Abstract
A fundamental role of the mammalian immune system is to eradicate pathogens while minimizing immunopathology. Instigating and maintaining immunological tolerance within the intestine represents a unique challenge to the mucosal immune system. Regulatory T cells are critical for continued immune tolerance in the intestine through active control of innate and adaptive immune responses. Dynamic adaptation of regulatory T-cell populations to the intestinal tissue microenvironment is key in this process. Here, we discuss specialization of regulatory T-cell responses in the intestine, and how a breakdown in these processes can lead to chronic intestinal inflammation.
The gastrointestinal tract is colonized by numerous commensal microbiota. Complex interactions among the microbiota, intestinal cells, and the immune system (including regulatory T cells) maintain this relationship.
The mammalian host harbors a vast and diverse commensal microbiota. The gastrointestinal tract is a site of preferential colonization by commensal organisms, consisting of fungal, viral, and bacterial species. Initial microbial colonization of the host occurs during birth and continues until a stable commensal microbiota is established during childhood (Tannock 2007). Colonization of the gastrointestinal tract is a vital triggering stimuli for maturation of the mucosal immune system, and the presence of a commensal microbiota further benefits the host by providing resistance to invading pathogens and metabolism of dietary components (Macpherson et al. 2005; Hooper et al. 2012). A dynamic molecular dialogue between microbiota and host ensures this colonization occurs as a state of mutualism, the breakdown of which can result in chronic pathologies of the gastrointestinal tract, such as inflammatory bowel diseases (IBD) (Kaser et al. 2010; Maloy and Powrie 2011). Complex interactions between the microbiota, mucosal immune system, and the intestinal tissue cells provide multiple layers of regulation that control intestinal immunity. Here, we focus on the role of regulatory T cells as key components of intestinal homeostasis and discuss how tissue-specific adaptations contribute to their function when patrolling this challenging frontier.
THE GASTROINTESTINAL TRACT
The intestine constitutes a vast network of nonlymphoid and secondary lymphoid tissues, and as such, is home to numerous populations of leukocytes. Reflecting the unique challenge of maintaining intestinal immune tolerance, many of these cellular populations are found to be enriched in, or exclusive to, the intestine. In addition to the array of hematopoietic cell populations critical for immune tolerance within the intestine, the intestinal mucosa is anatomically specialized to promote homeostasis (Hill and Artis 2010; Harrison and Maloy 2011). The intestinal epithelium consists of a single layer of specialized epithelial cell subsets derived from multipotent and highly proliferative Lgr5+ stem cells located within the intestinal crypts. Although the cellular composition of the intestinal epithelium varies with anatomical location, both the colon and small intestine possess populations of absorptive and secretory cells, including enterocytes, colonocytes, goblet, endocrine, and Paneth cells (Wright 2000; Simons and Clevers 2011). Goblet-cell secretion of membrane tethered and soluble mucus components creates not only a viscous protective barrier, but a matrix loaded with secretory IgA and Paneth cell-derived antimicrobial peptides (AMP) to forge a layer impermeable to the majority of intestinal bacteria (Hill and Artis 2010). Pattern-recognition receptors (PRR), including Toll-like receptors (TLR) and Nod-like receptors (NLR), are germ-line-encoded sensors of microbial and host-derived danger signals (Schroder and Tschopp 2010). Tonic PRR-signaling within IEC drives cell-intrinsic proliferation, survival, AMP production and fortification of intercellular tight junctions, limiting bacterial translocation to the lamina propria (Maloy and Powrie 2011). Furthermore, homeostatic PRR-signaling within the intestinal epithelium serves to regulate not only localization but also composition of the microbiota. For example, deficiency in the cytosolic PRR, NLRP6, results in an altered intestinal microbiota with elevated abundance of the bacterial phyla Bacteroidetes (Prevotellaceae) and TM7 (Elinav et al. 2011). NLRP6-deficient mice have increased susceptibility to experimental DSS (dextran sodium sulphate) colitis; elegant cross-fostering and cohousing experiments revealed the colitogenic microbiota to be both vertically and horizontally transmissible. Thus, the intestinal epithelium uses multiple pathways to influence the localization and composition of the microbiota to promote intestinal tolerance. Dysbiosis, an imbalance in the composition of microbial communities, occurs in patients with IBD, although whether this is cause or a consequence of the inflammatory environment remains unclear (Kaser et al. 2010).
ANTIGEN-PRESENTING CELLS PROMOTE INTESTINAL TOLERANCE
In line with important roles in host defense and tolerance, the intestine contains abundant and heterogeneous populations of myeloid antigen-presenting cells (APC). Subsets from distinct developmental origins vary in anatomical localization, phenotype, and function (Coombes and Powrie 2008; Varol et al. 2010). Recent studies of intestinal APC have used the differential expression of CD103 (αE integrin) and CX3CR1 (receptor for Fractalkine [CX3CL1]) to identify two major tissue resident populations, both of which appear to contribute to intestinal tolerance in distinct ways (Varol et al. 2010). Thus, CD103+ dendritic cells (DC) can take up intestinal antigens and following CCR7-dependent migration to the mesenteric lymph node (MLN), initiate T-cell responses with an intestinal tropism through induction of intestinal homing receptors CCR9 and α4β7 (Iwata et al. 2004; Johansson-Lindbom et al. 2005; Jaensson et al. 2008). Under homeostatic conditions, CD103+ DC preferentially promote regulatory T-cell responses (Coombes et al. 2007; Sun et al. 2007; Schulz et al. 2009). However, this function is not hardwired, and as discussed below, is controlled by the intestinal microenvironment providing a mechanism through which the balance between tolerance and immunity can be controlled (Laffont et al. 2010). In addition to effects on T-cell-mediated immunity, CD103+ DC also promote T-cell-independent IgA class switch recombination by intestinal B cells contributing to host defense and intestinal barrier function (Uematsu et al. 2008). In contrast, intestinal CX3CR1+ APC (consisting of dendritic cells and macrophages) are thought to be nonmigratory and poor primers of naïve T cells (Schulz et al. 2009), although this has recently been challenged (Diehl et al. 2013). CX3CR1+ APC accumulate within the lamina propria following microbial colonization where they promote intestinal tolerance through a variety of mechanisms (Niess and Adler 2010). CX3CR1+ APC lie adjacent to the epithelial barrier, and project transepithelial processes into the intestine to sample luminal antigens (Rescigno et al. 2001; Niess et al. 2005). Highly phagocytic in nature, colonic macrophages aid intestinal tolerance through clearance of apoptotic cells and debris, while also acting to expand populations of regulatory T cells (Murai et al. 2009; Hadis et al. 2011). Together the data indicate that lymphoid and tissue dwelling populations of APC collaborate to instigate and maintain a tolerogenic intestinal microenvironment.
