Abstract
Since its discovery more than 25 years ago, numerous studies have established that the MET receptor is unique among tyrosine kinases. Signaling through MET is necessary for normal development and for the progression of a wide range of human cancers. MET activation has been shown to drive numerous signaling pathways; however, it is not clear how MET signaling mediates diverse cellular responses such as motility, invasion, growth, and angiogenesis. Great strides have been made in understanding the pleotropic aspects of MET signaling using three-dimensional molecular structures, cell culture systems, human tumors, and animal models. These combined approaches have driven the development of MET-targeted therapeutics that have shown promising results in the clinic. Here we examine the unique features of MET and hepatocyte growth factor/scatter factor (HGF/SF) structure and signaling, mutational activation, genetic mouse models of MET and HGF/SF, and MET-targeted therapeutics.
The MET receptor and its only ligand, hepatocyte growth factor/scatter factor (HGF/SF), drive numerous signaling pathways that mediate diverse cellular responses. MET is involved in a wide range of human cancers and is a highly promising therapeutic target.
Since the discovery of the receptor tyrosine kinase MET and its ligand, hepatocyte growth factor/scatter factor (HGF/SF), numerous studies have established the significant role of this receptor/ligand pair in tumor growth and metastasis. The last 25 years of work has made it clear that MET is unique among receptor tyrosine kinases (RTKs), yet the MET receptor activates many signaling pathways that are common to other RTKs. Why MET activation produces growth in one cell type and invasion in another is still unclear, yet the variety of responses to MET signaling is what makes this receptor so frequently associated with malignant growth. Understanding the relationships between MET activation and its downstream signaling effectors is critical to the development of successful therapeutics for a wide range of malignancies.
The MET oncogene was first identified in the early 1980s in a human osteosarcoma tumor cell line that was exposed to N-methyl-N′-nitro-N-nitrosoguanidine, which produced a chromosomal translocation and a novel fusion protein, between a region called the translocated promoter region (TPR) on chromosome 1 and MET kinase domain on chromosome 7. Here the activation of the MET tyrosine kinase domain occurs through the dimerization domain from the TPR (Cooper et al. 1984; Park et al. 1986). Isolation of the full-length proto-oncogene revealed that MET was a unique receptor tyrosine kinase (Park et al. 1986).
The ligand was discovered first as a mitogenic factor of liver cells called hepatocyte growth factor (HGF), and it was shortly after determined to be the same as the motogenic factor called scatter factor (SF). The ligand, commonly referred to as HGF/SF, is the only ligand for the MET receptor (Stoker et al. 1987; Nakamura et al. 1989; Bottaro et al. 1991; Weidner et al. 1991; Gherardi et al. 2006). Under normal physiological conditions, HGF/SF is predominantly produced by mesenchymal cells and acts in a paracrine fashion on MET-expressing epithelial cells (Jeffers et al. 1996). The proliferative and motogenic effects observed in these early studies were some of the first indications of the varied roles that MET signaling has in tumor growth and metastasis.
Embryonic development and tissue regeneration are normal physiological processes that parallel the mechanisms of growth and invasion that occur during tumor progression. Several studies have shown that MET-HGF/SF signaling is essential for embryonic development and regeneration. Depending on the cellular context, MET signaling induces cell proliferation, motility, scattering, angiogenesis, or invasion. These pleotropic attributes are what make MET signaling essential in both normal development and tumor progression. During development, paracrine MET signaling drives the epithelial-to-mesenchymal transition (EMT) of myogenic progenitor cells and is crucial for placenta and liver development (Bladt et al. 1995; Schmidt et al. 1995; Uehara et al. 1995). MET signaling is also critical for liver regeneration and wound repair in skin (Chmielowiec et al. 2007). The signaling networks that drive the developmental processes of EMT, wound healing, and invasion are exploited in tumor cells to promote invasive growth.
The expression and/or activation of MET and HGF/SF have been implicated in the development of numerous human cancers (www.vai.org/met), including carcinomas (breast, colon, gastric, renal, pancreatic, bladder, liver, lung, prostate, ovarian, etc.), sarcomas (osteosarcoma, rhabdomyosarcoma), hematopoietic malignancies (multiple myeloma, lymphoma, chronic myeloid leukemia), melanomas, and central nervous system tumors (glioblastomas and astrocytomas) (Birchmeier et al. 2003; Corso et al. 2005; Gherardi et al. 2012). Uncontrolled MET signaling can occur through overexpression of HGF/SF or MET, mutational activation of MET, autocrine signaling, or gene amplification. Numerous in vitro and in vivo studies have shown that MET signaling plays a key role in tumorigenic growth, metastasis, and therapeutic resistance. It is crucial that we develop an in-depth understanding of how MET signaling regulates both normal and tumorigenic cell processes to develop successful therapeutic strategies. In this review, we will discuss the unique features of MET and HGF/SF structure and signaling, mutational activation, genetic mouse models of MET and HGF/SF, and MET-targeted therapeutics.
