Abstract
K2 or Spice is an emerging drug of abuse that contains synthetic cannabinoids, including JWH-018 and JWH-073. Recent reports indicate that monohydroxylated metabolites of JWH-018 and JWH-073 retain high affinity and activity at cannabinoid type-1 receptors (CB1Rs), potentially contributing to the enhanced toxicity of K2 compared to marijuana. Since the parent compounds also bind to cannabinoid type-2 receptors (CB2Rs), this study investigated the affinity and intrinsic activity of JWH-018, JWH-073 and several monohydroxylated metabolites at human CB2Rs (hCB2Rs). The affinity of cannabinoids for hCB2Rs was determined by competition binding studies employing CHO-hCB2 membranes. Intrinsic activity of compounds was assessed by G-protein activation and adenylyl cyclase (AC)-inhibition in CHO-hCB2 cells. JWH-073, JWH-018 and several of their human metabolites exhibit nanomolar affinity and act as potent agonists at hCB2Rs. Furthermore, a major omega hydroxyl metabolite of JWH-073 (JWH-073-M5) binds to CB2Rs with 10-fold less affinity than the parent molecule, but unexpectedly, is equipotent in regulating AC-activity when compared to the parent molecule. Finally, when compared to CP-55,940 and Δ9-tetrahydrocannabinol (Δ9-THC), JWH-018, JWH-018-M5 and JWH-073-M5 require significantly less CB2R occupancy to produce similar levels of AC-inhibition, indicating that these compounds may more efficiently couple CB2Rs to AC than the well characterized cannabinoid agonists examined. These results indicate that JWH-018, JWH-073 and several major human metabolites of these compounds exhibit high affinity and demonstrate distinctive signaling properties at CB2Rs. Therefore, future studies examining pharmacological and toxicological properties of synthetic cannabinoids present in K2 products should consider potential actions of these drugs at both CB1 and CB2Rs.
Keywords: Drug abuse, Drug metabolism, K2, Synthetic cannabis, Spice, Δ9-Tetrahydrocannabinol
Introduction
K2 has emerged as a very popular drug of abuse that is heavily marketed to young teens and first-time drug users as “legal marijuana” (Vardakou et al., 2010; Seely et al., 2011). Various formulations of K2 products are sold at “head shops” and internet sites under the brand names of Spice, Spice Dream, or Yucatan Fire (Auwarter et al., 2009). Most K2 preparations consist of inert plant materials laced with a mixture of several synthetic cannabinoid compounds possessing psychoactive properties similar to those produced by Δ9-tetrahydrocannabinol (Δ9-THC) found in marijuana. Δ9-THC produces psychotropic actions by activating CB1 cannabinoid receptors (CB1Rs) in the CNS (Fujiwara and Egashira, 2004). Although structurally distinct from Δ9-THC, the synthetic compounds found in K2 products are derivatives of the well characterized aminoalkylindole (AAI) chemical class of ligands that also bind and activate CB1Rs (Manera et al., 2008). Therefore, the abuse liability of both Δ9-THC and K2-aminoalkylindoles (K2-AAIs) results from their ability to potently and efficaciously activate CB1Rs. As many as 10 different K2-AAIs are reported to be present in various K2 preparations, but two compounds that are commonly detected are JWH-018 [1-pentyl-3-(1-naphthoyl)indole)] and JWH-073 [1-butyl-3-(1-naphthoyl)indole)] (Vardakou et al., 2010).
Although controversial, marijuana has been used medicinally for centuries (Zuardi, 2006). In marked contrast, virtually no research has evaluated the safety or efficacy of any K2-AAI and several reports from Europe and the U.S. suggest that the clinical effects of K2 products are not only distinct from those caused by marijuana, but also pose significant health risks. For example, unlike effects typically observed with marijuana, some K2 smokers experience extreme paranoia, hallucinations, agitation, anxiety, seizures, elevated blood pressure and even death (Auwarter et al., 2009; Zimmermann et al., 2009; Every-Palmer, 2010; Muller et al., 2010; Vardakou et al., 2010). Therefore, understanding the basic pharmacological and toxicological properties of K2-AAIs is needed to provide insight into potential mechanisms underlying the unique adverse effect profile of these compounds. Such information should also aid in development of efficacious pharmacotherapies to reduce the serious consequences following use of these dangerous designer drugs of abuse.
Our laboratory has recently reported an important and unusual characteristic of K2-AAI metabolism that may contribute to the distinct clinical profile of the K2 products. Specifically, phase I metabolism of JWH-018 and JWH-073 produces several monohydroxylated metabolites that not only retain high nanomolar binding affinity for CB1Rs, but also exhibit a range of intrinsic activity from neutral antagonism to full agonism (Brents et al., 2011; Brents et al., 2012). These observations are unexpected given that Δ9-THC metabolism results in production of only a single major active metabolite (11-OH-Δ9-THC) with reduced CB1R affinity (Kochanowski and Kala, 2005).
In addition to acting as agonists at CB1Rs (Atwood et al., 2010; Atwood et al., 2011), JWH-018 and JWH-073 are known to bind with high affinity to CB2Rs, the second major cannabinoid receptor subtype (Chin et al., 1999; Aung et al., 2000). In contrast to CB1Rs, CB2Rs are expressed in highest density outside the CNS on immune cells (Klein et al., 2003) and regulate many important physiological processes ranging from inflammation to bone formation (Patel et al., 2010). Although also present in relatively low numbers in the brain and/or spinal cord, increasing evidence indicates that activation of CB2Rs in the CNS modulates the addictive properties of several drugs of abuse, including cocaine (Xi et al., 2011), alcohol (Onaivi et al., 2008) and nicotine (Gamaleddin et al., 2012). Since metabolism of JWH-073 and JWH-018 unexpectedly produces metabolites retaining significant affinity and activity at CB1Rs, it is important to determine if the parent compounds and/or metabolites of these drugs might be active at CB2Rs as well.
Although JWH-018 and JWH-073 have been reported to act as agonists at CB1Rs (Atwood et al., 2010; Atwood et al., 2011; Brents et al., 2011; Brents et al., 2012), no studies to date have examined the pharmacological properties of these K2 synthetic cannabinoids or their metabolites at CB2Rs. Therefore, the purpose of this study was to determine the affinity and intrinsic activity of JWH-018, JWH-073 and several of their major phase I hydroxylated metabolites at human CB2Rs. We report that JWH-018, JWH-073 and several of their human metabolites exhibit high affinity and demonstrate distinctive signaling properties at CB2Rs. These results indicate that future studies examining pharmacological and toxicological properties of synthetic cannabinoids present in K2 products should consider potential actions of these drugs at both CB1 and CB2Rs.
Methods
Cell Culture
Chinese hamster ovary (CHO) cells stably expressing human CB2Rs (CHO-hCB2) or human mu-opioid receptors (CHO-hMOR), generated in our laboratory (Shoemaker et al., 2005a) were cultured in DMEM medium containing 10% fetal calf serum, 100 units/ml penicillin, 100mg/ml streptomycin and 0.5 mg/ml geneticin (G418) in a humidified atmosphere of 5% CO2, 95 % air at 37°C. Cells from passages 5–15 were used in all experiments.
Membrane Preparation
CHO-hCB2 cells were homogenized in ice-cold buffer (50 mM Hepes, pH 7.4, 3 mM MgCl2, and 1 mM EGTA) by 10 strokes employing a 40 ml Dounce glass homogenizer as detailed elsewhere (Shoemaker et al., 2005b). In brief, samples were centrifuged at 40,000 × g for 10 min at 4°C and homogenized similarly twice more. Samples were resuspended in Hepes buffer (50 mM, pH 7.4), aliquoted and stored at −80°C. Protein concentration was determined by employing the BCA™ Protein Assay (Thermo Scientific, Rockford, IL).