Foxp3+ Treg IN INTESTINAL IMMUNE TOLERANCE
The intestine contains a high frequency of regulatory T-cell populations and early studies revealed their importance in preventing intestinal inflammation (Izcue et al. 2009). More recent studies have emphasized the importance of Foxp3 (Forkhead box P3)-expressing populations in particular. Foxp3 is a transcription factor vital for the induction and maintenance of a regulatory T-cell phenotype (Josefowicz et al. 2012a). Mutations in the X-linked gene encoding Foxp3 illustrate the essential role for Foxp3+ Treg cells in immunological tolerance in both mice and humans (Ziegler 2006). The majority of patients with IPEX (immune dysregulation polyendocrinopathy enteropathy X-linked) syndrome bear loss-of-function mutations within the FOXP3 gene and suffer an early onset fatal autoimmunity (Chatila et al. 2000; Bennett et al. 2001; Wildin et al. 2001). Although IPEX syndrome affects many organs and tissues, the intestine is most frequently affected, emphasizing the key role of Foxp3+ Treg cells in establishing and maintaining tolerance within the intestine (Gambineri et al. 2003).
Induction of Foxp3 expression and acquisition of the regulatory T-cell program occurs at both thymic and extrathymic locations. Thymus-derived Foxp3+ Treg cells (tTreg) differentiate in response to high-affinity TCR (T-cell antigen receptor) interactions with MHC (major histocompatibility complex) class II presented self-antigens (Stritesky et al. 2012; Xing and Hogquist 2012). Extrathymic acquisition of Treg function and Foxp3 expression is also dependent on TCR-signaling but also strongly influenced by environmental factors (Bilate and Lafaille 2012). Indeed, there is convincing evidence that the intestinal microenvironment is a preferential site of antigen-induced Treg (iTreg) development, most likely reflecting high immune challenge and the continuous need for active immune suppression to maintain intestinal homeostasis.
Indeed, results in several mouse models of colitis have shown that disruption of regulatory T-cell networks can promote chronic intestinal inflammation in the presence of a triggering intestinal microbiota (Izcue et al. 2009). We have further dissected control of colitis by regulatory T cells using a T-cell transfer model. In this model, adoptive transfer of naïve CD4+ CD45RBHi T cells to a lymphopenic host results in cachexia and severe intestinal inflammation (Powrie et al. 1993). Transfer of Foxp3+ Treg, or originally, populations enriched in regulatory potential (CD45RBLo and then CD45RBLo CD25+) are sufficient to prevent the onset of, or “cure” ongoing, intestinal inflammation in this and other models (Powrie et al. 1993, 1994; Maloy et al. 2003). Development of colitis is dependent on the cytokine IL-23, which functions to promote Th1 and Th17 cell-mediated pathogenic effector responses as well as restrain iTreg differentiation in the intestine (Hue et al. 2006; Izcue et al. 2008; Ahern et al. 2010).
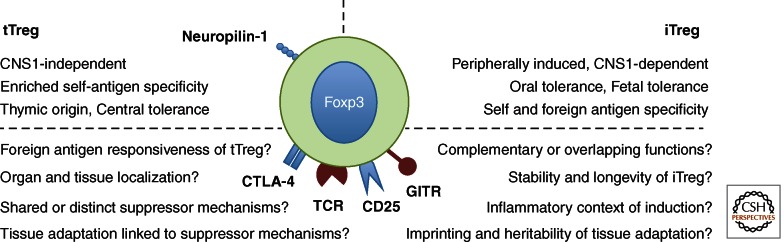
The presence of tTreg and iTreg populations in the intestine raises the question of the functional roles of these populations (Fig. 1). This has been difficult to decipher, as there are few markers that distinguish between these two populations. In vitro activation of mature naive CD4+ T cells in the presence of TGF-β1 and IL-2 is now a commonly used method of Foxp3+ iTreg generation. Although these cells show some facets of Treg function, as assessed in vitro, the genetic composition of these cells is vastly different to those generated in different tissue contexts in vivo (Haribhai et al. 2009; Feuerer et al. 2010). Helios, a putative tTreg marker and Ikaros transcription factor family member, is encoded by Ikzf2, a gene enriched in the Treg cell signature (Hill et al. 2007; Thornton et al. 2010). However, it is now apparent that a substantial proportion of experimentally induced Foxp3+ iTreg can express Helios, and as such, its use as a marker of Treg origin should be employed with caution (Verhagen and Wraith 2010; Gottschalk et al. 2012). In the absence of inflammation, the use of neuropilin-1 as a selective marker of tTreg appears promising; mucosa-derived iTreg are Nrp-1lo compared with their tTreg Nrp-1Hi counterparts (Weiss et al. 2012; Yadav et al. 2012). However, the robustness of neuropilin-1 as a marker of tTreg and its function in this context will require further experimental investigation.
Figure 1.
Properties of Foxp3+ Treg from thymic and extrathymic origins.
Despite these caveats, sophisticated genetic models have provided strong support for collaboration between iTreg and tTreg populations in control of intestinal inflammation. Thus, efficient prevention of T-cell transfer colitis by tTreg cells requires deviation toward an iTreg phenotype by the colitogenic transferred T cells (Haribhai et al. 2009). A nonredundant role for iTreg in maintenance of intestinal tolerance under homeostatic conditions has been shown through a series of elegant genetic manipulations of the Foxp3 locus. Within the Foxp3 locus, conserved noncoding DNA sequences (CNS) promote size, stability, and composition of the Foxp3+ Treg population (Zheng et al. 2010). In vivo, CNS3 deficiency leads to drastically decreased thymic output of Foxp3+ tTreg, and CNS2-dependent mechanisms appear responsible for heritable maintenance of Foxp3 expression in proliferating Treg. Foxp3 CNS1 contains a TGF-β/NFAT response element, and synergistic binding of Smad3 and NFAT at CNS1 are suggested to be required for Foxp3 induction (Tone et al. 2008). In contrast, CNS1-deficient mice have unaffected tTreg populations, but defective induction of Foxp3+ iTreg, particularly within the GALT (gut-associated lymphoid tissue). This highly selective blockade in extrathymic Foxp3+ Treg induction did not lead to fatal autoimmune disorders, nor exacerbated Th1 or Th17 responses in the intestine, as may be expected if there is loss of intestinal immune tolerance. Instead, complete loss of intronic Foxp3 CNS1 manifested as pronounced Th2 responses, primarily at mucosal sites (Josefowicz et al. 2012b). In line with this, aged CNS1-deficient mice develop B-cell-dependent intestinal inflammation with antibodies to food and intestinal antigens, elevated IgE and IgA, and accumulation of Gata3+ CD4+ T cells producing IL-4, IL-5, and IL-13. These data are consistent with earlier studies showing a key role for iTreg cell development in oral tolerance toward nominal antigens and control of allergic responses, and illustrate the importance of immune deviation toward a Foxp3+ Treg program for mucosal tolerance (Curotto de Lafaille et al. 2008). The reason for the deviation toward Th2-mediated pathology in the absence of iTreg may reflect the prevailing environmental conditions, such as, for example, the lack of particular microbial stimuli required to elicit strongly inflammatory Th1/Th17 responses. Indeed, it will be of interest to test whether infection with known colitogenic bacteria elicits exacerbated inflammation in CNS1-deficient mice. Alternatively, it is possible that deficiency in iTreg is not sufficient to drive a pathogenic Th1/Th17 response to the microbiota and that there are multiple layers of regulation, including tTreg cells and IgA among others, that contribute to this important function (Feng et al. 2010).