STRUCTURAL CHARACTERISTICS OF MET AND HGF/SF
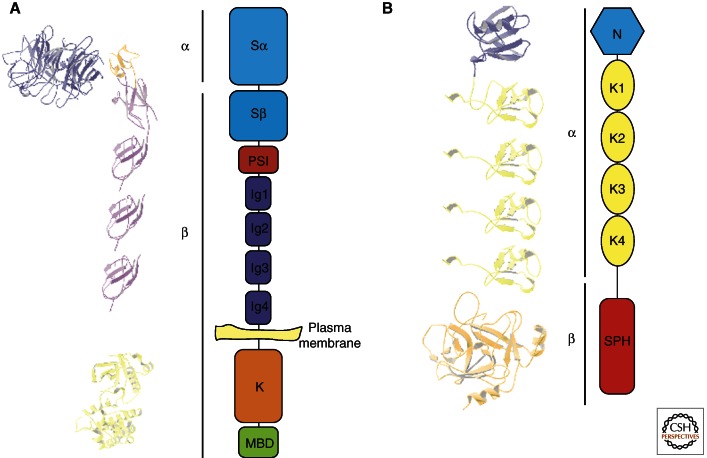
MET is a disulfide-linked heterodimer that is created by cleavage of the precursor into a shorter extracellular α chain and a longer β chain (Fig. 1A). The extracellular portion consists of a seven-bladed β-propeller Sema domain atop four immunoglobulin-like (Ig-like) repeated domains (Love et al. 2003). The Sema domain, which is also present in plexins, semaphorins, and integrins, contains the binding sites for HGF/SF and is linked to the Ig-like repeats by a short cysteine-rich PSI domain (Stamos et al. 2004; Holmes et al. 2007). The remainder of the receptor is composed of a juxtamembrane domain, a tyrosine kinase domain, and a carboxy-terminal tail that is involved in downstream signaling. Structurally, the MET receptor is similar to RON (recepteur d’origine nantais) in humans, Stk (RON murine homolog), f-Stk (RON feline homolog), and c-sea, a cell-surface receptor in chickens (Ronsin et al. 1993; Camp et al. 2005).
Figure 1.
Structural characteristics of MET and HGF/SF. (A) Structure of the MET receptor (α and β refer to subunits present after proteolytic cleavage). MET is expressed at the plasma membrane: The extracellular portion consists of the sema domain, a PSI domain, and four immunoglobulin-like (Ig-like) repeated domains; the intracellular region contains the tyrosine kinase domain and the multifunctional binding domain. The three-dimensional models (left) were generated using the following coordinates from the Protein Data Bank: 1SHY (sema domain), 2UZY (PSI and Ig1, and 2), and 1R1W (kinase domain). The Ig3 and Ig4 domains are modeled as copies of the Ig2 domain. (B) Functional domains of HGF/SF. HGF/SF contains an amino-terminal domain (N), four tandem repeats of kringle domains (K1–K4), and a serine protease homology domain (SPH). The three-dimensional models (left) were generated using the following coordinates from the Protein Data Bank: 1NK1 (N and K1) and 1SHY (HGF/SF β chain). K2–4 are represented as copies of K1.
HGF/SF is also a polyprotein that structurally belongs to the serine protease family and is most closely related to macrophage stimulating factor (MSP) and plasminogen. HGF/SF is composed of an α chain that contains the amino-terminal domain, four tandem repeats of kringle domains, and a serine protease-like β chain that lacks catalytic activity (Fig. 1B) (Donate et al. 1994). The amino-terminal domain also contains a high-affinity binding site for heparin and dermatan sulfate (Deakin and Lyon 1999a,b; Lietha et al. 2001; Lyon et al. 2004). Similar to plasminogen and MSP, HGF/SF is made as an inactive, single-chain precursor that is proteolytically converted into an active heterodimer. HGF/SF can be activated by a number of proteases, including hepatocyte growth factor activator, plasma kallikrein, and coagulation factor XIa (Shimomura et al. 1993; Peek et al. 2002), and it can bind with high affinity to MET in an active or inactive state (Gherardi et al. 2006). Activation of HGF/SF occurs by cleavage at Arg494, which produces a sulfhydryl-linked heterodimer of the NK4α domain with the β subunit serine protease domain.