Competition Receptor Binding Assays
Competition receptor binding assays were conducted as previously described (Shoemaker et al., 2007; Brents et al., 2012). Briefly, the cannabinoid agonist [3H]CP-55,940 (0.2 nM) and the competing non-radioactive ligands were allowed to equilibrate for 90 min at room temperature, in 1ml of binding buffer (50mM TRIS and 0.05% BSA), containing 25 µg of CHO-hCB2 homogenates and 5 mM MgCl2. Non-specific binding was defined by inclusion of 10 µM of the high affinity cannabinoid ligand WIN-55,212-2. After incubation, assay mixes were filtered through glass fiber filters, washed five times and radioactivity determined by liquid scintillation spectrophotometry.
[35S]GTPγS Binding Assay
[35S]GTPγS binding assays were performed as described previously (Shoemaker et al., 2007) in a buffer containing 20 mM Hepes, 100 mM NaCl, 10 mM MgCl2, 20 units/L adenosine deaminase and 0.05% BSA at pH 7.4. Each binding reaction contained 25 µg of CHO-hCB2 homogenates, cannabinoid ligands, 0.1 nM [35S]GTPγS and 10 µM of GDP. Non-specific binding was defined by the addition of 10 µM of non-radioactive GTPγS. Samples were incubated for 30 min at 30°C, rapidly filtered through glass fiber filters and radioactivity quantified by liquid scintillation spectrophotometry.
Adenylyl Cyclase (AC) assay
CHO-hCB2 cells were plated in (17mm) 24 well plates at a density of 4 × 106 cells per plate and cultured for 24 hrs prior to the assay as detailed elsewhere (Shoemaker et al., 2005b). On the day of the assay, cells were preincubated for 6 hrs at 37°C in DMEM containing 0.9% NaCl, 500 µM 3-isobutyl-1-methyl xanthine and 2.5 µCi/ml of [3H]adenine. The pre-incubation mix was removed and cannabinoid ligands were added for 15 min in a Krebs-Ringer-Hepes solution (10 mM Hepes, 110 mM NaCl, 25 mM Glucose, 55 mM Sucrose, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, pH 7.4) containing 3-isobutyl-1-methyl xanthine and 30 µM forskolin. Reactions were terminated by adding 50 µl 2.2N HCl. [3H]cAMP was isolated by alumina column chromatography and radioactivity quantified by liquid scintillation spectrophotometry.
Materials
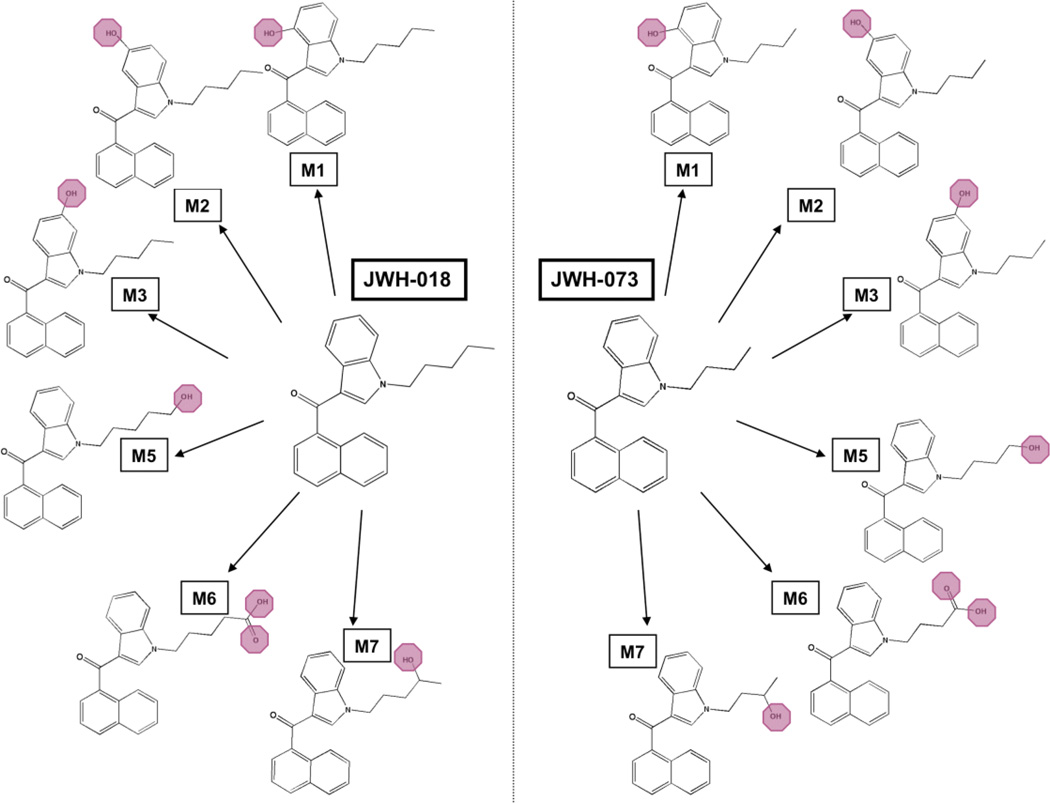
Dulbeco’s modified eagle’s medium (DMEM), penicillin/streptomycin (10,000 IU/mL and 10,000 µg/mL) and geneticin (G418) were purchased from Fisher Scientific (Pittsburg, PA). Fetal calf serum was obtained from Gemini Bioproducts (West Sacramento, CA). GTPγS and GDP were purchased from EMD Chemical (Gibbstown, NJ), and Sigma Aldrich (St. Louis, MO), respectively. Cayman Chemical (Ann Arbor, MI) synthesized and verified the structures of JWH-018, JWH-073 and their respective metabolites (M1–M7, Figure 1) through mass spectrometry and NMR. Δ9-THC was supplied by the National Institute on Drug Abuse (NIDA, Bethesda, MD). CP-55,940, DAMGO and morphine were obtained from Tocris Biosciences (Ellisville, MO). [3H]CP-55,940 (144 Ci/mmole) was purchased from PerkinElmer (Waltham, MA), [3H]adenine (26 Ci/mmole) was procured from Vitrax (Placenia, CA) and [35S]GTPγS (1250 Ci/mmole) was obtained from American Radiolabeled Chemicals (St. Louis, MO).
Figure 1. Structures of synthetic cannabinoids and metabolites examined in the present study.
Major metabolites of the synthetic cannabinoids JWH-018 and JWH-073 are monohydroxylated around the indole ring (M1, M2, and M3), monohydroxylated on the alkyl chain (M5 and M7), or monocarboxylated on the alkyl chain (M6). The metabolites oxidized on the alkyl chain (M5–M7) are of particular interest due to their confirmed presence as major urinary metabolites produced after use of JWH-018 and/or JWH-073 (Chimalakonda et al., 2011).
