat): ν = 3074, 3034, 2915, 2855, 1631, 1442, 1399, 1371, 1291, 1175, 1040, 931, 891, 826, 790 cm−1. HRMS (ESI-TOF): calc’d for C16H24O [M+H+] 233.1900, found 233.1910.
Data for (±)-71 as a 3:2 mixture of inseparable diastereomers: Appearance: Slightly yellow oil. TLC: Rf = 0.79–0.82 (silica gel, 1:3 EtOAc/hexanes). Major diastereomer, 1H NMR (400 MHz, CDCl3): δ 9.72 (d, J = 1.3 Hz, 1 H), 4.88 (m, 1 H), 4.50 (m, 1 H), 2.47 (m, 1 H), 2.12 (m, 2 H), 1.79 (m, 1 H), 1.74 (dd, J = 1.5, 0.9 Hz, 3 H), 1.69 (m, 1 H), 1.65 (s, 3 H), 1.64 (s, 3 H), 1.58 (m, 1 H), 1.52–1.44 (m, 1 H), 1.44–1.34 (m, 1 H), 1.34–1.07 (m, 3 H), 1.02 (ddd, J = 9.4, 5.2, 1.7 Hz, 1 H), 0.53 (dd, J = 9.3, 4.5 Hz, 1 H), 0.28 (t, J = 4.9 Hz, 1 H) ppm. Minor diastereomer, 1H NMR (400 MHz, CDCl3): δ 9.66 (d, J = 1.5 Hz, 1 H), 4.88 (m, 1 H), 4.50 (m, 1 H), 2.74 (m, 1 H), 2.12 (m, 2 H), 1.79 (m, 1 H), 1.75 (dd, J = 1.5, 0.9 Hz, 3 H), 1.69 (m, 1 H), 1.66 (s, 3 H), 1.65 (s, 3 H), 1.58 (m, 1 H), 1.52–1.44 (m, 1 H), 1.44–1.34 (m, 1 H), 1.34–1.07 (m, 3 H), 0.93 (ddd, J = 9.0, 6.7, 5.6 Hz, 1 H), 0.48 (dd, J = 8.8, 4.8 Hz, 1 H), 0.20 (t, J = 5.2 Hz, 1 H) ppm. Major diastereomer, 13C NMR (151 MHz, CDCl3): δ 204.1, 146.8, 136.6, 124.8, 113.0, 48.7, 40.3, 28.1, 27.6, 22.9, 22.9, 21.9, 20.3, 19.6, 19.6, 16.7, 16.4 ppm. Minor diastereomer, 13C NMR (151 MHz, CDCl3): δ 204.3, 146.8, 136.4, 124.9, 113.1, 45.6, 40.3, 28.2, 27.9, 22.9, 21.9, 20.4, 20.1, 19.6, 18.9, 16.5, 14.4 ppm. IR (neat): ν = 3073, 2925, 2855, 2712, 1726, 1631, 1447, 1371, 1301, 1172, 1123, 1076, 1021, 892, 833 cm−1. HRMS (ESI-TOF): calc’d for C17H26O [M+H+] 247.2056, found 247.2048.
Data for (±)-72: Appearance: Slightly yellow oil. TLC: Rf = 0.50–0.53 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.39 (d, J = 6.5 Hz, 1 H), 4.89 (dq, J = 3.0, 1.5 Hz, 1 H), 4.51 (dq, J = 2.7, 0.8 Hz, 1 H), 4.13 (bs, 2 H), 2.17 (dtd, J = 26.2, 13.3, 5.3 Hz, 2 H), 2.03 (dt, J = 17.1, 6.5 Hz, 1 H), 1.90 (dd, J = 12.8, 7.2 Hz, 1 H), 1.87–1.76 (m, 1 H), 1.75 (dd, J = 1.5, 0.9 Hz, 3 H), 1.66 (s, 3 H), 1.65 (s, 3 H), 1.50 (td, J = 12.6, 5.4 Hz, 1 H), 1.45 (td, J = 12.5, 6.1 Hz, 1 H), 1.32 (bs, 1 H), 1.26 (m, 1 H), 1.00 (dd, J = 8.4, 4.2 Hz, 1 H), 0.80 (t, J = 4.2 Hz, 1 H), 0.64 (dd, J = 8.4, 4.2 Hz, 1 H) ppm. 13C NMR (151 MHz, CDCl3): δ 146.7, 140.6, 136.7, 124.9, 117.5, 113.0, 67.4, 38.8, 28.5, 24.2, 23.2, 22.9, 21.9, 21.6, 19.6, 19.2, 16.7 ppm. IR (neat): ν = 3347 (br), 3072, 2913, 2853, 1661, 1631, 1442, 1371, 1168, 1069, 1050, 1005, 931, 892, 813 cm−1. HRMS (ESI-TOF): calc’d for C17H26O [M+H+] 247.2056, found 247.2053.