Together, the above results suggest important roles for both tTreg and iTreg populations in maintaining intestinal homeostasis. Whether there is shared responsibility or specific functional roles of these developmentally distinct populations is not clear. One obvious possibility is that tTreg and iTreg populations contribute different antigen specificities, increasing the frequency of potentially responsive Treg populations that can be activated. It is known that Foxp3+ Treg cells use a broad αβ-TCR repertoire distinct from that of Foxp3− effector T cells (Hsieh et al. 2006; Pacholczyk et al. 2006). Recent studies indicate that colonic Foxp3+ Treg use a TCR repertoire distinct from Foxp3+ Treg found in systemic tissues, suggesting that the local antigenic milieu shapes the colonic Foxp3+ Treg pool (Lathrop et al. 2011). As may be expected, microbial specificities are increased within colonic Foxp3+ Treg populations, and transgenic mice bearing a TCR repertoire derived from colonic Foxp3+ Treg could not select thymic Foxp3+ Treg, suggesting they are specific for exogenous antigens. Furthermore, when T cells bearing these TCR were genetically prevented from adopting a Treg cell fate, they developed colitogenic potential. These data are compatible with the notion that the intestinal microenvironment supports local differentiation of microbe-reactive iTreg as a means of preventing development of inflammatory effector responses. This local adaptation to the intestinal microenvironment provides one layer of control that may be complemented by tTreg cells, some of which may also be reactive with microbial antigens. Indeed, it would be intestinal to determine whether thymectomized germ-free mice also show accumulation of Foxp3+ Treg on colonization. Further understanding of the specificity of intestinal Foxp3 expressing cell populations is required to fully understand the role of these cells in antigen-specific tolerance induction.
TOLEROGENIC MODULES USED BY INTESTINAL Foxp3+ Treg
Although a source of early debate, it is now widely accepted that Foxp3+ Treg cells use multiple regulatory mechanisms to control host immune responses. Thus, production of regulatory cytokines (TGF-β, IL-10, and IL-35), modulation of APC function (LAG-3, CTLA-4, Granzyme/Perforin), and alteration of cellular metabolism (CD25, CD39/CD73, IDO induction in DC), have all been implicated in Treg cell function in distinct inflammatory settings (Vignali et al. 2008; Shevach 2009). We focus briefly on IL-10 and TGF-β as key cytokine pathways necessary for immune tolerance within the intestine.
IL-10, a regulatory cytokine, is produced by multiple leukocyte populations and protects the host by limiting inflammatory responses to microbial challenge (Saraiva and O’Garra 2010). IL-10 signals via a cytokine receptor complex consisting of IL-10Rα and IL-10Rβ subunits, promoting STAT3 activation via Jak1 and Tyk2 (Glocker et al. 2011). In contrast with the fatal consequences of Foxp3 deficiency, genetic ablation of IL-10 or IL-10R leads to colitis in the presence of a triggering microbiota (Kuhn et al. 1993; Davidson et al. 1996; Sellon et al. 1998). Mutations in genes encoding IL-10 or IL-10R subunits are found in patients with very severe early-onset IBD (eo-IBD) and are associated with immune hyperactivation within the intestine (Glocker et al. 2009). Many of these patients are unresponsive to immunosuppressive therapies and require bone marrow transplantation. Recent studies suggest up to 15% of eo-IBD cases are attributable to mutations in the IL-10/IL-10R axis, clearly illustrating the important role of this pathway in intestinal homeostasis (Kotlarz et al. 2012). Single nucleotide polymorphisms in IL10 have also been associated with susceptibility to adult ulcerative colitis and Crohn’s disease, although in this setting the genetic contribution to disease is modest (Franke et al. 2008, 2010; Jostins et al. 2012).
IL-10 production is enriched within mucosal tissues and CD4+ T cells at these sites are an abundant source. Furthermore, both Foxp3+ and Foxp3− populations contribute to the production of IL-10 within the gastrointestinal tract. However, strikingly there is compartmentalization of this response, with high levels of IL-10 production in the colon primarily restricted to Foxp3+ cells and the reverse in the small intestine where Foxp3− cells represent the major source (Uhlig et al. 2006; Maynard et al. 2007). Functional studies support the expression data as the adoptive transfer of IL-10−/− CD4+ T cells to lymphopenic hosts results in severe intestinal inflammation even in the face of host derived IL-10 production, and development of colitis ensues following specific deletion of IL-10 from CD4+ T cells (Davidson et al. 1996; Asseman et al. 1999; Roers et al. 2004). Early studies showed that IL-10-deficient Treg cells could prevent, but not cure, colitis in a T-cell transfer model (Asseman et al. 1999; Uhlig et al. 2006). In a lymphocyte-replete model, deletion of IL-10 from Foxp3+ Treg also leads to intestinal inflammation, albeit with less severity than deletion in total CD4+ T cells, implicating CD4+ Foxp3− cells as a functional source of IL-10 that contributes to intestinal homeostasis (Rubtsov et al. 2008).
HOW DOES IL-10 ACT TO PROMOTE INTESTINAL TOLERANCE?
The IL-10 receptor complex is expressed on innate and adaptive immune cell populations within the intestine and acts to control intestinal CD4+ T cells through direct and indirect mechanisms. Myeloid-specific ablation of STAT3 leads to spontaneous colitis driven by dysregulated CD4+ T-cell responses. In this setting, defective IL-10R signaling in colonic macrophages results in elevated levels of costimulatory molecules CD80/86 and hyperactivation of colitogenic CD4+ T cells (Takeda et al. 1999; Kayama et al. 2012). Direct IL-10R signaling into CD4+ T cells also serves to limit colitogenic responses within the intestine. Intestinal Th17 and Foxp3+ Treg express IL-10Rα, and CD4-specific expression of a dominant-negative IL-10Rα, results in selective expansion of Th17 and IFN-γ/IL-17A coproducing populations during intestinal inflammation (Huber et al. 2011). Under these circumstances, IL-10 production from both Foxp3+ and Foxp3− CD4+ T cells is required to control colitis.