Statistical Analysis
Curve fitting and statistical analyses were performed using GraphPad Prism (version 4.0b, GraphPad Software Inc., San Diego, CA). Non-linear regression for one-site competition was used to determine the IC50 for competition receptor binding. The Cheng-Prusoff equation (Cheng and Prusoff, 1973) was used to convert the experimental IC50 values to Ki values, a quantitative measure of receptor affinity. Curve fitting of concentration-effect curves via non-linear regression was also employed to determine the IC50 (a measure of potency) and Imax (a measure of efficacy) for all AC experiments. Data are expressed as mean ± SEM, calculated from a minimum of 3 replicates. A one-way ANOVA, followed by Dunnett’s or Tukey’s post-hoc test, was used to determine statistical significance (P<0.05) between three or more groups. When only two groups were compared, the Student’s t-test was employed, with (P<0.05) as the minimal level of significance. A one-sample t-test was used to determine whether the amount of G-protein activation or AC-inhibition produced by a 10 µM concentration of compounds in CHO-hMOR cells was significantly different than basal levels.
Results
Monohydroxylated Metabolites of K2 Synthetic Cannabinoids Retain High Affinity for CB2Rs
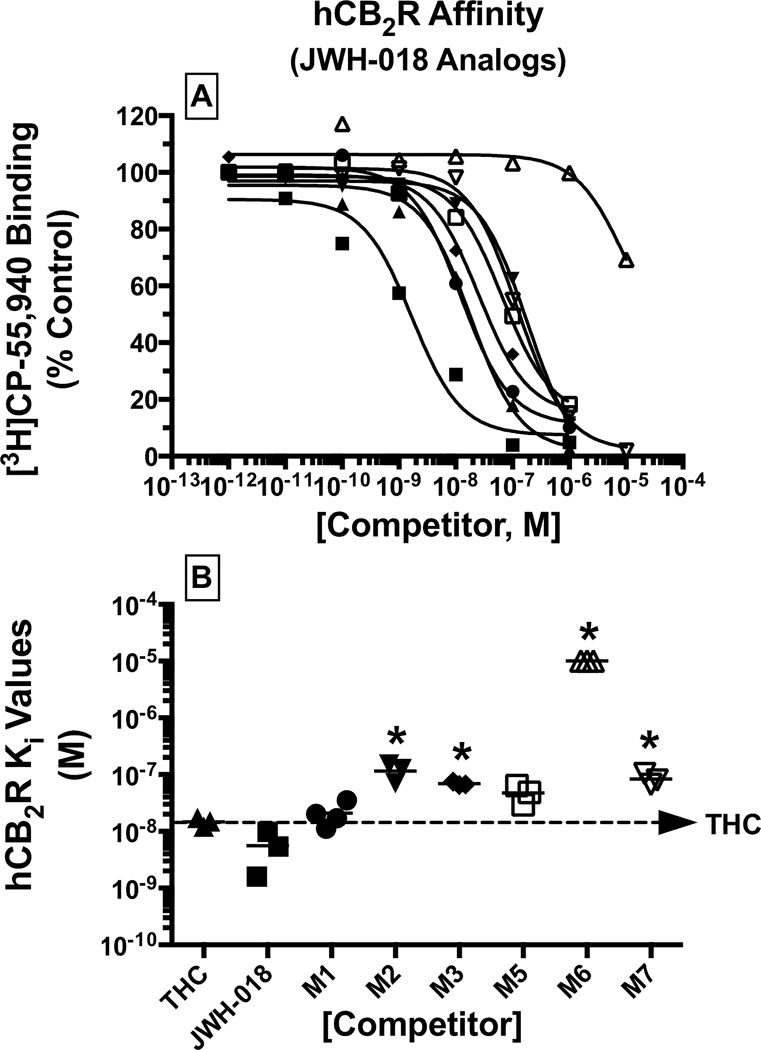
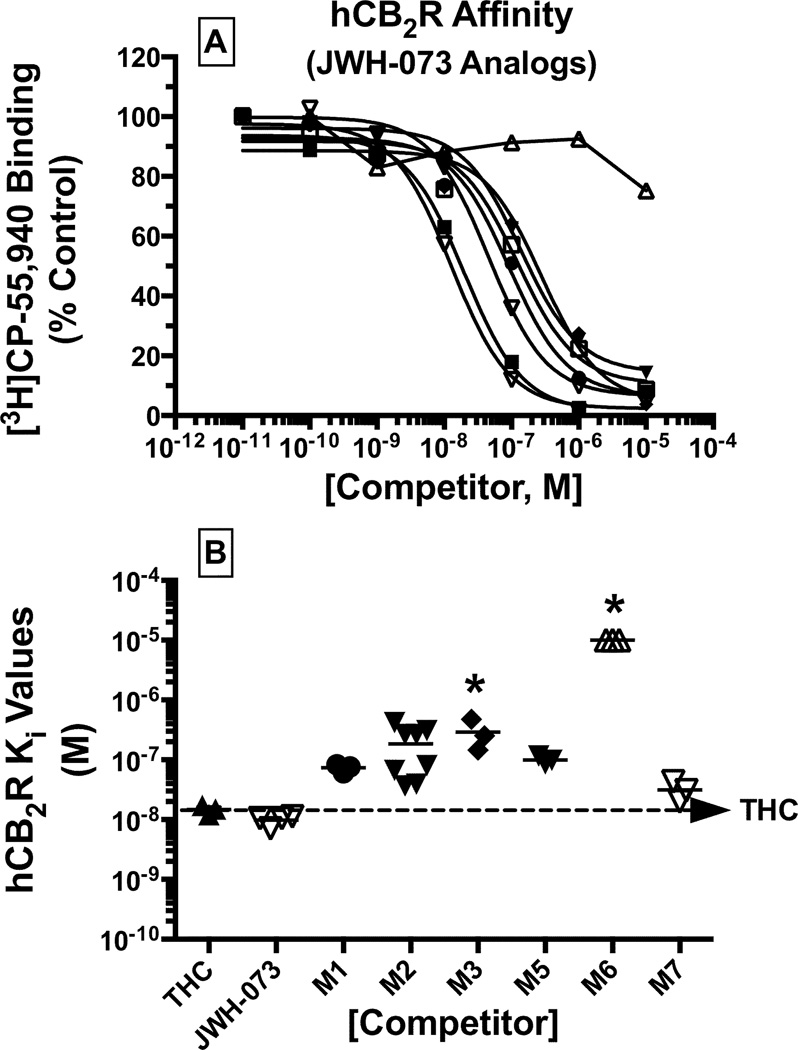
A measure of CB2R affinity (Ki) for each metabolite was derived from the IC50 values obtained from [3H]CP-55,940 competition binding studies (Figures 2A and 3A; Table 1). Monohydroxylated metabolites of JWH-018 bound to CB2Rs with Ki values ranging from 21 nM (M1, high affinity) to 115 nM (M2, intermediate affinity) (Figure 2B; Table 1). The rank order of CB2R affinity for these compounds was JWH-018 ≥ Δ9-THC ≥ M1 ≥ M5 > M3 ≥ M7 > M2 >> M6 (Figure 2). The affinity of the monohydroxylated metabolites of JWH-073 for CB2Rs similarly ranged from Ki values of 31 nM (M7, high affinity) to 291 nM (M3, intermediate affinity) (Figure 3B, Table 1). Interestingly, the rank order of CB2R affinity for the JWH-073 metabolites was slightly different from that of the JWH-018 compounds, specifically with JWH-073 ≥ Δ9-THC ≥ M7 ≥ M1 ≥ M5 ≥ M2 > M3 >> M6 (Figure 3).
Figure 2. Monohydroxylated metabolites of JWH-018 retain nanomolar CB2R affinity.
A measure of CB2R affinity (Ki) for JWH-018 and respective metabolites, was obtained by evaluating competition binding between increasing concentrations of each test compound and the radiolabeled cannabinoid [3H]CP-55,940 in CHO-hCB2 membranes [A]. Ki values (mean ± SEM) are provided in Table 1 [B].