4.2.6. Allylated cyclopropane ketone 73
To a flame-dried 10 mL microwave vial equipped with a stir bar was added ketone (±)-57 (18.0 mg, 0.0775 mmol, 1.0 equiv.) in THF (500 µL) and cooled to 0 °C. Lithium diethylamide (2.0 M, synthesized by adding nBuLi to diethylamine; 40 µL, 0.080 mmol, 1.0 equiv.) was added and was stirred at 0 °C for 30 min. A solution of freshly prepared allyl iodide (30 µL, 0.33 mmol, 4.2 equiv.) in THF (200 µL) was added and this reaction mixture was warmed to room temperature overnight. The reaction was quenched with saturated aqueous NH4Cl (1 mL), diluted with H2O (1 mL) and extracted with EtOAc (3 × 1 mL). The combined organic layers was washed with H2O (2 mL) then brine (2 mL), dried over MgSO4, and then evaporated to dryness in vacuo. Purification by PTLC (gradient of EtOAc/hexanes) yielded four separate compounds (in order of decreasing Rf), (±)-74 (1.4 mg, 5% yield, ~90% pure), (±)-73a (3.6 mg, 17% yield), (±)-73b (2.6 mg, 12% yield) and recovered (±)-57 (8.9 mg, 49%). While 73a and 73b are C5-epimers of one another, the relative stereochemistry at C5 for 73a and 73b is unknown.
Data for (±)-74: The full compound characterization has not been obtained. Its structure has been assigned solely by its mass analysis and an analogy to the formation of allylated products 63 and 64 (see Figure 8). Appearance: Slightly yellow oil. TLC: Rf = 0.80–0.83 (silica gel, 1:3 EtOAc/hexanes). HRMS (ESI-TOF): calc’d for C22H32O [M+H+] 313.2526, found 313.2520.
Data for (±)-73a: Appearance: Slightly yellow oil. TLC: Rf = 0.77–0.80 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.67 (ddt, J = 17.3, 10.2, 7.2 Hz, 1 H), 5.06–4.99 (m, 2 H), 4.90 (dq, J = 3.0, 1.5 Hz, 1 H), 4.51 (dq, J = 2.7, 0.9 Hz, 1 H), 2.50 (dddt, J = 13.6, 6.7, 3.9, 1.2 Hz, 1 H), 2.23 (m, 1 H), 2.14 (t, J = 8.4 Hz, 2 H), 2.02 (m, 1 H), 1.95 (m, 1 H), 1.85–1.70 (m, 2 H), 1.74 (dd, J = 1.5, 0.9 Hz, 3 H), 1.65 (s, 6 H), 1.61 (d, J = 10.2, 4.5 Hz, 1 H), 1.50–1.30 (m, 4 H), 0.85 (dd, J = 10.2, 5.2 Hz, 1 H) ppm. 13C NMR (151 MHz, CDCl3): δ 209.9, 146.4, 136.0, 135.9, 125.5, 117.1, 113.4, 46.0, 37.6, 35.6, 33.7, 27.9, 27.7, 25.6, 22.9, 22.9, 21.9, 19.7, 15.8 ppm. IR (neat): ν = 3075, 2916, 2855, 1684, 1640, 1442, 1371, 1335, 1263, 1213, 1171, 1073, 997, 910, 892 cm−1. HRMS (ESI-TOF): calc’d for C19H28O [M+H+] 273.2213, found 273.2210.
Data for (±)-73b: Appearance: Slightly yellow oil. TLC: Rf = 0.74–0.77 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.79–5.68 (m, 1 H), 5.06–5.03 (m, 1 H), 5.01 (t, J = 1.2 Hz, 1 H), 4.90 (dq, J = 3.0, 1.5 Hz, 1 H), 4.53–4.50 (m, 1 H), 2.50–2.42 (m, 1 H), 2.17–1.98 (m, 4 H), 1.92–1.76 (m, 3 H), 1.74 (dd, J = 1.5, 0.9 Hz, 3 H), 1.65 (s, 6 H), 1.61–1.56 (m, 2 H), 1.38–1.32 (m, 2 H), 1.27–1.24 (m, 1 H), 1.02 (dd, J = 10.0, 5.2 Hz, 1 H) ppm. 13C NMR (151 MHz, CDCl3): δ 211.2, 146.4, 136.5, 135.9, 125.5, 116.9, 113.4, 43.2, 38.5, 34.8, 33.2, 30.4, 28.1, 26.2, 24.9, 22.9, 21.9, 21.6, 19.7 ppm. IR (neat): ν = 3074, 2918, 2856, 1684, 1639, 1443, 1371, 1320, 1265, 1228, 1205, 1167, 996, 909, 892 cm−1. HRMS (ESI-TOF): calc’d for C19H28O [M+H+] 273.2213, found 273.2213.