In addition to effects on effector T cells, there is also accumulating evidence that IL-10R signaling into Foxp3+ cells is important for their function. Thus, IL-10Rβ−/− Foxp3+ Treg cells fail to protect mice from intestinal inflammation following naïve T-cell transfer (Murai et al. 2009). In that study, IL-10Rβ−/− Treg cells lost Foxp3 expression and regulatory function following transfer into a lymphopenia-induced disease setting. In a genetic model, specific deletion of IL-10Rα in Foxp3+ Treg cells resulted in spontaneous intestinal inflammation characterized by selective dysregulation of Th17 cells (Chaudhry et al. 2011). However, in that and other lymphocyte replete settings, Foxp3+ Treg cells show remarkable stability and are not observed to lose expression of Foxp3 (Rubtsov et al. 2010; Chaudhry et al. 2011). Whether the loss of Foxp3 expression in lymphopenia-driven colitis represents a failure to maintain the Treg program or is a specific consequence of the cell transfer system requires further study, especially given the interest in Treg cell therapy in the clinical setting. Finally, it should not be overlooked that many other cell types produce IL-10, including B cells and macrophages, and there is evidence that this plays a functional role in intestinal homeostasis (Mizoguchi et al. 2002; Murai et al. 2009; Hadis et al. 2011).
TRANSFORMING GROWTH FACTOR (TGF)-β
The transforming growth factor (TGF)-β family of cytokines have developmental and physiological roles throughout the mammalian lifespan. Encoded by individual genes, the TGF-β family consists of three isoforms, TGF-β1, TGF-β2, and TGF-β3, which signal via the TGF-βR complex (TGF-βRI and TGF-βRII). TGF-βR activation triggers intrinsic kinase activity and phosphorylation of downstream signaling components, of which transcription factors Smad2/3 are an example (Derynck and Zhang 2003). Inhibitory Smads, for example, Smad7, act to negatively regulate TGF-βR signaling, making this a finely tuned system. Further control occurs at the posttranscriptional level as TGF-β is secreted to the cell surface in a latent complex, requiring further posttranslational modification to become biologically active. Activation of the latent TGF-β1 complex requires enzymatic cleavage of latency-associated peptides, or integrin-mediated conformational change. The dominant role for TGF-β1 in immunological tolerance is highlighted by the T-cell-dependent, early onset, fatal autoimmune disease of TGF-β1−/− mice that occurs within weeks of birth (Shull et al. 1992; Diebold et al. 1995; Letterio et al. 1996; Kobayashi et al. 1999). Similar to Foxp3 deficiency, complete ablation of TGF-β1 production, or TGF-βR expression in T cells, leads to catastrophic autoinflammatory disease early after birth. In contrast with complete loss of this pathway, quantitative reductions in mice with impaired but not abolished TGF-β regulatory pathways leads to intestinal inflammation, indicating the intestine is particularly sensitive to TGF-β signaling. Genetic perturbation of Smad signaling, through loss of Smad3/4, or forced expression of Smad7 results in intestinal inflammation, implicating active TGF-β in intestinal tolerance. Likewise, inability to activate latent TGF-β in either T cell or myeloid cells results in loss of intestinal homeostasis. Mice with APC-specific ablation of integrin subunits, αv or β8, or T-cell-specific deletion of convertase furin are prone to development of intestinal inflammation (Lacy-Hulbert et al. 2007; Travis et al. 2007; Yang et al. 2007; Pesu et al. 2008). In addition to the importance of T-cell-produced TGF-β1, it is also crucial that T cells can respond to this cytokine (Li et al. 2006). Naïve T cells unable to respond to TGF-β cannot be controlled by Treg cells (Fahlen et al. 2005); however, whether TGF-β mediates suppression by inhibition of proliferation, acquisition of colitogenic potential, or generation of iTreg cell populations warrants further investigation. Furthermore, the distinct signaling pathways initiated by different TGF-β isoforms remains to be examined in this and multiple other contexts.
Foxp3+ Treg-CELL SPECIALIZATION IN THE INTESTINE
An emerging concept in T-cell biology is that of adaptation to the tissue environment and context-dependent specialization of the adaptive immune response is well documented (Littman and Rudensky 2010). Differentiation of CD4+ T cells to distinct effector populations is dependent on appropriate TCR signaling, costimulation, and surrounding cytokine milieu. Expression of lineage-specifying transcription factors T-bet, Gata3, and ROR(γ)t is required for Th1, Th2, and Th17 responses, respectively (Zheng and Flavell 1997; Szabo et al. 2000; Ivanov et al. 2006). However, expression of these differentiation programs is not mutually exclusive and it is increasingly appreciated that interplay between these factors contributes to the fate and flexibility of effector T-cell responses (Oestreich and Weinmann 2012). As has been described for effector T-cell populations, it is now evident that Foxp3+ Treg can acquire expression of transcription factor modules that allow context-dependent immune suppression (Barnes and Powrie 2009). For example, in response to IFN-γ or IL-27, Foxp3+ Treg cells up-regulate expression of the Th1 cell transcription factor, T-bet (Koch et al. 2009; Hall et al. 2012). T-bet expression in Foxp3+ Treg promotes CXCR3 expression and migration to Th1-inflamed sites facilitating control of the Th1 response (Koch et al. 2009). Equally, the reliance of Th17 cells on STAT3-dependent cytokines for their differentiation is mirrored by the STAT3-dependency of Foxp3+ Treg-mediated control. In that case, targeted deletion of STAT3 (or IL-10Rα, which signals via STAT3) in Foxp3+ Treg lead to severe intestinal inflammation dominated by accumulation of Th17 cells (Chaudhry et al. 2009, 2011).
In contrast with the Th1-context-dependent expression of T-bet in Foxp3+ Treg, the majority of peripheral Foxp3+ Treg express transcription factors IRF4 and/or Gata3, suggesting these transcription factors may play more general roles in Treg cell function (Zheng et al. 2009; Wang et al. 2011; Wohlfert et al. 2011; Rudra et al. 2012). Indeed, the specific loss of IRF4 in Foxp3+ Treg results in an autoimmune syndrome characterized by mucosal Th2 responses, similar in reported phenotype to mice deficient in Foxp3 CNS1 (Zheng et al. 2009). IRF4 seems to be an important pioneer transcription factor involved in multiple T-cell effector phenotypes including Th2, Th17, and Follicular Helper T-cell (TFH) differentiation (Lohoff et al. 2002; Rengarajan et al. 2002; Huber et al. 2008; Bollig et al. 2012; Glasmacher et al. 2012). Based on these findings, IRF4 may play multiple roles in Foxp3+ Treg cells that promote specific modules of suppression. In support of this, Blimp-1 is a regulator of IL-10 production and its expression in Treg is dependent on IRF4 (Cretney et al. 2011). Furthermore, IRF4-deficient Foxp3+ Treg cells are unable to control Th1 and Th17 responses in a T-cell transfer model of colitis (Zheng et al. 2009). Further understanding of the various molecular mechanisms through which IRF4 expression in Foxp3+ Treg cells contributes to mucosal immune tolerance is required.