*Significantly different from the Ki value determined for Δ9-THC (P<0.05; ANOVA + Dunnett’s post-hoc comparison).
Figure 3. Monohydroxylated metabolites of JWH-073 retain nanomolar CB2R affinity.
A measure of CB2R affinity (Ki) for JWH-073 and respective metabolites, was obtained by evaluating competition binding between increasing concentrations of each test compound and the radiolabeled cannabinoid [3H]CP-55,940 in CHO-hCB2 membranes [A]. Ki values (mean ± SEM) are provided in Table 1 [B].
*Significantly different from the Ki value determined for Δ9-THC (P<0.05; ANOVA + Dunnett’s post-hoc comparison).
Table 1.
Comparison of CB2R affinity (Ki), potency for AC-Inhibition (IC50) and Fractional Receptor Occupancy (FRO) for 50% AC-Inhibition for JWH-018, JWH-073 and metabolites
| Drug | CB2R Affinity (Ki, nM) |
Potency for AC-Inhibition (IC50, nM) |
FRO for 50% AC-Inhibition (%)a |
|
|---|---|---|---|---|
| CP-55,940 | 0.4 ± 0.2 (3) | 3.3 ± 1.4 (5) | 81.7 ± 6.7 (5) | |
| Δ9-THC | 14.8 ± 1.5 (3) | 57.9 ± 19.8 (5) | 71.7 ± 7.8 (5) | |
| JWH-018 | 5.6 ± 2.4 (3) | 3.6 ± 2.2 (6) | 19.0 ± 5.3 (5) | |
| M1 | 20.9 ± 5.1 (4) | N.D. | N.D. | |
| M2 | 115.1 ± 23.4 (3) | N.D. | N.D. | |
| M3 | 69.3 ± 3.2 (3) | N.D. | N.D. | |
| M5 | 47.4 ± 10.1 (3) | 17.7 ± 5.5 (4) | 25.5 ± 7.2 (4) | |
| M6 | >10,000 (3) | N.D. | N.D. | |
| M7 | 83.7 ± 10.6 (3) | N.D. | N.D. | |
| JWH-073 | 9.8 ± 0.9 (4) | 16.9 ± 1.9 (5) | 62.5 ± 3.0 (5) | |
| M1 | 73.4 ± 6.7 (3) | N.D. | N.D. | |
| M2 | 184.7 ± 51.8 (8) | N.D. | N.D. | |
| M3 | 291.3 ± 96.9 (3) | N.D. | N.D. | |
| M5 | 96.0 ± 7.1 (3) | 13.3 ± 2.0 (3) | 12.1 ± 1.6 (3) | |
| M6 | >10,000 (3) | N.D. | N.D. | |
| M7 | 31.4 ± 6.7 (3) | N.D. | N.D. | |
The data are presented as the mean ± SEM (number of replications)
The formula employed to calculate FRO = [DRUG] / (Ki + [DRUG]) (Tallarida, 1990)
N.D. = Not determined
Monohydroxylated Metabolites of K2 Synthetic Cannabinoids Act as Partial and Full Agonists at CB2Rs to Activate G-proteins in CHO-hCB2 Membranes
To determine the intrinsic activity of JWH-018, JWH-073 and their monohydroxylated metabolites, the ability of each compound to activate G-proteins in CHO-hCB2 membranes was examined (Figure 4, Table 2). To compare the maximal level of efficacy attainable by all compounds, a single receptor-saturating concentration (10 µM) of each drug was examined. At 10 µM, CP-55,940 (a CB1R/CB2R full agonist), JWH-018 (Figure 4A), JWH-073 (Figure 4C), and all of the respective monohydroxylated metabolites examined significantly activated G-proteins above basal levels. The efficacy for G-protein activation varied for individual metabolites and ranged from partial to full agonists (Figure 4, Table 2). There was no statistical difference (p<0.05; ANOVA + Dunnett’s post-hoc test) in the level of G-protein activation produced by the full agonist CP-55,940 and most metabolites tested. The level of G-protein activation ranged from 17.6 to 79.7 fmole/mg, compared to 96.1 ± 1.6 fmole/mg produced by CP-55,940 (Table 2). Two metabolites of JWH-018 (M2 and M7; Figure 4A) and JWH-073 (M1 and M2; Figure 4C) exhibited partial agonist activity, producing significantly less increase in [35S]GTPγS binding when compared to CP-55,940 (Table 2). G-protein activation studies were not conducted for the monocarboxylated M6 metabolites of JWH-018 and JWH-073 because these metabolites lack affinity for CB2Rs (Figures 2 and 3).
Figure 4. Monohydroxylated metabolites of JWH-018 and JWH-073 activate G-proteins via CB2 receptors.
A measure of maximal efficacy (EMAX) for CB2R-mediated G-protein activation produced by JWH-018 [A] and [B], JWH-073 [C] and [D] and respective metabolites, was obtained by evaluating [35S]GTPγS binding produced by a receptor saturating concentration (10 µM) of each test compound in CHO-hCB2 [A] and [C] or CHO-hMOR [B] and [D] membranes. G-protein activation produced by the MOR agonist DAMGO (10 µM) was evaluated as a positive control in CHO-hMOR membranes. The mean ± SEM of [35S]GTPγS binding produced by test compounds is presented as fmole/mg. EMAX values (mean ± SEM) are provided in Table 2.
*Significantly different from G-protein activation produced by CP-55,940 (P<0.05; ANOVA + Dunnett’s post-hoc comparison).
**Significantly different from basal G-protein activity (P<0.05; One sample t-test).
Table 2.
Maximal Efficacy of JWH-018 and JWH-073 metabolites for regulation of G-protein and AC-activity via CB2Rs
| Drug | CB2R Efficacy (EMAX) | ||
|---|---|---|---|
| G-protein Activation (fmole/mg) |
AC-Inhibition (% Inhibition) |
||
| CP-55,940 | 96.1 ± 1.6 (13) | 67.3 ± 3.5 (6) | |
| Δ9-THC | N.D. | 49.2 ± 1.4 (5) | |
| JWH-018 | 60.3 ± 11.4 (7) | 59.3 ± 4.0 (4) | |
| M1 | 48.0 ± 11.3 (5) | 46.9 ± 3.2 (4) | |
| M2 | 38.0 ± 10.4 (6) | 55.1 ± 2.7 (4) | |
| M3 | 79.7 ± 20.4 (6) | 57.7 ± 1.4 (4) | |
| M5 | 38.4 ± 8.5 (5) | 53.5 ± 4.6 (4) | |
| M6 | N.D. | N.D. | |
| M7 | 15.0 ± 4.7 (4) | 62.7 ± 2.5 (5) | |
| JWH-073 | 40.1 ± 4.2 (3) | 62.6 ± 2.7 (3) | |
| M1 | 20.3 ± 3.0 (3) | 49.1 ± 3.8 (4) | |
| M2 | 17.6 ± 5.7 (4) | 55.8 ± 4.9 (4) | |
| M3 | 48.5 ± 3.8 (3) | 56.8 ± 5.7 (4) | |
| M5 | 31.0 ± 1.0 (3) | 54.2 ± 5.9 (4) | |
| M6 | N.D. | N.D. | |
| M7 | 48.2 ± 10.9 (3) | 54.4 ± 6.2 (6) | |
The data are presented as the mean ± SEM (number of replications)
N.D. = Not determined
CB2R specificity was not assessed with selective CB2R antagonist/inverse agonists because three different CB2R antagonists (e.g., AM-630, JTE-907 and SR-144528) acted as inverse agonists, producing concentration-dependent decreases in basal G-protein activity when administered alone (data not shown). Therefore, to determine the CB2R-mediated specificity of G-protein activation by the synthetic cannabinoids examined, the ability of all compounds to modulate [35S]GTPγS binding in homogenates from CHO cells stably expressing human mu-opioid (but not CB2) receptors (Figure 4B and 4D) was examined. Serving as a positive control, the mu-opioid receptor (MOR) agonist DAMGO (10 µM) significantly activated G-proteins in CHO-hMOR homogenates (22 ± 3.0 fmol/mg). Most importantly, a 10 µM concentration of JWH-018 (Figure 4B), JWH-073 (Figure 4D) cannabinoids and all respective metabolites failed to significantly alter G-protein activity in CHO-hMOR membranes not expressing hCB2Rs. These data demonstrate that G-protein activation produced by all synthetic cannabinoids examined in CHO-hCB2 membranes does indeed occur due to selective activation of CB2Rs.