4.2.7. Acroleinated cyclopropane ketone 75
To a flame-dried 10 mL microwave vial equipped with a stir bar was added ketone (±)-57 (18.0 mg, 0.0775 mmol, 1.0 equiv.) in THF (500 µL) and cooled to 0 °C. Lithium diethylamide (2.0 M, synthesized by adding nBuLi to diethylamine; 40 µL, 0.080 mmol, 1.0 equiv.) was added and was stirred at 0 °C for 30 min. A solution of freshly distilled acrolein (distilled over CaSO4; 20 µL, 0.30 mmol, 3.9 equiv.) in THF (200 µL) was added and this reaction mixture was warmed to room temperature overnight. The reaction was quenched with saturated aqueous NH4Cl (1 mL), diluted with H2O (1 mL) and extracted with EtOAc (3 × 1 mL). The combined organic layers was washed with H2O (2 mL) then brine (2 mL), dried over MgSO4, and then evaporated to dryness in vacuo. Purification by PTLC (gradient of EtOAc/hexanes) yielded (±)-75 (6.0 mg, 29%). Appearance: Slightly yellow oil. TLC: Rf = 0.71–0.73 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 7.12 (d, J = 11.6 Hz, 1 H), 6.56 (ddd, J = 16.7, 11.6, 10.1 Hz, 1 H), 5.63 (d, J = 16.8 Hz, 1 H), 5.51 (d, J = 10.0 Hz, 1 H), 4.91 (dq, J = 3.0, 1.5 Hz, 1 H), 4.52 (dq, J = 2.7, 0.9 Hz, 1 H), 2.76 (dd, J = 17.2, 5.3 Hz, 1 H), 2.25 (m, 1 H), 2.15 (t, J = 8.4 Hz, 2 H), 2.05 (ddd, J = 13.3, 5.6, 1.8 Hz, 1 H), 1.82 (td, J = 13.6, 5.6 Hz, 1 H), 1.79 (dd, J = 9.4, 4.1 Hz, 1 H), 1.74 (dd, J = 1.4, 0.9 Hz, 3 H), 1.65 (s, 6 H), 1.53–1.36 (m, 3 H), 1.00 (dd, J = 9.5, 5.0 Hz, 1 H) ppm. 13C NMR (151 MHz, CDCl3): δ 199.1, 146.4, 136.0, 135.1, 132.2, 131.4, 125.9, 125.5, 113.4, 37.7, 33.6, 30.1, 27.9, 24.5, 22.9, 22.1, 21.9, 19.7, 17.6 ppm. IR (neat): ν = 3075, 2917, 2856, 1670, 1630, 1611, 1580, 1444, 1372, 1319, 1264, 1228, 989, 892, 875 cm−1. HRMS (ESI-TOF): calc’d for C19H26O [M+H+] 271.2056, found 271.2055.
4.2.8. Methylated alcohol 76
To a flame-dried 5 ml microwave vial was added a solution of (±)-61 (40.0 mg, 0.139 mmol, 1.00 equiv.) in PhMe (900 µL) and this was cooled to 0 °C. MeMgBr solution (2.8 M; 200 µL, 0.560 mmol, 4.03 equiv.) was then added dropwise, and the reaction mixture was stirred at 0 °C for 10 min. The reaction was quenched with saturated aqueous NH4Cl (0.5 mL), diluted with H2O (1 mL) and extracted with EtOAc (3 × 1 mL). The combined organic layers was washed with H2O (2 mL) then brine (2 mL), dried over Na2SO4, and then evaporated to dryness in vacuo. Purification by silica gel flash chromatography (gradient of EtOAc/hexanes) yielded (±)-76 (25.3 mg, 60%). Appearance: Slightly yellow oil. TLC: Rf = 0.64–0.68 (silica gel, 1:3 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 4.00 (s, 1 H, D2O exchangeable), 2.87 (s, 1 H), 2.74 (ddd, J = 14.5, 11.5, 5.2 Hz, 1 H), 2.49 (m, 1 H), 2.44 (t, J = 12.2 Hz, 1 H), 2.14 (m, 1 H), 2.05–1.85 (m, 1 H), 1.79 (m, 1 H), 1.78 (s, 3 H), 1.66 (dddd, J = 14.0, 4.1, 2.9, 1.5 Hz, 1 H), 1.51 (td, J = 13.2, 3.7 Hz, 1 H), 1.39 (m, 1 H), 1.29 (dt, J = 15.2, 5.3 Hz, 1 H), 1.25 (s, 3 H), 1.24 (dtd, J = 13.2, 3.5, 1.5, 1 H), 1.14 (s, 3 H), 1.11 (m, 1 H), 1.08 (s, 3 H), 1.06 (s, 3 H) ppm. 13C NMR (151 MHz, CDCl3): δ 223.6, 137.3, 129.9, 72.2, 64.8, 57.1, 42.8, 40.7, 40.4, 39.5, 38.0, 32.7, 30.2, 28.4, 26.9, 25.1, 24.7, 21.9, 18.7, 18.6 ppm. IR (neat): ν = 3504, 2962, 2900, 1661, 1460, 1380, 1369, 1327, 1176, 1137, 1093, 1044, 949, 896, 732 cm−1. HRMS (ESI-TOF): calc’d for C20H32O2 [M+H+] 305.2475, found 305.2466.