The newly created amino terminus of the serine protease β domain is an integral part of the catalytic site and prevents protease activity from other serine proteases. After cleavage, the available amino terminus inserts into the “activation pocket” of the protease domain, enabling the HGF/SF β chain to bind to the Sema domain of MET (Kirchhofer et al. 2004; Carafoli et al. 2005). Binding of HGF/SF to MET results in receptor oligomerization at the plasma membrane and subsequent autophosphorylation of the activation loop (tyrosines 1234 and 1235) within the intracellular kinase domain (Gonzatti-Haces et al. 1988; Blume-Jensen and Hunter 2001; Bardella et al. 2004). The autophosphorylation destabilizes the loop, allowing substrate access and phosphorylation of tyrosines 1349 and 1356 within the carboxy-terminal domain (Schiering et al. 2003; Wang et al. 2005). These carboxy-terminal tyrosines serve as the multifunctional docking sites for various signaling adaptors, as discussed below.
MET activation by HGF/SF is a complex process; for example, HGF/SF can bind to MET in either an active or an inactive state, and it can be activated by a number of proteases. In fact, the uncleavable mutant form of HGF/SF (arginine 494 mutated to glutamic acid) can even function as a competitive inhibitor to wild-type HGF/SF (Gherardi et al. 2006). In addition, the α chain of HGF/SF is susceptible to proteolysis by coagulation factor Xa (and possibly by other proteases), generating N-domain and kringle fragments that possess their own intrinsic activity in some circumstances (Rubin et al. 2001; Pediaditakis et al. 2002; Shen et al. 2008). HGF/SF also has two shorter splice variants, the amino-terminal domain plus either one or two kringle domains (NK1 and NK2) (Stahl et al. 1997), which can act as agonists or antagonists depending on the conditions and assay used (Montesano et al. 1998). Heparin/dermatan sulfate can also have a dominant effect on NK1, NK2, and the affinity of the α chain for MET (Deakin and Lyon 1999b; Lyon et al. 2004). Two possible HGF/SF dimer interfaces, an α-chain- and a β-chain-based dimer, may induce MET oligomerization and activation (Chirgadze et al. 1999; Stamos et al. 2004). The discovery of these alternative ligand–receptor interactions has led to the development of a number of HGF/SF-based agonist/antagonist derivatives that may have therapeutic value in wound healing or cancer treatment (Lietha et al. 2001; Tolbert et al. 2007; Youles et al. 2008).
MET ACTIVATION, SIGNALING, AND REGULATION
MET activation induces complex signaling events that depend on the cellular context and produce a variety of cellular responses. For example, treatment of epithelial cell lines with HGF/SF can induce proliferation, anchorage-independent growth, cell scattering, survival, or tubulogenesis. Tubulogenesis is a complex process that involves cellular proliferation, motility, differentiation, and polarization (Birchmeier et al. 1997). Induction of these biological responses through MET requires the cooperation of several intracellular adaptors and signaling effectors. The strength and duration of these signals are tightly controlled through interactions with diverse signal modifiers, expression levels, and subcellular localization and degradation.