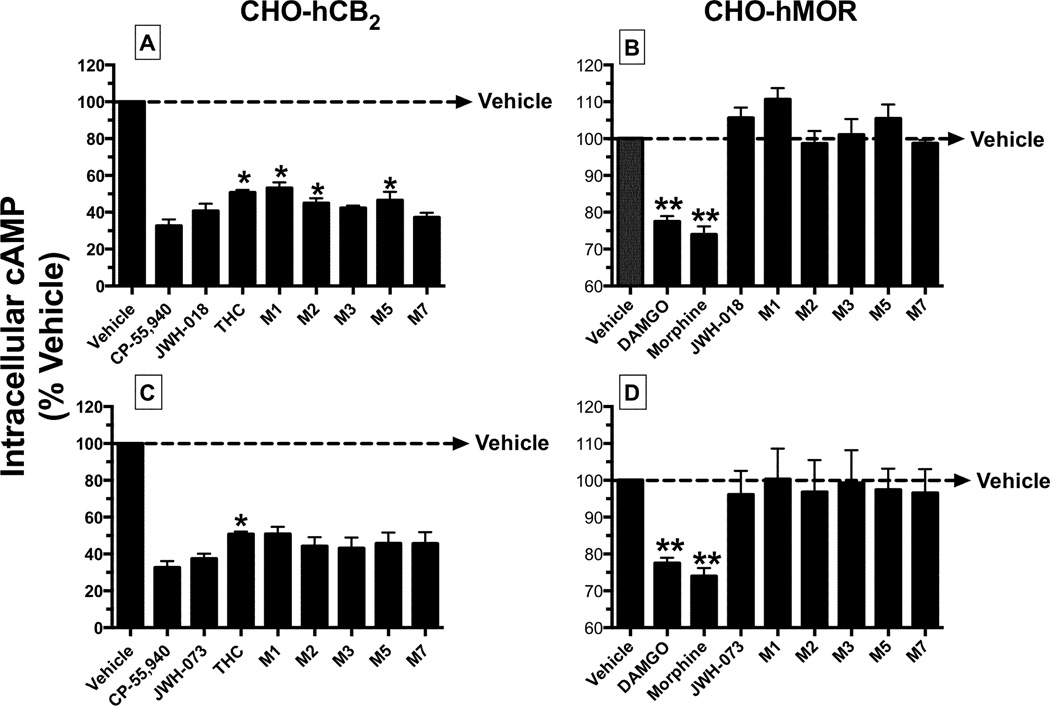
Monohydroxylated Metabolites of K2 Synthetic Cannabinoids Also Act as Partial and Full Agonists at CB2Rs to Regulate Adenylyl Cyclase (AC)-Activity in intact CHO-hCB2 Cells
As a second measure of intrinsic activity, the ability of JWH-018, JWH-073 and metabolites to regulate the activity of AC (a downstream intracellular effector) in intact CHO-hCB2 cells was next examined (Figure 5A and 5C, Table 2). All cannabinoids significantly lowered intracellular cAMP levels when tested at a single receptor-saturating concentration (10 µM) (Figure 5). The maximal efficacy for inhibition of AC-activity produced by the JWH-018 metabolites ranged from partial to full agonism. Specifically, when compared to the full CB1R/CB2R agonist CP-55,940 (67.3 ± 3.5%), all JWH-018 metabolites except M1, M2 and M5 produced equivalent levels of AC-inhibition (Figure 5A, Table 2). Therefore, Δ9-THC, JWH-018-M1, JWH-018-M2 and JWH-018-M5 acted as partial CB2R agonists in this assay. Interestingly, JWH-073 and all of its metabolites examined acted as full agonists in this assay (Figure 5B, Table 2).
Figure 5. Monohydroxylated metabolites of JWH-018 and JWH-073 inhibit forskolin-stimulated adenylyl cyclase (AC)-activity via CB2Rs in intact cells.
A measure of maximal efficacy (EMAX) for CB2R-mediated inhibition of forskolin-stimulated AC-activity produced by JWH-018 [A] and [B], JWH-073 [C] and [D] and respective metabolites, was obtained by evaluating the level of intracellular cAMP levels in response to vehicle or a receptor saturating concentration (10 µM) of each test compound in intact CHO-hCB2 [A] and [C] or CHO-hMOR [B] and [D] cells. AC-inhibition produced by the MOR agonists DAMGO (10 µM) and morphine (10 µM) was evaluated as positive controls in CHO-hMOR cells. The mean ± SEM of AC-inhibition produced by test compounds is presented. EMAX values (mean ± SEM) are provided in Table 2.
*Significantly different from percent of AC-inhibition produced by CP-55,940 (P<0.05; ANOVA + Dunnett’s post-hoc comparison).
**Significantly different from basal cAMP levels (P<0.05; One sample t-test).
As noted previously for [35S]GTPγS binding experiments, all CB2R antagonists examined similarly exhibited inverse agonist activity in this assay, producing significant elevations of intracellular cAMP levels above basal levels when tested alone (data not shown). Therefore, CB2R specificity was demonstrated by examining the ability of all compounds to modulate AC-activity in CHO-hMOR cells (Figure 5B and 5D). Although a 10 µM concentration of both MOR agonists DAMGO and morphine significantly inhibited AC-activity, JWH-018 (Figure 5B), JWH-073 (Figure 5D) and all respective metabolites failed to alter intracellular cAMP levels in intact CHO-hMOR cells not expressing hCB2Rs.