4.2.9. Taxadienyl xanthate (+)-81
To a flame-dried 10 mL microwave vial equipped with a stir bar were added KOtBu (15.0 mg, 0.134 mmol, 2.0 equiv.) and THF (1 mL). This was cooled to 0 °C, upon which taxadienol (+)-80 (19.5 mg, 0.0676 mmol, 1.0 equiv.) was added as a solution in THF (0.5 mL), and warmed to room temperature over 2 h. The reaction mixture was cooled to 0 °C, upon which freshly distilled CS2 (100 µL, 1.66 mmol, 24 equiv.) was added and stirred for 1 h at 0 °C. Finally, MeI (50 µL, 0.803 mmol, 12 equiv.) was added and the reaction was warmed to room temperature over 1 h. The reaction was quenched with saturated aqueous NH4Cl (0.5 mL), diluted with H2O (1 mL) and extracted with EtOAc (3 × 1 mL). The combined organic layers was washed with H2O (2 mL) then brine (2 mL), dried over Na2SO4, and then evaporated to dryness in vacuo. Purification by silica gel flash chromatography (gradient of EtOAc/hexanes) yielded (+)-81 (13.1 mg, 51%). Appearance: Yellow oil. TLC: Rf = 0.42–0.44 (silica gel, hexanes). 1H NMR (400 MHz, CDCl3): δ 6.39 (d, J = 4.1 Hz, 1 H), 5.32 (s, 1 H), 3.22 (s, 1 H), 2.81 (td, J = 13.6, 5.2 Hz, 1 H), 2.69 (td, J = 14.2, 5.2 Hz, 1 H), 2.60 (s, 3 H), 2.34 (dd, J = 9.3, 3.9 Hz, 1 H), 2.30 (d, J = 9.6 Hz, 1 H), 2.22 (d, J = 8.8 Hz, 1 H), 2.20–2.06 (m, 2 H), 2.05–1.94 (m, 2 H), 1.85 (ddd, J = 18.6, 10.5, 3.6 Hz, 1 H), 1.80 (s, 3 H), 1.71 (s, 3 H), 1.46 (s, 3 H), 1.42 (ddd, J = 15.3, 5.0, 3.0 Hz, 1 H), 1.37 (ddd, J = 14.8, 10.6, 4.1 Hz, 1 H), 1.15 (m, 1 H), 1.03 (s, 3 H), 0.99 (s, 3 H) ppm. 13C NMR (151 MHz, CDCl3): δ 215.2, 137.8, 136.7, 128.9, 122.4, 90.2, 46.4, 41.8, 40.3, 38.2, 38.2, 38.2, 32.4, 28.9, 26.2, 25.4, 24.8, 24.6, 23.9, 22.1, 21.4, 19.6 ppm. IR (neat): ν = 3048, 3004, 2919, 2882, 2857, 1459, 1376, 1250, 1212, 1079, 1052, 963, 895, 821, 784 cm−1. HRMS (ESI-TOF): calc’d for C22H34OS2 [M+H+] 379.2124, found 379.2140. Optical rotation: [α]D20 (c 5.0, CHCl3) = +1.4°, taken on a 91% ee sample.
4.2.10. Dimered enone 85
During the formation of enone 42 from bromodiene 11 and vinylcyclohexenone 56, varying amounts of a bis-addition product has been observed, sometimes not at all, sometimes in large quantities of ~20% yield. This product has been characterized and was found to have the structure shown in Figure 20. Appearance: Yellow oil. TLC: Rf = 0.28–0.31 (silica gel, 1:1 EtOAc/hexanes). 1H NMR (400 MHz, CDCl3): δ 5.85 (s, 1 H), 5.82 (s, 1 H), 4.99 (dq, J = 2.9, 1.4 Hz, 1 H), 4.54 (dq, J = 2.7, 0.9 Hz, 1 H), 2.40–2.30 (m, 4 H), 2.30–2.20 (m, 7 H), 2.10–2.00 (m, 2 H), 2.00–1.90 (m, 4 H), 1.73 (dd, J = 1.4, 0.9 Hz, 3 H), 1.68 (s, 3 H), 1.66 (s, 3 H), 1.65 (m, 1 H), 1.57 (m, 1 H) ppm. 13C NMR (151 MHz, CDCl3): δ 199.9, 199.8, 168.4, 165.7, 145.3, 133.9, 128.0, 127.0, 125.7, 114.9, 46.2, 37.8, 37.4, 36.0, 34.6, 30.0, 29.1, 27.2, 23.0, 22.8, 22.6, 22.2, 20.4 ppm. IR (neat): ν = 3073, 2931, 2866, 1663, 1621, 1453, 1427, 1372, 1345, 1324, 1252, 1190, 1132, 964, 886 cm−1. HRMS (ESI-TOF): calc’d for C23H32O2 [M+H+] 341.2475, found 341.2485.
4.3. X-ray crystallographic data
Crystallographic data for (+)-10, (−)-10, (−)-61 and (−)-86 have been deposited with the Cambridge Crystallographic Data Centre. Copies of the data can be obtained free of charge from http://www.ccdc.cam.ac.uk/products/csd/request/ with CCDC # 837815 for (+)-10, # 932623 for (−)-10, # 932622 for (−)-61, and # 840165 for (−)-86.