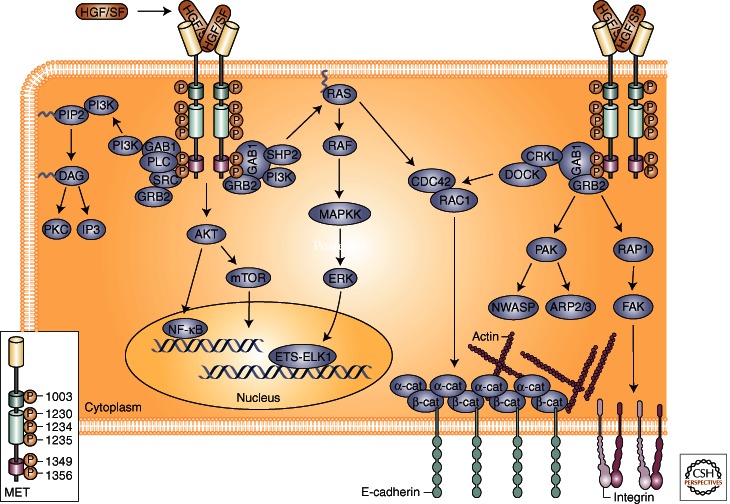
For ligand-mediated receptor activation, HGF/SF binds to the extracellular domain of MET, resulting in receptor dimerization and trans-phosphorylation of tyrosines Y1234 and Y1235 within the kinase domain. Phosphorylation of these residues initiates a conformational change within the receptor, resulting in the phosphorylation of Y1349 and Y1356. These carboxy-terminal residues are part of a unique bidentate docking site that is necessary for MET signaling. Upon MET activation, the multifunctional docking site is capable of interacting with several adaptor proteins and direct kinase substrates, including Gab1, Grb2, phosphatidylinositol 3-kinase (PI3K), Shc, Src, Shp2, Ship1, and Stat3 (Fig. 2). Scaffolding adaptors contain several phosphorylation sites that facilitate the recruitment of proteins having Src-homology-2 (SH2) or phosphotyrosine-binding (PTB) domains. The binding of specific substrates to the MET carboxy-terminal docking site creates a scaffold of signaling effectors that govern cellular responses to MET activation.
Figure 2.
MET-HGF/SF signaling. The MET receptor is activated at the plasma membrane by binding of HGF/SF to the extracellular region of MET. Upon dimerization and activation, tyrosine phosphorylation occurs at Tyr1003 in the juxtamembrane CBL-binding site (shown in green), Tyr1230, Tyr1234, and Tyr1235 in the active site of the kinase (shown in light green), and Tyr1349 and Tyr1356 in the bidentate docking site (shown in pink). MET can then mediate several intracellular signaling pathways through a diverse array of adaptors and downstream effectors. The Gab1 and Grb2 adaptor proteins are critical mediators of MET activation and signaling through RAS-MAPK, PI3K-AKT, RAC1, and PAK pathways drive distinct cellular responses including proliferation, cell survival, and migration. (From Gherardi et al. 2006; reprinted, with permission, from Nature Publishing Group, © 2012.)
Gab1 is the major substrate for MET in epithelial cells, and several studies have shown its necessity for downstream signaling through MET (Maroun et al. 1999; Sachs et al. 2000). Embryos that are nullizygous for Gab1 have the same lethal defects as animals nullizygous for MET and HGF/SF (Itoh et al. 2000; Sachs et al. 2000). The interaction between Gab1 and MET is distinctive in that it can occur either directly or indirectly through Grb2. Direct interaction occurs through a 13-amino-acid MET-binding domain (MBD) on Gab1 that binds to Y1349 in the MET multifunctional docking site (Schaeper et al. 2000). The MBD is a unique motif that is not conserved in other Gab family members, yet it promotes sustained interaction and phosphorylation on MET activation (Maroun et al. 2000). This interaction leads to the activation of several signaling cascades through Gab1, including Shp2, PI3K, PLCγ, and Crk. Activation of these pathways is able to induce diverse cellular responses necessary for normal and tumorigenic cell growth. For instance, Gab1-mediated activation of PI3K and AKT is known to promote cell survival and cell migration (Rosario and Birchmeier 2003), whereas Crk couples with Rap1 and Rac to mediate cell motility and branching morphogenesis (Lamorte et al. 2002a,b; Rodrigues et al. 2005). The binding of the phosphatase Shp2 to Gab1 is known to up-regulate the Ras/ERK/MAPK pathway, leading to branching morphogenesis and proliferation (Maroun et al. 2000; Schaeper et al. 2000).
In addition to Gab1, the Grb2 and Shc adaptor proteins are critical mediators of MET activation. Grb2 and Shc associate with MET and other RTKs through their respective SH2 and PTB domains (Furge et al. 2000). Grb2 is able to directly bind to MET through its SH2 and SH3 domains, but is also recruited indirectly through Shc. Grb2 links activated MET receptors with multiple downstream signaling pathways, such as Ras/ERK and PI3K/AKT (Lowenstein et al. 1992; Rozakis-Adcock et al. 1992; Gu et al. 2000; Ong et al. 2001). Recent studies have shown that recruitment of Shc, but not Grb2, to MET induces VEGF expression (Saucier et al. 2004). Therefore, binding of Shc to MET may be a crucial angiogenic switch during tumor growth.