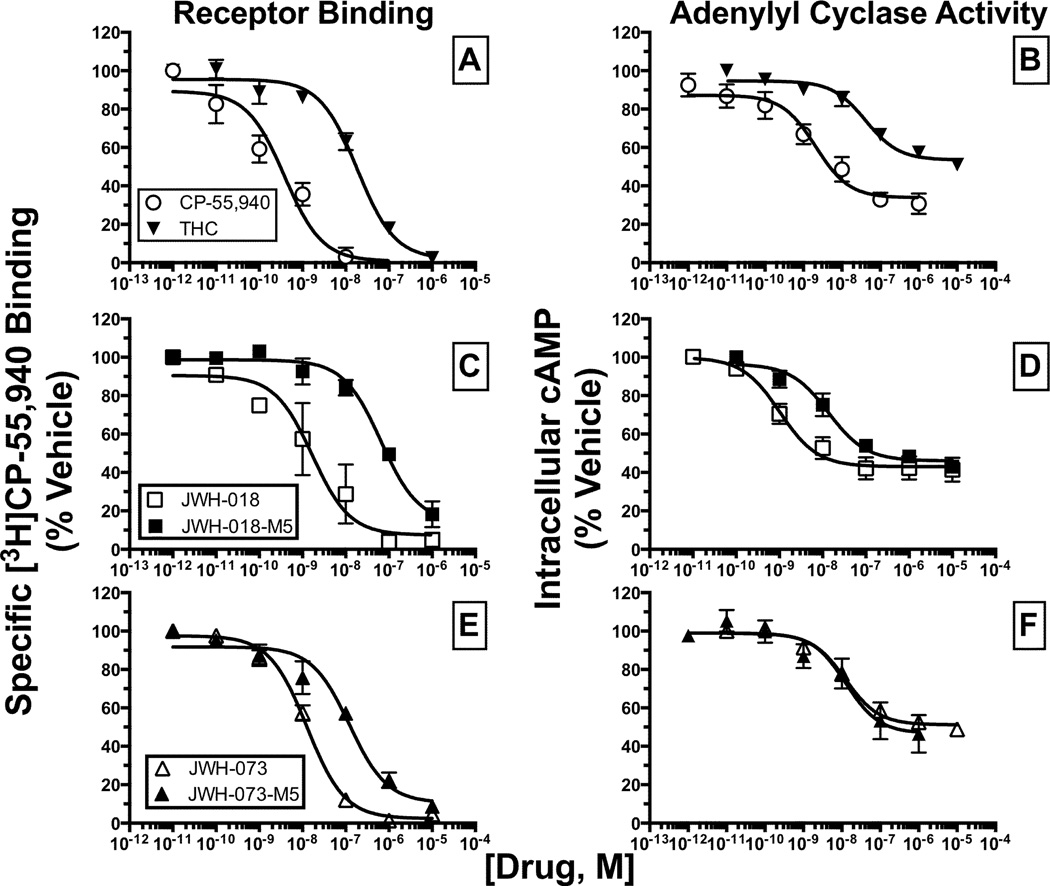
In addition to efficacy, the potency with which JWH-018, JWH-073 and the M5 metabolite of each synthetic cannabinoid to regulate AC-activity in CHO-hCB2 cells was examined (Figure 6B, 6D and 6F). The omega monohydroxylated (M5) metabolites of JWH-018 and JWH-073 were chosen for these studies because these molecules are reported as major circulating metabolites found in human blood (Chimalakonda et al., 2011). The full CB1R/CB2R agonist CP-55,940 and partial CB1R/CB2R agonist Δ9-THC produced a concentration-dependent reduction in intracellular cAMP levels in intact CHO-hCB2 cells with high potency (IC50 = 3.3 and 57.9 nM, respectively; Figure 6B, Table 1). JWH-018, JWH-073 and each respective M5 metabolite also potently inhibited AC-activity with IC50 values ranging from 19.0 to 63.3 nM (Figure 6D and 6F, Table 1).
Figure 6. The omega hydroxyl metabolite (M5) of JWH-073 produces equipotent AC-inhibition compared with its parent, despite having 10-fold lower affinity for CB2Rs.
The affinity (Ki) of cannabinoids for CB2Rs [A], [C] and [E] determined by receptor binding, were compared with the respective potency (IC50) for inhibition of AC-activity [B], [D] and [F] in CHO-hCB2 cells. All Ki and IC50 values (mean ± SEM) were derived from non-linear regression analysis of the curves presented and specific values are provided in Table 1.
As anticipated, the relative rank order of potency for CP-55,940 and Δ9-THC to regulate AC-activity (3.3 versus 58.9 nM, respectively; Figure 6B, Table 1) was consistent with the rank order of affinity of these two cannabinoids for CB2Rs (0.4 versus 14.8 nM, respectively; Figure 6A). Similarly, the affinity of the M5 metabolite of JWH-018 for CB2Rs was reduced approximately 10-fold relative to the parent compound (5.6 versus 47.4 nM, respectively; Figure 6C), and this was reflected by an almost 5-fold lower potency to regulate AC-activity (3.6 versus 17.7 nM, respectively; Figure 6D). In marked contrast, although the M5 metabolite of JWH-073 also bound to CB2Rs with 10-fold less affinity than the parent molecule (9.8 vs 96.0 nM, respectively; Figure 6E, Table 1), it unexpectedly was equipotent in regulating AC-activity when compared to JWH-073 (16.9 vs 13.3 nM, respectively; Figure 6F).
K2 Synthetic Cannabinoids and Metabolites Require Significantly Less Fractional Receptor Occupancy (FRO) of CB2Rs to Produce Similar Levels of AC-Inhibition Than Well Characterized Cannabinoids
To quantify potential differences in coupling efficiency, the fractional receptor occupancy (FRO) required to produce half-maximal regulation of AC-activity (e.g., the FRO at AC-IC50) was determined for selected cannabinoids (Figure 7, Table 1). Our laboratory has previously used differences in FRO to quantify agonist-directed-trafficking of response produced by endocannabinoids acting at CB2Rs (Shoemaker et al., 2005b). The law of mass action predicts that, at equilibrium, the FRO is the proportion of all receptors that are bound by drug at a given concentration (Tallarida, 1990). FRO is calculated using the following formula: FRO = [DRUG] / (Ki + [DRUG]). Based on these assumptions, it would be predicted that half of the receptors are occupied when the concentration of a ligand employed is equal to the Ki of the drug being investigated. Applying this concept to quantify CB2R coupling to AC, it was observed that the established CB2R cannabinoid agonists CP-55,940 and Δ9-THC required similar, but near full occupancy of CB2Rs to produce half-maximal regulation of AC-activity in intact CHO-hCB2 cells (81.7 and 71.7%, respectively; Figure 7, Table 1). In marked contrast, JWH-018 and its M5 metabolite required only approximately 20% CB2R occupancy to produce similar levels of AC-inhibition (Figure 7). Finally, although JWH-073 required a level of CB2R occupancy to inhibit AC-activity more similar to that of CP-55,940 and Δ9-THC (e.g., 62.5%, Table 1), the hydroxylated JWH-073-M5 metabolite required activation of remarkably fewer CB2Rs (e.g., 12.1%, Table 1) to produce levels of AC-inhibition similar to that of its parent molecule.
Figure 7. JWH-018, JWH-018-M5 and JWH-073-M5 require significantly less fractional receptor occupancy (FRO) of CB2Rs to produce half-maximal inhibition of AC than established cannabinoids.
The law of mass action predicts that, at equilibrium, the FRO is the proportion of all receptors that are bound by drug at a given concentration (Tallarida, 1990). FRO is calculated using the following formula: FRO = [DRUG] / (Ki + [DRUG]). Applying this concept to quantify CB2R coupling to AC, the fractional receptor occupancy (FRO) required to produce half-maximal regulation of AC-activity was determined for the indicated cannabioids. All FRO values (mean ± SEM) are presented in Table 1.
a,bBars identified with different letters are significantly different (P≤0.05, ANOVA + Tukey’s post-hoc test to compare all treatment combinations).
Discussion
The present study provides the first in vitro characterization of JWH-018, JWH-073 and several phase I hydroxylated metabolites of each at CB2Rs. We report that the parent compounds and several metabolites of these K2 synthetic cannabinoids exhibit high affinity, act as potent agonists, and demonstrate distinct signaling properties at CB2Rs. Therefore, future studies examining pharmacological and toxicological properties of synthetic cannabinoids present in K2 products should consider potential actions of these drugs at both CB1 and CB2Rs.