Acknowledgements
We thank D.-H. Huang and L. Pasternack for assistance in NMR spectroscopy, G. Siuzdak for assistance in mass spectrometry, A. Rheingold (UCSD) for assistance in X-ray crystallography, and M. Wasa and J.-Q. Yu for chiral HPLC assistance. We thank N. Wilde for valuable technical assistance. We particularly thank G. Stephanopoulos (MIT) for providing a bioengineered sample of taxadiene. Financial support for this work was provided by the NIH/NIGMS (GM-097444), NSERC (doctoral fellowship for Y. I.), MEC-Fulbright program (postdoctoral fellowship for A. M.), and Bristol–Myers Squibb (unrestricted research support).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.a) Isolation of Taxol® (1): Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. b) Isolation of taxusin: Miyazaki M, Shimizu K, Mishima H, Kurabayashi M. Chem. Pharm. Bull. 1968;16:546–548. c) Isolation of taxadiene (8): Koepp AE, Hezari M, Zajicek J, Vogel BS, LaFever RE, Lewis NG, Croteau R. J. Biol. Chem. 1995;270:8686–8690. doi: 10.1074/jbc.270.15.8686.. For selected reviews on Taxol® (1), see: Nicolaou KC, Dai W-M, Guy RK. Angew. Chem. Int. Ed. 1994;33:15–44. Kingston DGI, Jagtap PG, Yuan H, Samala L. Prog. Chem. Org. Nat. Prod. 2002;84:53–225. doi: 10.1007/978-3-7091-6160-9_2.. For selected reviews in the isolation of taxane natural products, see: Miller RW. J. Nat. Prod. 1980;43:425–437. Baloglu E, Kingston DGI. J. Nat. Prod. 1999;62:1448–1472. doi: 10.1021/np990176i. Shigemori H, Kobayashi J. J. Nat. Prod. 2004;67:245–256. doi: 10.1021/np030346y. Shi Q-W, Kiyota H. Chem. Biodivers. 2005;2:1597–1623. doi: 10.1002/cbdv.200590131. Wang Y-F, Shi Q-W, Dong M, Kiyota H, Gu Y-C, Cong B. Chem. Rev. 2011;111:7652–7709. doi: 10.1021/cr100147u.
- 2.Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 3. For a debate on synthetic biology versus synthetic chemistry, see: Keasling JD, Mendoza A, Baran PS. Nature. 2012;492:188–189. doi: 10.1038/492188a.
- 4. The total synthesis of Taxol® (1) has been achieved seven times (along with one formal synthesis). Total synthesis by K. C. Nicolaou: Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, Claiborne CF, Renaud J, Couladouros EA, Paulvannan K, Sorensen EJ. Nature. 1994;367:630–634. doi: 10.1038/367630a0. Nicolaou KC, Nantermet PG, Ueno H, Guy RK, Couladouros EA, Sorensen EJ. J. Am. Chem. Soc. 1995;117:624–633. Nicolaou KC, Liu JJ, Yang Z, Ueno H, Sorensen EJ, Claiborne CF, Guy RK, Hwang CK, Nakada M, Nantermet PG. J. Am. Chem. Soc. 1995;117:634–644. Nicolaou KC, Yang Z, Liu JJ, Nantermet PG, Claiborne CF, Renaud J, Guy RK, Shibayama K. J. Am. Chem. Soc. 1995;117:645–652. Nicolaou KC, Ueno H, Liu JJ, Nantermet PG, Yang Z, Renaud J, Paulvannan K, Chadha R. J. Am. Chem. Soc. 1995;117:653–659.. Total synthesis by R. A. Holton: Holton RA, Somoza C, Kim HB, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S. J. Am. Chem. Soc. 1994;116:1597–1598. Holton RA, Kim HB, Somoza C, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S. J. Am. Chem. Soc. 1994;116:1599–1600.. Total synthesis by S. J. Danishefsky: Masters JJ, Link JT, Snyder LB, Young WB, Danishefsky SJ. Angew. Chem. Int. Ed. 1995;34:1723–1726. Danishefsky SJ, Masters JJ, Young WB, Link JT, Snyder LB, Magee TV, Jung DK, Isaacs RCA, Bornmann WG, Alaimo CA, Coburn CA, Di Grandi MJ. J. Am. Chem. Soc. 1996;118:2843–2859.. Total synthesis by P. A. Wender: Wender PA, Badham NF, Conway SP, Floreancig PE, Glass TE, Gränicher C, Houze JB, Jänichen J, Lee D, Marquess DG, McGrane PL, Meng W, Mucciaro TP, Mühlebach M, Natchus MG, Paulsen H, Rawlins DB, Satkofsky J, Shuker AJ, Sutton JC, Taylor RE, Tomooka K. J. Am. Chem. Soc. 1997;119:2755–2756. Wender PA, Badham NF, Conway SP, Floreancig PE, Glass TE, Houze JB, Krauss NE, Lee D, Marquess DG, McGrane PL, Meng W, Natchus MG, Shuker AJ, Sutton JC, Taylor RE. J. Am. Chem. Soc. 1997;119:2757–2758.. Total synthesis by T. Mukaiyama: Shiina I, Saitoh K, Fréchard-Ortuno I, Mukaiyama T. Chem. Lett. 1998;27:3–4. Mukaiyama T, Shiina I, Iwadare H, Saitoh M, Nishimura T, Ohkawa N, Sakoh H, Nishimura K, Tani Y-i, Hasegawa M, Yamada K, Saitoh K. Chem. Eur. J. 1999;5:121–161.. Total synthesis by I. Kuwajima: Morihira K, Hara R, Kawahara S, Nishimori T, Nakamura N, Kusama H, Kuwajima I. J. Am. Chem. Soc. 1998;120:12980–12981. Kusama H, Hara R, Kawahara S, Nishimori T, Kashima H, Nakamura N, Morihira K, Kuwajima I. J. Am. Chem. Soc. 2000;122:3811–3820.. Total synthesis by Y. shi, only described in a PhD. thesis by his graduate student: Lim J. PhD. Dissertation. Harvard University; 2000. . Formal synthesis by T. Doi: Doi T, Fuse S, Miyamoto S, Nakai K, Sasuga D, Takahashi T. Chem. Asian J. 2006;1:370–383. doi: 10.1002/asia.200600156.