Although MET signaling is primarily mediated by the Ras-MAPK and PI3K-AKT pathways (Bertotti et al. 2009), other downstream effectors such as NF-κB, β-catenin, and STAT3 have been linked to MET. NF-κB contributes to HGF/SF-mediated proliferation and tubulogenesis through ERK1/2 and p38 MAPK (Muller et al. 2002). Another study showed that MET activation of NF-κB is able to protect cells from apoptosis through AKT (Fan et al. 2005). STAT3 (signal transducer and activator of transcription 3) is also associated with MET. We have found that MET-mediated STAT3 activation in SK-LMS-1 (human leiomyosarcoma cells) and Madin-Darby canine kidney (MDCK) epithelial cells is required for anchorage-independent growth and tumorigenesis, whereas it has no effect on branching morphogenesis (Zhang et al. 2002). Others have reported that STAT3 mediates anchorage-independent growth and is required for branching morphogenesis, but we found no such activity (Boccaccio et al. 1998; Zhang et al. 2002).
MET is also able to initiate biological responses indirectly by interacting with proteins at the plasma membrane. Crosstalk occurs between MET and the developmental Wnt-β-catenin pathway in which MET directly interacts with E-cadherin and induces nuclear localization of β-catenin (Monga et al. 2002; Apte et al. 2006; Reshetnikova et al. 2007). Recent work has shown that in colon cancer, HGF-producing myofibroblasts activate β-catenin-dependent transcription and stimulate cancer stem cell (CSC) populations (Vermeulen et al. 2010). Further, MET interaction with the hyaluron receptor CD44 creates a complex with the ERM proteins (ezrin, radixin, and moesin) and the actin cytoskeleton (Orian-Rousseau et al. 2002), and in some cell lines, interaction with CD44 is required for Ras/MAPK signaling. MET also interacts with the death receptor Fas, which maintains homeostasis in many tissues. MET is able to prevent Fas-mediated apoptosis in hepatocytes by sequestering Fas (Wang et al. 2002; Zou et al. 2007). This is a novel relationship between growth factor receptors and cytokine receptors in controlling apoptosis.
Tumor angiogenesis is an essential response to hypoxic conditions that allows tumors to progress beyond the limitations of the normal vasculature. Angiogenesis is largely mediated by the vascular endothelial growth factor receptor (VEGFR) family and the hypoxia-inducible factors (HIF). Several studies have shown that MET signaling can promote angiogenesis through induction of VEGFA expression and suppression of thrombospondin 1 (TSP1), a negative regulator of angiogenesis (Bussolino et al. 1992; Grant et al. 1993; Zhang et al. 2003; Abounader and Laterra 2005). By inducing angiogenic promoters and inhibiting angiogenic suppressors, MET ensures angiogenesis during tumor progression. In recent years, it has been shown that hypoxic conditions induce MET transcription and amplify MET signaling and MET-mediated invasion in several types of carcinomas (Pennacchietti et al. 2003). These observations have significant clinical implications: for instance, will antiangiogenic therapies induce hypoxia-mediated MET activation? On the other hand, the combined inhibition of angiogenesis and MET is being evaluated in both preclinical and clinical studies.
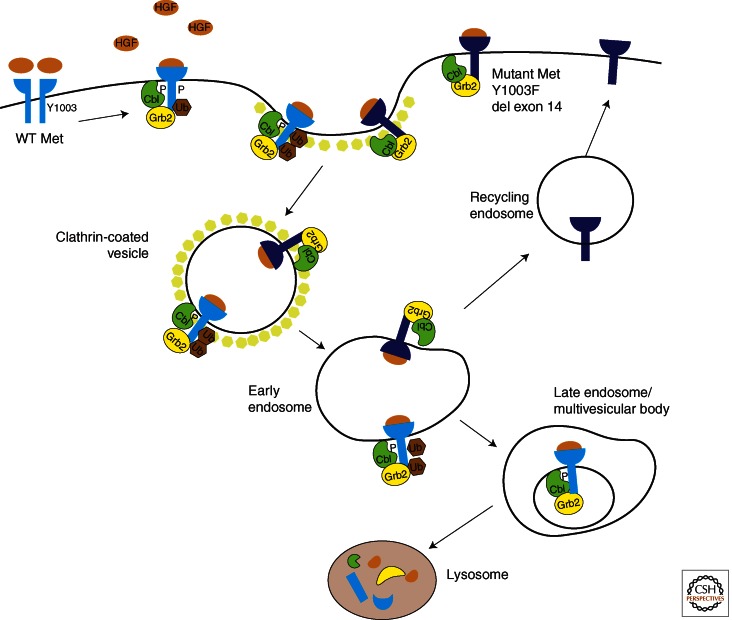