The first novel finding reported by this study is the observation that both JWH-073 and JWH-018 act as full agonists at human CB2Rs. It has been known for over a decade that JWH-018 and JWH-073 bind to CB1 and CB2Rs with high affinity (Chin et al., 1999; Aung et al., 2000). However, since these initial studies, very little novel information has been reported concerning the cellular and/or molecular mechanisms that mediate the actions of these common synthetic cannabinoids found in K2 products. Due to the recent emergence of abuse of K2 products (Vardakou et al., 2010; Seely et al., 2011), new reports have demonstrated that both JWH-018 (Atwood et al., 2010; Brents et al., 2011) and JWH-073 (Atwood et al., 2011; Brents et al., 2012) act as potent and efficacious agonists at CB1Rs. These findings were expected based on the reported abuse liability of these compounds (Lindigkeit et al., 2009). While this limited knowledge concerning the action of JWH-018 and JWH-073 at CB1Rs is useful, no information other than receptor binding affinity (Chin et al., 1999; Aung et al., 2000) is available concerning the action of these synthetic cannabinoids at CB2Rs. To address the action of these synthetic cannabinoids at CB2Rs, the first set of experiments in this study examined the affinity and intrinsic activity of these compounds at human CB2Rs stably expressed in CHO cells. Both compounds bind with high affinity to CB2Rs and demonstrate intrinsic activity similar to that of the full CB1/CB2 agonist CP-55,940, when either activation of G-proteins or regulation of the intracellular effector AC was evaluated. Therefore, similar to other well characterized cannabinoids such as CP-55-940, WIN-55,512-2 and HU-210 (Pertwee et al., 2010), JWH-018 and JWH-073 also act as high affinity, non-selective, full agonists at both CB1 and CB2Rs.
A second significant observation of the present study is that several phase I hydroxylated JWH-018 and JWH-073 metabolites examined exhibit a range of intrinsic activity at hCB2Rs, acting as partial to full agonists. Our laboratory has recently reported that several phase I hydroxylated metabolites of both JWH-018 and JWH-073 unexpectedly retain high affinity and activity for CB1Rs (Brents et al., 2011; Brents et al., 2012), suggesting that formation of potent, biologically active metabolites may contribute to the distinct actions of these compounds when compared to Δ9-THC. In the present study, the second series of experiments were conducted to determine the affinity and intrinsic activity of several human metabolites of JWH-018 and JWH-073 at CB2Rs. It was found that all but one of these phase I hydroxylated metabolites of JWH-018 and JWH-073 bind with high nanomolar affinity, activate G-proteins and inhibit AC-activity via CB2Rs. As such, similar to previously reported observations with CB1Rs (Brents et al., 2011; Brents et al., 2012), human metabolites of synthetic cannabinoids found in K2 products also bind with high affinity and act as potent agonists at CB2Rs receptors.
A third noteworthy result detailed in the current study is an observation of distinct signaling properties exhibited by JWH-073-M5, a major metabolite of JWH-073. Specifically, although M5 expectedly binds with approximately 10-fold less affinity than the parent JWH-073 molecule, this metabolite surprisingly retains potency for regulation of AC-activity similar to that of JWH-073. Since M5 also exhibits high affinity for CB2Rs (e.g., Ki = 96.0 nM), sufficient formation of M5 in target tissues could result in additive effects with other synthetic cannabinoids present. Interestingly, this scenario seems plausible because the omega monohydroxylated (M5) metabolite of both JWH-018 and JWH-073 are major metabolites excreted in human urine conjugates after use of the parent compounds (Chimalakonda et al., 2011).
A fourth important finding of this study is that the “efficiency” by which classical and synthetic cannabinoids couple CB2Rs to the intracellular effector AC might be different. Initial evidence to support this hypothesis was provided by comparison of the fractional receptor occupancy (e.g., FRO) required for regulation of AC-activity by K2 synthetic cannabinoids, when compared to established CB2 agonists. For example, when CB2R affinity (e.g., Ki value) of individual compounds was qualitatively compared to the potency of these same compounds to regulate AC-activity (e.g., IC50 value), it was observed that the concentration of the well characterized cannabinoids CP-55,940 and Δ9-THC required to produce half-maximal inhibition of AC-activity (e.g.., 3.3 and 57.9 nM, respectively; Table 1) was significantly greater than Ki values calculated for these ligands for CB2Rs (e.g., 0.4 and 14.8 nM, respectively). In marked contrast, this relationship appeared to be reversed when examining similar measures for the synthetic cannabinoids. For example, the IC50 values (e.g., 3.6 – 17.7 nM) for the synthetic cannabinoids examined were similar, or in most cases lower, than their respective Ki values (e.g., 5.6 – 96.0 nM). These observations suggest that several K2 synthetic cannabinoids may more efficiently couple CB2Rs to regulation of AC-activity than well characterized classical cannabinoid agonists.
To provide quantification for hypothesized potential differences in coupling efficiency, we compared the FRO (Tallarida, 1990) required for regulation of AC-activity by K2 synthetic cannabinoids, relative to that required by other well established cannabinoids. Our laboratory has used this method previously to demonstrate agonist-directed-trafficking of response (ADTR) by endocannabinoids acting at CB2Rs (Shoemaker et al., 2005b). The data reported in the present study show that JWH-018, JWH-018-M5 and JWH-073-M5 all require occupancy of significantly fewer hCB2Rs to produce equivalent levels of AC-activity regulation, when compared to CP-55,940 or Δ9-THC. This suggests that these K2 synthetic cannabinoids may more “efficiently” couple hCB2Rs to AC-regulation, and/or other effectors, when compared to classical or non-classical cannabinoids. It should be noted that the FRO values determined empirically here may not fully reflect the absolute FRO values occurring in vivo due to differences in conditions employed for the receptor binding and AC assays. However, since all drugs in this study were evaluated using the same conditions in each assay, comparison of relative FRO values between drugs examined here should nevertheless provide measures that allow accurate comparisons of receptor-effector coupling. Interestingly, similar to data obtained here for K2 synthetic cannabinoids, the endocannabinoids 2-glycerol and noladin ether also require occupancy of significantly fewer CB2Rs to regulate AC-activity than CP-55,950 (Shoemaker et al., 2005b). Although the mechanism(s) responsible for potential differences in coupling efficiency are unknown, it is possible that individual K2 synthetic cannabinoids preferentially bind to and activate distinct CB2R conformations (Console-Bram et al., 2012) that are different from those bound by classical cannabinoids, that then transduce signals to intracellular effectors with varying degrees of efficiency.
When all significant findings of this study are considered collectively, the data suggest that acute and/or chronic exposure to the K2 synthetic cannabinoids with distinct signaling characteristics reported here might produce several previously unappreciated effects mediated by action at CB2Rs. First, due to the high affinity and “efficiency” of coupling of K2 synthetic cannabinoids when compared to Δ9-THC, relatively low doses of these compounds might produce prolonged CB2R-mediated effects, resulting from formation of metabolites that retain affinity and activity similar to that of the parent compounds. Interestingly, exhaustion and difficulties in thinking persist in users for as long as 24-hours after initial K2 exposure (Auwarter et al., 2009), suggesting that metabolites of K2 synthetic cannabinoids shown to be active at CB1Rs (Brents et al., 2011; Brents et al., 2012) may also retain biological activity for prolonged periods of time. These previous reports, coupled with the present observations, indicate that use of K2 synthetic cannabinoids may result in sustained activation of not only CB1Rs, but CB2Rs as well. However, these suggestions cannot be confirmed until results from comprehensive pharmacokinetic studies of JWH-018, JWH-073 and their metabolites are known.