- 5.The total synthesis of a less oxidized taxane, taxusin, has been accomplished three times. Total synthesis by R. A. Holton: Holton RA, Juo RR, Kim HB, Williams AD, Harusawa S, Lowenthal RE, Yogai S. J. Am. Chem. Soc. 1988;110:6558–6560.. Total synthesis by I. Kuwajima: Hara R, Furukawa T, Horiguchi Y, Kuwajima I. J. Am. Chem. Soc. 1996;118:9186–9187. Hara R, Furukawa T, Kashima H, Kusama H, Horiguchi Y, Kuwajima I. J. Am. Chem. Soc. 1999;121:3072–3082.. Total synthesis by L. A. Paquette: Paquette LA, Zhao M. J. Am. Chem. Soc. 1998;120:5203–5212. Paquette LA, Wang H-L, Su Z, Zhao M. J. Am. Chem. Soc. 1998;120:5213–5225.
- 6.The total synthesis of the least oxidized natural taxane, taxadiene (8), has only been reported once prior to our own work in reference 12: R. M. Williams: Rubenstein SM, Williams RM. J. Org. Chem. 1995;60:7215–7223.
- 7.Many syntheses of the taxane skeleton have been reported, and selected reports are shown here. Synthesis efforts by K. J. Shea: Shea KJ, Davis PD. Angew. Chem. Int. Ed. 1983;22:419–420. Shea KJ, Gilman JW, Haffner CD, Dougherty TK. J. Am. Chem. Soc. 1986;108:4953–4956. Shea KJ, Haffner CD. Tetrahedron Lett. 1988;29:1367–1370. Jackson RW, Higby RG, Gilman JW, Shea KJ. Tetrahedron. 1992;48:7013–7032. Jackson RW, Shea KJ. Tetrahedron Lett. 1994;35:1317–1320.. Synthesis efforts by J. D. Winkler: Winkler JD, Kim HS, Kim S. Tetrahedron Lett. 1995;36:687–690. Winkler JD, Holland JM, Peters DA. J. Org. Chem. 1996;61:9074–9075. Winkler JD, Kim HS, Kim S, Ando K, Houk KN. J. Org. Chem. 1997;62:2957–2962. doi: 10.1021/jo961620e.. Synthesis efforts by A. G. Fallis: Tjepkema MW, Wilson PD, Wong T, Romero MA, Audrain H, Fallis AG. Tetrahedron Lett. 1995;36:6039–6042. Fallis AG. Pure Appl. Chem. 1997;69:495–500. Tjepkema MW, Wilson PD, Audrain H, Fallis AG. Can. J. Chem. 1997;75:1215–1224. Forgione P, Wilson PD, Yap GPA, Fallis AG. Synthesis. 2000:921–924. Villalva-Servín NP, Laurent A, Yap GPA, Fallis AG. Synlett. 2003:1263–1266. Laurent A, Villalva-Servín NP, Forgione P, Wilson PD, Smil DV, Fallis AG. Can. J. Chem. 2004;82:215–226. Villalva-Servín NP, Laurent A, Fallis AG. Can. J. Chem. 2004;82:227–239.. Synthesis efforts by P. Magnus: Frost C, Linnane P, Magnus P, Spyvee M. Tetrahedron Lett. 1996;37:9139–9142. Magnus P, Westwood N, Spyvee M, Frost C, Linnane P, Tavares F, Lynch V. Tetrahedron. 1999;55:6435–6452.. Synthesis efforts by G. Pattenden: Hitchcock SA, Houldsworth SJ, Pattenden G, Pryde DC, Thomson NM, Blake AJ. J. Chem. Soc. Perkin Trans. 1998;1:3181–3206.. Synthesis efforts by A. J. Phillips: Phillips AJ, Morris JC, Abell AD. Tetrahedron Lett. 2000;41:2723–2727.. Synthesis efforts by P. R. Jenkins: Brown PA, Jenkins PR. J. Chem. Soc. Perkin Trans. 1986;1:1303–1309. Bonnert RV, Jenkins PR. J. Chem. Soc. Perkin Trans. 1989;1:413–418.