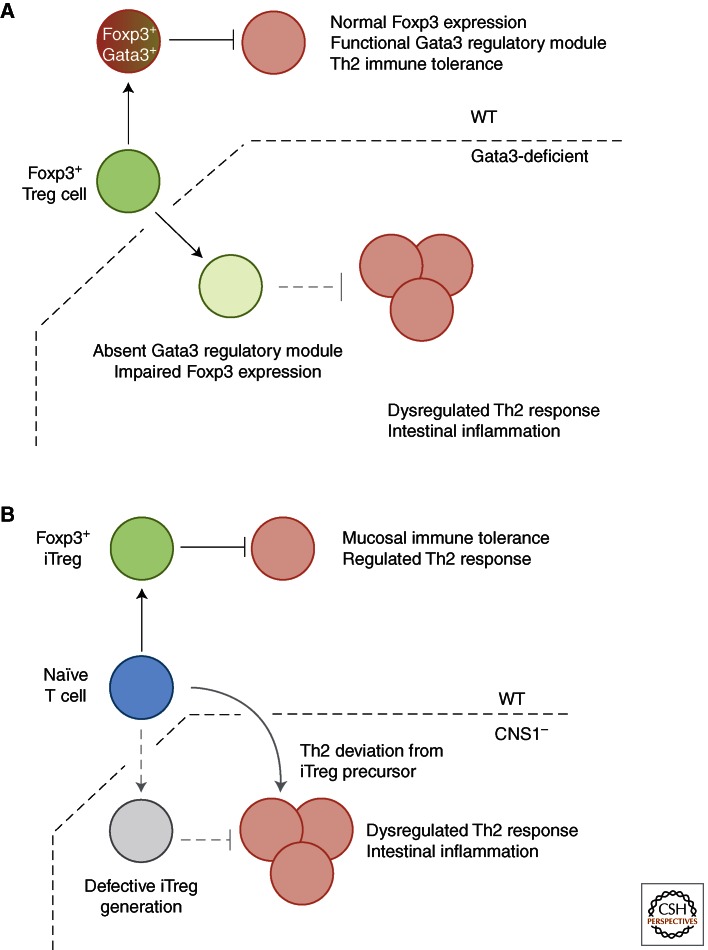
Beyond its essential role in embryogenesis, within the hematopoietic system, the transcription factor Gata3 is required for thymic T-cell development and mature CD4+ T cell-differentiation to a Th2 lineage (Zheng and Flavell 1997). In addition, this lineage-specifying factor promotes immune tolerance through transcriptional enhancer activity at Foxp3 CNS2 to maintain Foxp3 expression in regulatory T cells (Wang et al. 2011). Genetic ablation of Gata3 in Foxp3+ Treg results in a systemic autoimmune syndrome, dermatitis, and intestinal inflammation (dependent on experimental system) because of reduced expression of Foxp3 mRNA and protein on a per-cell basis and associated decrease in regulatory capability (Wang et al. 2011; Wohlfert et al. 2011; Rudra et al. 2012). Alongside decreased Foxp3 expression, Foxp3+ Treg-specific ablation of Gata3 results in uncontrolled intestinal Th2 responses, with increased frequencies of Gata3+, IL-4, 5, and 13 producing populations in the intestinal lamina propria (Rudra et al. 2012) (Fig. 2A).
Figure 2.
Foxp3+ Treg specialization on microenvironment adaptation maintains mucosal immune tolerance. (A) Gata3-dependent mechanisms promote Foxp3 expression and regulation of mucosal Th2 responses. Whether these mechanisms are distinct is not known. (B) CNS1 deficiency and loss of Foxp3+ iTreg-mediated regulation of mucosal Th2 responses may occur through cell-extrinsic (absolute requirement for Foxp3+ iTreg in control of Th2 responses) or cell-intrinsic (naïve T-cell precursors to Foxp3+ iTreg deviate to Th2 phenotype in absence of CNS1) mechanisms.
Although it is clear that Foxp3 and Gata3 cooperate to promote intestinal tolerance, the precise mechanisms are not known. Foxp3 mediates its effects at a molecular level through multimeric protein complexes, and directly regulates the expression of many of its interacting partners, including Gata3 (Rudra et al. 2012). Cooperation between Foxp3 and Gata3 promotes the Treg transcriptional program and subset stability. In the case of CNS1-deficient mice, it is possible that reduced Foxp3 expression during iTreg induction leads to sole binding of Gata3 at classically Foxp3/Gata3 cobound sites, resulting in an aberrant Gata3 transcriptional response that leads to mucosal Th2 pathologies. As such, the mucosal Th2 response observed in CNS1-deficient mice may represent a cell intrinsic failure of effector T cells to divert their fate to a Foxp3+ iTreg phenotype, rather than specific requirement for Foxp3+ iTreg cells to control aberrant Th2 responses to the local antigenic milieu (Fig. 2B).
It is important to note that expression levels of effector T-cell transcription factors by Foxp3+ Treg cells are low relative to their Foxp3− counterparts, suggesting that Foxp3+ Treg cells may use this strategy to adopt some, but not all aspects of distinct effector programs. For example, the acquisition of homing receptors, CXCR3 in the example of T-bet, without production of proinflammatory cytokine IFN-γ, renders Foxp3+ Treg cells able to migrate, but not contribute, to Th1-driven pathologies. In the instance of T-bet+ Foxp3+ Treg, incomplete commitment to an effector phenotype is regulated by suppression of IL-12Rβ2 expression required for STAT4-mediated acquisition of effector function (Koch et al. 2012). The mechanisms by which IFN-γ/STAT1 signals in effector T cells, but not Foxp3+ Treg, lead to IL-12Rβ2 expression remain to be elucidated. As yet, whether the role of effector T-cell-associated transcription factors in Foxp3+ cells is similar for tTreg and iTreg populations remains to be examined. Interestingly, Gata3 has been shown to inhibit the induction of Foxp3 in TGF-β-induced iTreg (Mantel et al. 2007), in comparison to the requirement for maintenance of Foxp3 in mature Treg.
Adaptation of Foxp3+ Treg to the tissue microenvironment and the ability to use multiple regulatory mechanisms is key to instigating and maintaining active immunological tolerance. Distinct antigen specificities, enrichment in regulatory cytokine production and utilization of effector transcription factor networks show that adaptation of Foxp3+ Treg to the tissue microenvironment is critical in maintaining intestinal immune tolerance. The role of the host immune system in continuous tolerance to the microbiota is evident; yet, the commensal microbiota itself is intimately involved in the dynamic molecular dialogue required for immune tolerance at both mucosal and peripheral sites.