Due to high expression of CB2Rs in a variety of immune cell types (Galiegue et al., 1995), a second unanticipated consequence of K2 use may be modulation of immune function. Prolonged marijuana use is associated with immune-suppression (Klein et al., 2003; Klein and Cabral, 2006) and chronic cannabis use has been reported to result in failure to mount an appropriate immune response to certain viruses (Huemer et al., 2007). Such effects on the immune system produced by Δ9-THC has been suggested to occur via activation of CB2Rs (McKallip et al., 2002). In addition to these potential negative consequences on immune function, activation of CB2Rs can also result in pronounced anti-inflammatory actions (Klein, 2005) and thus selective CB2R ligands are being developed for several potential therapeutic uses (Guindon and Hohmann, 2008). For example, CB2-selective cannabinoid agonists markedly improve symptoms associated with several inflammatory diseases including endotoxic shock (Mathison et al., 2004), athlerosclerosis (Steffens et al., 2005), multiple sclerosis (Walter and Stella, 2004), Alzheimer’s disease (Benito et al., 2003; Ramirez et al., 2005) and amyotrophic lateral sclerosis (Shoemaker et al., 2007). Therefore, future studies may be needed to determine whether prolonged activation of CB2Rs by long-term K2 synthetic cannabinoid abuse produces unanticipated consequences on immune system function.
Finally, several recent studies demonstrate that although CB2Rs are expressed in relatively low numbers within the CNS, they appear to nevertheless modulate addictive properties of several drugs of abuse, including cocaine (Xi et al., 2011), alcohol (Onaivi et al., 2008) and nicotine (Gamaleddin et al., 2012). Most reports indicate that activation of centrally located CB2Rs decreases abuse related behaviors (Morales and Bonci, 2012). However, abuse of high efficacy, long-acting K2 synthetic cannabinoids for extended periods of time might result in compensatory CB2R down-regulation and desensitization (Howlett, 2005), potentially resulting in an unanticipated increase in drug use. In any case, because these drugs are rarely abused alone (Seely et al., 2011), the potential for chronic exposure to K2 synthetic cannabinoids to alter the development, expression and/or maintenance of dependence resulting from concurrent use of marijuana, other synthetic cannabinoids and additional drugs of abuse should be considered.
Highlights.
-
➢
JWH-018 and JWH-073 are common synthetic cannabinoids present in abused K2 products.
-
➢
JWH-018, JWH-073 and their human metabolites have high affinity for CB2 receptors.
-
➢
JWH-018, JWH-073 and their human metabolites are potent agonists at CB2 receptors.
-
➢
JWH-018, JWH-073 and their human metabolites exhibit distinct CB2 signaling properties.
-
➢
Studies of JWH-018 and JWH-073 should consider actions at both CB1 and CB2 receptors.
Acknowledgments
This research was supported in part by the Association of Public Health Laboratories [Grant Innovations in Quality Public health Laboratory Practice] (JHM), the Centers for Disease Control [Contract 200-2007-21729] (JHM), an award from the University of Arkansas for Medical Sciences Translational Research Institute, which is funded by the National Center for Research Resources [1 UL 1RR029884, Curtis Lowery, PI, JHM and PLP, Co-I].
Abbreviations
- AC
adenylyl cyclase
- BSA
bovine serum albumin
- CB1R
cannabinoid type-1 receptor
- CB2R
cannabinoid type-2 receptor
- CHO-hCB2
CHO cells expressing human CB2Rs
- Δ9-THC
delta-9-tetrahydrocannabinol
- FRO
fractional receptor occupancy
- GDP
guanosine diphosphate
- GTPγS
guanosine 5'-O-[gamma-thio]triphosphate
- GPCR
G-protein coupled receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors declare that they have no financial or personal conflicts of interest that influenced, or could be perceived to have influenced, this work.
References
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of 'Spice' herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br. J. Pharmacol. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. CP47,497-C8 and JWH073, commonly found in '0Spice' herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur. J. Pharmacol. 2011;659:139–145. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60:133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. 'Spice' and other herbal blends: harmless incense or cannabinoid designer drugs? J. Mass Spectrom. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J. Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Mono-hydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem. Pharmacol. 2012;83:952–961. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:c21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, Fifer EK, Lapoint J, Nelson LS, Hoffman RS, James LP, Radominska-Pandya A, Moran JH. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal. Chem. 2011;83:6381–6388. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CN, Murphy JW, Huffman JW, Kendall DA. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J. Pharmacol. Exp. Ther. 1999;291:837–844. [PubMed] [Google Scholar]
- Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: nomenclature and pharmacological principles. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105:1859–1860. doi: 10.1111/j.1360-0443.2010.03119.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Egashira N. New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application. J. Pharmacol. Sci. 2004;96:362–366. doi: 10.1254/jphs.fmj04003x2. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One. 2012;7:e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Huemer HP, Himmelreich A, Honlinger B, Pavlic M, Eisendle K, Hopfl R, Rabl W, Czerny CP. "Recreational" drug abuse associated with failure to mount a proper antibody response after a generalised orthopoxvirus infection. Infection. 2007;35:469–473. doi: 10.1007/s15010-007-6194-9. [DOI] [PubMed] [Google Scholar]
- Klein T, Newton C, Larsen K, Lu L, Perkins I, Liang N, Friedman H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nature Reviews of Immunology. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J. Neuroimmune Pharmacol. 2006;1:50–64. doi: 10.1007/s11481-005-9007-x. [DOI] [PubMed] [Google Scholar]
- Kochanowski M, Kala M. Tetrahydrocannabinols in clinical and forensic toxicology. Przegl. Lek. 2005;62:576–580. [PubMed] [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T. Spice: a never ending story? Forensic Sci. Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Manera C, Tuccinardi T, Martinelli A. Indoles and related compounds as cannabinoid ligands. Mini Rev. Med. Chem. 2008;8:370–387. doi: 10.2174/138955708783955935. [DOI] [PubMed] [Google Scholar]
- Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br. J. Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Martin BR, Nagarkatti M, Nagarkatti PS. Delta(9)-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J. Pharmacol. Exp. Ther. 2002;302:451–465. doi: 10.1124/jpet.102.033506. [DOI] [PubMed] [Google Scholar]
- Morales M, Bonci A. Getting to the core of addiction: Hooking CB2 receptor into drug abuse? Nat. Med. 2012;18:504–505. doi: 10.1038/nm.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H, Sperling W, Kohrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr. Res. 2010;118:309–310. doi: 10.1016/j.schres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB(2) receptors in health and disease. Curr. Med. Chem. 2010;17:1393–1410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol. Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Prather PL, James LP, Moran JH. Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol. Interv. 2011;11:36–51. doi: 10.1124/mi.11.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J. Pharmacol. Exp. Ther. 2005a;314:868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 2005b;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Seely KA, Reed RL, Crow JP, Prather PL. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J. Neurochem. 2007;101:87–98. doi: 10.1111/j.1471-4159.2006.04346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Control and oscillation in ligand receptor interactions according to the law of mass action. Life Sci. 1990;46:1559–1568. doi: 10.1016/0024-3205(90)90389-9. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol. Lett. 2010;197:157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br. J. Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB receptors modulate cocaine's actions in mice. Nat. Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of "spice gold". Dtsch. Arztebl. Int. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW. History of cannabis as a medicine: a review. Rev. Bras. Psiquiatr. 2006;28:153–157. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]