- 8.Chen K, Baran PS. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 9.For selected reviews in C–H activation, see: Shilov AE, Shul'pin GB. Chem. Rev. 1997;97:2879–2932. doi: 10.1021/cr9411886. Crabtree RH. J. Chem. Soc., Dalton Trans. 2001:2437–2450. Jia C, Kitamura T, Fujiwara Y. Acc. Chem. Res. 2001;34:633–639. doi: 10.1021/ar000209h. Dick AR, Sanford MS. Tetrahedron. 2006;62:2439–2463. Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. Gutekunst WR, Baran PS. Chem. Soc. Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. Newhouse T, Baran PS. Angew. Chem. Int. Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. Wencel-Delord J, Droge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666.
- 10.Chen K, Ishihara Y, Morón Galán M, Baran PS. Tetrahedron. 2010;66:4738–4744. [Google Scholar]
- 11.Ishihara Y, Baran PS. Synlett. 2010:1733–1745. [Google Scholar]
- 12.Mendoza A, Ishihara Y, Baran PS. Nat. Chem. 2012;4:21–25. doi: 10.1038/nchem.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Paquette LA, Zon G. J. Am. Chem. Soc. 1974;96:224–233. [Google Scholar]; b) Belmont DT, Paquette LA. J. Org. Chem. 1985;50:4102–4107. [Google Scholar]; c) Reichel CJ, Bryson TA. Heterocycles. 1982;18:277–280. [Google Scholar]; d) Munslow WD, Reusch W. J. Org. Chem. 1982;47:5096–5099. [Google Scholar]; e) Bose G, Barua PMB, Chaudhuri MK, Kalita D, Khan AT. Chem. Lett. 2001;30:290–291. [Google Scholar]; f) Ramanarayanan GV, Shukla VG, Akamanchi KG. Synlett. 2002:2059–2061. [Google Scholar]; g) Jyothi D, HariPrasad S. Synlett. 2009:2309–2311. [Google Scholar]; h) Johnson CR, Adams JP, Braun MP, Senanayake CBW, Wovkulich PM, Uskokovic MR. Tetrahedron Lett. 1992;33:917–918. [Google Scholar]; i) Sha C-K, Huang S-J. Tetrahedron Lett. 1995;36:6927–6928. [Google Scholar]; j) Benhida R, Blanchard P, Fourrey J-L. Tetrahedron Lett. 1998;39:6849–6852. [Google Scholar]; k) Rafferty RJ, Williams RM. J. Org. Chem. 2012;77:519–524. doi: 10.1021/jo202139k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Rathke MW. Org React. 1975;22:423–460. [Google Scholar]; b) Gaudemar M. Tetrahedron Lett. 1983;24:2749–2752. [Google Scholar]; c) Inoue M, Sasaki M, Tachibana K. J. Org. Chem. 1999;64:9416–9429. [Google Scholar]; d) Gabriel T, Wessjohann L. Tetrahedron Lett. 1997;38:1363–1366. [Google Scholar]; e) Pettit GR, Grealish MP. J. Org. Chem. 2001;66:8640–8642. doi: 10.1021/jo010530t. [DOI] [PubMed] [Google Scholar]; f) Lambert TH, Danishefsky SJ. J. Am. Chem. Soc. 2005;128:426–427. doi: 10.1021/ja0574567. [DOI] [PubMed] [Google Scholar]
- 15.Krenske EH, Perry EW, Jerome SV, Maimone TJ, Baran PS, Houk KN. Org. Lett. 2012;14:3016–3019. doi: 10.1021/ol301083q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellville DJ, Wirth DW, Bauld NL. J. Am. Chem. Soc. 1981;103:718–720. [Google Scholar]
- 17.Cain CM, Cousins RPC, Coumbarides G, Simpkins NS. Tetrahedron. 1990;46:523–544. [Google Scholar]
- 18.a) Majetich G, Nishide H, Phillips RM, Yu J. Heterocycles. 2007;74:225–231. [Google Scholar]; b) Petersson MJ, Marchal C, Loughlin WA, Jenkins ID, Healy PC, Almesåker A. Tetrahedron. 2007;63:1395–1401. [Google Scholar]