MICROBIOTA AND DIETARY METABOLITES AFFECT INTESTINAL CD4+ T-CELL SUBSETS
Colonization of the host by the commensal microbiota and the presence of multiple dietary components is key to proper mucosal immune cell function (Fig. 3). Indeed, individual bacterial species promote differentiation of distinct intestinal T-cell populations. Reared in sterile surrounds, and on a diet free of live microbes, germ-free (GF) mice provide a useful experimental tool in understanding the relationship between host immune system and commensal microbiota. Controlled colonization of germ-free mice allows analysis of the role for individual or multiple bacterial species in mutualistic interactions with the host immune system.
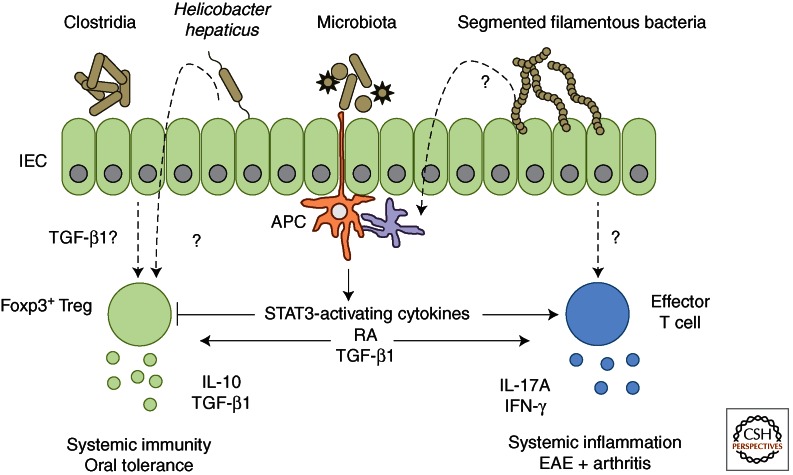
Figure 3.
Commensal microbiota and dietary metabolites influence intestinal T-cell adaptation to direct mucosal and peripheral immune responses. The intestinal mucosa is constantly exposed to a diverse array of foreign antigens, including dietary antigens, metabolites, and components of the commensal microbiota. The presence of distinct microbial species promotes specialized host immune responses through differentiation of appropriate effector and regulatory T-cell populations. Segmented filamentous bacteria promote effector T-cell accumulation, particularly of Th17 cells, within the intestine. Accumulation of Foxp3+ regulatory T-cell populations and induction of IL-10 production arise in response to colonization of germ-free mice with a restricted flora (Clostridiales IV and XIVa, or Altered Schaedler Flora), or conventionally housed mice with Helicobacter hepaticus. In both cases, the mechanism of induction and antigen-specificity of the response require further investigation. Antigen-presenting cell production of soluble mediators heavily influences the balance between effector and regulatory T-cell responses. Production of STAT3 activating cytokines (IL-6, IL-23, IL-21) favors effector T-cell differentiation, whereas retinoic acid (RA) and TGF-β1 production modulate the balance between effector and regulatory responses, depending on the inflammatory microenvironment and associated cytokine production.
Segmented filamentous bacteria (SFB) are Gram-positive members of the Clostridiales genus able to penetrate the small intestinal mucus layer and interact intimately with the epithelium to colonize the host. Colonization with SFB results in accumulation of effector T cells within the small intestine, primarily Th17, and to a lesser extent, Th1 cells (Gaboriau-Routhiau et al. 2009; Ivanov et al. 2009). The mechanisms by which SFB promotes effector T-cell polarization and accumulation within the intestine remain largely unknown. Whether direct interactions between SFB and the intestinal epithelium, host immune cells, or production of unique metabolic modulators is required for induction of effector T cells is not understood. Equally, whether the immune response to SFB colonization is antigen-specific remains to be established. Identifying the genetic attributes of SFB that drive this highly immunostimulatory response will be crucial to understanding how distinct bacterial species modulate the mucosal immune system.
Regulatory T-cell populations are also affected by microbial colonization, which can lead to accumulation of intestinal Foxp3+ Treg populations and induction of IL-10 production (Honda and Littman 2012). For example, Bacteroides fragilis is a human commensal able to induce IL-10 production from intestinal T cells and limit Th17 responses during intestinal inflammation (Round and Mazmanian 2010). Colonization of germ-free mice with a cocktail of 46 spore-forming murine Clostridia strains promotes accumulation of colonic Foxp3+ Treg (Atarashi et al. 2011). The nature of the exact strains, microbial attributes, and associated host molecular responses to this complex gnotobiotic mixture are as yet unknown. However, accumulation of Foxp3+ Treg and induction of IL-10 production are associated with increased colonic levels of active TGF-β1 in these gnotobiotic mice. As discussed earlier, although the reliability of Helios expression to determine tTreg from iTreg is still controversial, Clostridum-colonized gnotobiotic mice showed a shift from Helios+ Foxp3+ to Helios− Foxp3+ Treg following colonization, indicative of iTreg generation. The antigen-specificity of Clostridia-induced colonic Foxp3+ Treg was not assessed, but in the context of TCR repertoire of colonic Foxp3+ Treg, it is possible to hypothesize that these iTreg would bear TCR-specificity for Clostridia-derived epitopes. Furthermore, analysis of TCR repertoire in this setting may aid identification of individual Clostridia strains responsible for Foxp3+ Treg accumulation and/or IL-10 induction. In addition, colonization with a benign altered Schaedler flora (ASF) promoted accumulation of colonic Helios− Foxp3+ Treg that served to promote intestinal tolerance during experimental DSS colitis (Geuking et al. 2011). Similarly, infection with the mouse pathogen Helicobacter hepaticus induces an IL-10-producing Treg response, which when missing leads to bacteria-driven chronic intestinal inflammation (Kullberg et al. 2002). Together, these data suggest that induction of a Treg/IL-10-producing response may be an important part of host-commensal mutualism allowing persistence of microbes in the absence of a damaging inflammatory response. It is clearly of interest to further understand the molecular pathways and metabolites that control microbe-induced Treg function, as these may provide novel immune modulators and underlie the poorly characterized properties of probiotic bacteria. Clostridia are fermenting bacteria and it is notable that fermentation products such as short-chain fatty acids promote gut health (Maslowski et al. 2009). Whether iTreg induction and IL-10 production following bacterial colonization requires constant microbial exposure remains to be examined and the use of reversible colonization systems will be a useful tool to examine this question.
Retinoic acid (RA) is a metabolite of diet-derived vitamin A, a nutrient essential for multiple physiological processes including an array of immunological functions (Hall et al. 2011b). Within the GALT, RA promotes both regulatory and effector immune responses through imprinting of intestinal homing receptors α4β7 and CCR9 on activated T cells, and acts as a cofactor in the de novo generation of Foxp3+ iTreg by CD103+ DC (Coombes et al. 2007; Sun et al. 2007). Induction of Foxp3 expression by CD103+ DC is dependent on TGF-β1 and is enhanced by RA, accordingly CD103+ DC highly express mRNA for aldh1a1 and aldh1a2, the retinal metabolizing enzymes required for RA synthesis (Coombes et al. 2007). In vivo corroboration of the role for RA in iTreg induction stems from the observation that mice reared on a vitamin-A deficient diet are defective in iTreg generation on oral antigen exposure (Hall et al. 2011a). As such, during intestinal homeostasis, RA promotes regulatory responses to bolster immunological tolerance to dietary antigens. However, recent studies show context-dependent roles for this metabolite in intestinal immune tolerance. Thus, RA acts to promote effector responses in settings of intestinal inflammation. Oral infection with Toxoplasma gondii is controlled by a strong intestinal Th1 response, which is impaired in vitamin-A deficient mice (Suzuki et al. 1988; Hall et al. 2011a). Furthermore, normal tolerance to dietary antigens is disturbed in patients with Celiac disease, whereby aberrant immune responses to gluten, a component of wheat, result in intestinal inflammation. Contrary to its recognized role in oral tolerance, RA, in combination with IL-15, a cytokine highly abundant in the mucosa of patients with Celiac disease, promotes proinflammatory cytokine production from intestinal APC to drive intestinal inflammation (DePaolo et al. 2011). As such, RA acts as a proinflammatory coadjuvant in settings of intestinal stress, while promoting intestinal tolerance during homeostasis.
THE INTESTINAL MICROBIOTA AFFECTS SYSTEMIC IMMUNITY
The effect of the commensal microbiota on distinct lymphocyte subsets modulates immune function and tolerance beyond the intestinal microenvironment. Many models of autoimmunity or chronic inflammation are attenuated in the absence of a commensal microbiota (Chervonsky 2010). Although Th17 cells are strongly associated with immunopathology in multiple models of autoimmunity, colonization with SFB does not result in immunopathology and instead aids in protection from intestinal infection with murine pathogen Citrobacter rodentium (Ivanov et al. 2009). However, colonization with SFB is required for inflammation in models of intestinal, joint, and central nervous system pathologies (Stepankova et al. 2007; Wu et al. 2010; Lee et al. 2011). Therefore, SFB colonization can promote tissue pathology in experimental settings predisposed to chronic inflammation, either in the absence of Treg (colitis) or in autoimmune prone strains (arthritis and experimental autoimmune encephalomyelitis [EAE]). However, whether SFB can be classed as a true commensal species is contentious, given its intimate interaction with the intestinal epithelium. The regulatory response to SFB remains ill-defined. Although clearly highly immunostimulatory, SFB is not essential for tissue inflammation. Transgenic SJL/J mice that express a myelin peptide-specific TCR develop relapsing-remitting EAE under conventional, but not GF conditions, indicating a requirement for microbial colonization as a triggering factor for disease pathogenesis (Berer et al. 2011). The colonization of GF mice with commensal microbiota, but not monocolonization with SFB, results in the acquisition of relapsing-remitting disease, highlighting the requirement for microbiota-derived signals as priming stimuli to trigger tissue pathologies in settings already predisposed to chronic inflammation. In the context of intestinal inflammation, the explosion in knowledge and techniques used in detailing the intestinal microbiota will be fundamental to understanding whether dysbiosis represents a triggering factor or is a consequence of inflammation, particularly given the high frequency of IBD-susceptibility SNPs within the healthy population.
CONCLUSION
The mucosal immune system promotes intestinal tolerance by controlling localization and composition of the commensal microbiota. Equally, the commensal microbiota is a fundamental stimulus for priming and activation of both mucosal and peripheral immune responses. However, dysregulated immune responses to the commensal microbiota have profound effects on human health. The dynamic interactions between microbiota and the immune system represent the key to targeted future therapies in chronic intestinal inflammation. To use natural tolerogenic mechanisms occurring under homeostatic microbial-mammalian dialogue, we require not only greater understanding of how the immune system regulates the microbiota, but equally how the distinct microbial communities influence host immunity.
ACKNOWLEDGMENTS
The authors are grateful for support from the Wellcome Trust (PhD Studentship to O.J.H., Investigator Award to F.M.P.) and for helpful discussions from past and present members of the Powrie and Maloy research groups.
Footnotes
Editors: Diane J. Mathis and Alexander Y. Rudensky
Additional Perspectives on Immune Tolerance available at www.cshperspectives.org
REFERENCES
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F 2010. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33: 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190: 995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Powrie F 2009. Hybrid Treg cells: Steel frames and plastic exteriors. Nat Immunol 10: 563–564 [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–21 [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G 2011. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479: 538–541 [DOI] [PubMed] [Google Scholar]
- Bilate AM, Lafaille JJ 2012. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 30: 733–758 [DOI] [PubMed] [Google Scholar]
- Bollig N, Brustle A, Kellner K, Ackermann W, Abass E, Raifer H, Camara B, Brendel C, Giel G, Bothur E, et al. 2012. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc Natl Acad Sci 109: 8664–8669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 106: R75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY 2009. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326: 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, et al. 2011. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34: 566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervonsky AV 2010. Influence of microbial environment on autoimmunity. Nat Immunol 11: 28–35 [DOI] [PubMed] [Google Scholar]
- Coombes JL, Powrie F 2008. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 8: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, et al. 2011. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol 12: 304–311 [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ 2008. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29: 114–126 [DOI] [PubMed] [Google Scholar]
- Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM 1996. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med 184: 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, et al. 2011. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 471: 220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE 2003. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584 [DOI] [PubMed] [Google Scholar]
- Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T 1995. Early-onset multifocal inflammation in the transforming growth factor β1-null mouse is lymphocyte mediated. Proc Natl Acad Sci 92: 12215–12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR 2013. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 494: 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. 2011. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F 2005. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J Exp Med 201: 737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Wang L, Schoeb TR, Elson CO, Cong Y 2010. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med 207: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C 2010. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci 107: 5919–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, et al. 2008. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet 40: 1319–1323 [DOI] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. 2010. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 42: 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31: 677–689 [DOI] [PubMed] [Google Scholar]
- Gambineri E, Torgerson TR, Ochs HD 2003. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol 15: 430–435 [DOI] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ 2011. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34: 794–806 [DOI] [PubMed] [Google Scholar]
- Glasmacher E, Agrawal S, Chang AB, Murphy TL, Zeng W, Vander Lugt B, Khan AA, Ciofani M, Spooner CJ, Rutz S, et al. 2012. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science 338: 975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, et al. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361: 2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B 2011. IL-10 and IL-10 receptor defects in humans. Ann NY Acad Sci 1246: 102–107 [DOI] [PubMed] [Google Scholar]
- Gottschalk RA, Corse E, Allison JP 2012. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol 188: 976–980 [DOI] [PubMed] [Google Scholar]
- Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O 2011. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34: 237–246 [DOI] [PubMed] [Google Scholar]
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. 2011a. Essential role for retinoic acid in the promotion of CD4+ T cell effector responses via retinoic acid receptor α. Immunity 34: 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Grainger JR, Spencer SP, Belkaid Y 2011b. The role of retinoic acid in tolerance and immunity. Immunity 35: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, et al. 2012. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37: 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB 2009. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol 182: 3461–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OJ, Maloy KJ 2011. Innate immune activation in intestinal homeostasis. J Innate Immun 3: 585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Artis D 2010. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol 28: 623–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C 2007. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity 27: 786–800 [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR 2012. The microbiome in infectious disease and inflammation. Annu Rev Immunol 30: 759–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ 2012. Interactions between the microbiota and the immune system. Science 336: 1268–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol 7: 401–410 [DOI] [PubMed] [Google Scholar]
- Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Low E, Lohoff M 2008. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci 105: 20846–20851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O’Connor W Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, et al. 2011. Th17 cells express interleukin-10 receptor and are controlled by Foxp3– and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ 2006. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med 203: 2473–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanove II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133 [DOI] [PubMed] [Google Scholar]
- Ivanove II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21: 527–538 [DOI] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F 2008. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 28: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F 2009. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 27: 313–338 [DOI] [PubMed] [Google Scholar]
- Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, et al. 2008. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med 205: 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med 202: 1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY 2012a. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY 2012b. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491: 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS 2010. Inflammatory bowel disease. Annu Rev Immunol 28: 573–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, Kubo A, Ishii M, Okazaki T, Murakami M, et al. 2012. Intestinal CX3C chemokine receptor 1high (CX3CR1high) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci 109: 5010–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Yoshida K, Ward JM, Letterio JJ, Longenecker G, Yaswen L, Mittleman B, Mozes E, Roberts AB, Karlsson S, et al. 1999. β 2-microglobulin-deficient background ameliorates lethal phenotype of the TGF-β1 null mouse. J Immunol 163: 4013–4019 [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ 2009. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ 2012. T-bet+ Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity 37: 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, Pfeifer D, Kreipe H, Pfister ED, Baumann U, et al. 2012. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: Implications for diagnosis and therapy. Gastroenterology 143: 347–355 [DOI] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274 [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A 2002. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med 196: 505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO 2007. Ulcerative colitis and autoimmunity induced by loss of myeloid αv integrins. Proc Natl Acad Sci 104: 15823–15828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont S, Siddiqui KR, Powrie F 2010. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol 40: 1877–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS 2011. Peripheral education of the immune system by colonic commensal microbiota. Nature 478: 250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 108 (Suppl 1): 4615–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, Nakabayashi T, Mackall CL, Gress RE, Roberts AB 1996. Autoimmunity associated with TGF-β1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Invest 98: 2109–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA 2006. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25: 455–471 [DOI] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140: 845–858 [DOI] [PubMed] [Google Scholar]
- Lohoff M, Mittrucker HW, Prechtl S, Bischof S, Sommer F, Kock S, Ferrick DA, Duncan GS, Gessner A, Mak TW 2002. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc Natl Acad Sci 99: 11808–11812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Geuking MB, McCoy KD 2005. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology 115: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F 2011. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298–306 [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F 2003. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, et al. 2007. GATA3-driven Th2 responses inhibit TGF-β1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol 5: e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT 2007. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3– precursor cells in the absence of interleukin 10. Nat Immunol 8: 931–941 [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK 2002. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16: 219–230 [DOI] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M 2009. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 10: 1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Adler G 2010. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol 184: 2026–2037 [DOI] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307: 254–258 [DOI] [PubMed] [Google Scholar]
- Oestreich KJ, Weinmann AS 2012. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nat Rev Immunol 12: 799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L 2006. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity 25: 249–259 [DOI] [PubMed] [Google Scholar]
- Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, et al. 2008. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature 455: 246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol 5: 1461–1471 [DOI] [PubMed] [Google Scholar]
- Powrie F, Correa-Oliveira R, Mauze S, Coffman RL 1994. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med 179: 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH 2002. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med 195: 1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2: 361–367 [DOI] [PubMed] [Google Scholar]
- Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Muller W 2004. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med 200: 1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci 107: 12204–12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr, et al. 2008. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558 [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY 2010. Stability of the regulatory T cell lineage in vivo. Science 329: 1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, Leslie C, Shaffer SA, Goodlett DR, Rudensky AY 2012. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 13: 1010–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A 2010. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181 [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J 2010. The inflammasomes. Cell 140: 821–832 [DOI] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O 2009. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206: 3101–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66: 5224–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM 2009. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30: 636–645 [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. 1992. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 359: 693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons BD, Clevers H 2011. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 145: 851–862 [DOI] [PubMed] [Google Scholar]
- Stepankova R, Powrie F, Kofronova O, Kozakova H, Hudcovic T, Hrncir T, Uhlig H, Read S, Rehakova Z, Benada O, et al. 2007. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis 13: 1202–1211 [DOI] [PubMed] [Google Scholar]
- Stritesky GL, Jameson SC, Hogquist KA 2012. Selection of self-reactive T cells in the thymus. Annu Rev Immunol 30: 95–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J Exp Med 204: 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS 1988. Interferon-γ: The major mediator of resistance against Toxoplasma gondii. Science 240: 516–518 [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669 [DOI] [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10: 39–49 [DOI] [PubMed] [Google Scholar]
- Tannock GW 2007. What immunologists should know about bacterial communities of the human bowel. Semin Immunol 19: 94–105 [DOI] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM 2010. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 184: 3433–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M 2008. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9: 194–202 [DOI] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. 2007. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. 2008. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol 9: 769–776 [DOI] [PubMed] [Google Scholar]
- Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F 2006. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol 177: 5852–5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Zigmond E, Jung S 2010. Securing the immune tightrope: Mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol 10: 415–426 [DOI] [PubMed] [Google Scholar]
- Verhagen J, Wraith DC 2010. Comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells.” J Immunol 185: 7129; Response to comment, 7130 [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ 2008. How regulatory T cells work. Nat Rev Immunol 8: 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Su MA, Wan YY 2011. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35: 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, et al. 2012. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ Treg cells. J Exp Med 209: 1723–1742, S1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27: 18–20 [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, et al. 2011. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J Clin Invest 121: 4503–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NA 2000. Epithelial stem cell repertoire in the gut: Clues to the origin of cell lineages, proliferative units and cancer. Int J Exp Pathol 81: 117–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D 2010. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32: 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Hogquist KA 2012. T-cell tolerance: Central and peripheral. Cold Spring Harb Perspect Biol 4: a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, et al. 2012. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 209: 1713–1722, S1711–S1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS 2007. Absence of integrin-mediated TGF-β1 activation in vivo recapitulates the phenotype of TGF-β1-null mice. J Cell Biol 176: 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Flavell RA 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89: 587–596 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY 2009. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458: 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF 2006. FOXP3: Of mice and men. Annu Rev Immunol 24: 209–226 [DOI] [PubMed] [Google Scholar]