- 19.a) Corey EJ, Naef R, Hannon FJ. J. Am. Chem. Soc. 1986;108:7114–7116. [Google Scholar]; b) Feringa BL, Pineschi M, Arnold LA, Imbos R, de Vries AHM. Angew. Chem. Int. Ed. 1997;36:2620–2623. [Google Scholar]; c) Feringa BL. Acc. Chem. Res. 2000;33:346–353. doi: 10.1021/ar990084k. [DOI] [PubMed] [Google Scholar]; d) Alexakis A, Rosset S, Allamand J, March S, Guillen F, Benhaim C. Synlett. 2001:1375–1378. [Google Scholar]; e) Alexakis A, Polet D, Rosset S, March S. J. Org. Chem. 2004;69:5660–5667. doi: 10.1021/jo049359m. [DOI] [PubMed] [Google Scholar]; f) d’Augustin M, Palais L, Alexakis A. Angew. Chem. Int. Ed. 2005;44:1376–1378. doi: 10.1002/anie.200462137. [DOI] [PubMed] [Google Scholar]; g) Martin D, Kehrli S, d’Augustin M, Clavier H, Mauduit M, Alexakis A. J. Am. Chem. Soc. 2006;128:8416–8417. doi: 10.1021/ja0629920. [DOI] [PubMed] [Google Scholar]; h) Vuagnoux-d’Augustin M, Alexakis A. Chem. Eur. J. 2007;13:9647–9662. doi: 10.1002/chem.200701001. [DOI] [PubMed] [Google Scholar]; i) Hird AW, Hoveyda AH. J. Am. Chem. Soc. 2005;127:14988–14989. doi: 10.1021/ja0553811. [DOI] [PubMed] [Google Scholar]; j) Lee K-s, Brown MK, Hird AW, Hoveyda AH. J. Am. Chem. Soc. 2006;128:7182–7184. doi: 10.1021/ja062061o. [DOI] [PubMed] [Google Scholar]; k) May TL, Brown MK, Hoveyda AH. Angew. Chem. Int. Ed. 2008;47:7358–7362. doi: 10.1002/anie.200802910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebel H, Marcoux J-F, Molinaro C, Charette AB. Chem. Rev. 2003;103:977–1050. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]; b) Kakei H, Sone T, Sohtome Y, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2007;129:13410–13411. doi: 10.1021/ja076797c. [DOI] [PubMed] [Google Scholar]
- 21.Vázquez A, Williams RM. J. Org. Chem. 2000;65:7865–7869. doi: 10.1021/jo005537+. [DOI] [PubMed] [Google Scholar]
- 22.a) Hadjiarapoglou L, de Meijere A, Seitz H-J, Klein I, Spitzner D. Tetrahedron Lett. 1994;35:3269–3272. [Google Scholar]; b) Mash EA, Baron JA, Gregg TM, Nimkar SK. Tetrahedron. 1998;54:2669–2682. [Google Scholar]; c) Mash EA, Baron JA. J. Org. Chem. 1999;64:7412–7418. [Google Scholar]
- 23.a) Kobayashi S, Hachiya I. Tetrahedron Lett. 1992;33:1625–1628. [Google Scholar]; b) Kobayashi S, Hachiya I. J. Org. Chem. 1994;59:3590–3596. [Google Scholar]; c) Kobayashi S, Hachiya I, Yamanoi Y. Bull. Chem. Soc. Jpn. 1994;67:2342–2344. [Google Scholar]
- 24.Sierra MA, de la Torre MC. Dead Ends and Detours. 1st ed. Wiley-VCH; Weinheim: 2005. p. 290. [Google Scholar]
- 25.Flack HD, Bernardinelli G. Chirality. 2008;20:681–690. doi: 10.1002/chir.20473. [DOI] [PubMed] [Google Scholar]
- 26.A bioengineered sample of taxadiene (8) was obtained from Professor Stephanopoulos (MIT) as a kind gift; see the following reference for his manuscript: Ajikumar PK, Xiao W-H, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G. Science. 2010;330:70–74. doi: 10.1126/science.1191652.
- 27.Furrow ME, Myers AG. J. Am. Chem. Soc. 2004;126:5436–5445. doi: 10.1021/ja049694s. [DOI] [PubMed] [Google Scholar]
- 28.a) Boar RB, Joukhadar L, McGhie JF, Misra SC, Barrett AGM, Barton DHR, Prokopiou PA. J. Chem. Soc. Chem. Commun. 1978:68–69. [Google Scholar]; b) Deshayes H, Pete J-P. J. Chem. Soc. Chem. Commun. 1978:567–568. [Google Scholar]; c) Hansson L, Carlson R. Acta Chem. Scand. 1992;46:103–107. [Google Scholar]; d) Yasuda M, Onishi Y, Ueba M, Miyai T, Baba A. J. Org. Chem. 2001;66:7741–7744. doi: 10.1021/jo0158534. [DOI] [PubMed] [Google Scholar]
- 29.Scheiper B, Bonnekessel M, Krause H, Fürstner A. J. Org. Chem. 2004;69:3943–3949. doi: 10.1021/jo0498866. [DOI] [PubMed] [Google Scholar]
- 30.a) Hoffmann RW. Synthesis. 2006:3531–3541. [Google Scholar]; b) Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]; c) Young IS, Baran PS. Nat. Chem. 2009;1:193–205. doi: 10.1038/nchem.216. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Horiguchi T, Croteau R, Williams RM. Tetrahedron. 2008;64:6561–6567. doi: 10.1016/j.tet.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]