Regulation of RTK activation is necessary for preventing prolonged activation of downstream signaling cascades. Termination of RTK activation can occur through receptor dephosphorylation, sequestration, and degradation, or through signaling antagonists (e.g., Sprouty, Mimp, and Mig-6). Defective receptor trafficking and degradation can result in increased signaling and, ultimately, malignant transformation (Mosesson et al. 2008). Termination of MET signaling is predominantly controlled through receptor internalization and degradation. Several studies have shown that the ubiquitin ligase Cbl is central to down-regulation of the MET receptor (Peschard and Park 2007). Cbl is recruited to MET through Grb2, but it is also able to bind directly to MET through phosphorylated Y1003 (Fig. 3). Binding of Cbl to Y1003 results in receptor ubiquitination, internalization, and degradation. MET ubiquitination is crucial to maintaining physiological MET activation levels, because it has been shown that mutations within the Cbl-binding domain are oncogenic (Abella et al. 2005; Peschard and Park 2007). Further studies are needed in both cellular and animal models for us to have a complete understanding of the intricacies of MET regulation and downstream signaling.
Figure 3.
Down-regulation of the MET receptor. Following ligand binding and dimerization, MET Y1003 is phosphorylated and recruits the ligase Cbl. Cbl induces receptor ubiquitination and internalization. MET is processed through the endosomal pathway and is eventually degraded by lysosomal proteases. Receptors that are not ubiquitinated can be recycled back to the plasma membrane. Mutation or deletion of Y1003 allows MET to avoid lysosomal degradation and MET is recycled back to the plasma membrane.
MUTATIONAL ACTIVATION OF MET
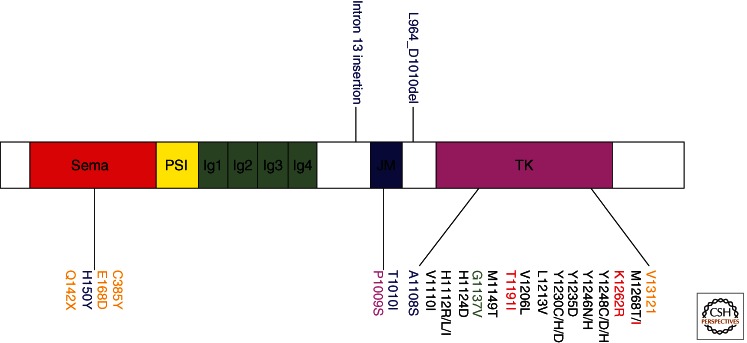
The first activating mutations found within MET were identified through a genome-wide scan of families with hereditary papillary renal carcinoma (HPRC) (Schmidt et al. 1997). This was the first genetic evidence demonstrating the oncogenicity of MET in humans. The missense mutations identified in HPRC patients flank the critical tyrosines Y1234 and Y1235 within the kinase domain (Fig. 4). Both germline and somatic mutations have been identified within the kinase domain, and several studies have shown that these mutations induce constitutive receptor activation (Jeffers et al. 1997, 1998; Schmidt et al. 1999). MET kinase domain mutations have also been observed in childhood hepatocellular carcinomas, metastatic head and neck cancers, gastric carcinomas, and squamous cell cancers (Park et al. 1999; Di Renzo et al. 2000; Lee et al. 2000; Aebersold et al. 2003). Numerous studies have delved into the mechanism by which mutational activation of MET is oncogenic, and it has been shown that mutationally activated MET can be ligand-dependent or -independent (Jeffers et al. 1998; Michieli et al. 1999; Wang et al. 2001).
Figure 4.
MET mutations identified in human cancers. Color coding of the mutation symbols above and below the MET protein diagram identifies the type of human tumor: black, papillary renal cell carcinoma; red, hepatocellular carcinoma; green, glioma; blue, lung carcinoma; purple, gastric carcinoma; orange, cancer unknown primary origin (CUP). Genetic alterations were found in the following tumors: L964_D1010del, lung adenocarcinoma; A1108S, squamous lung carcinoma; E168D, small cell lung carcinoma; intron 13 insertion 9IVS13-(52-52)insCT, small cell lung carcinoma; T1010I, small cell lung carcinoma. Note that T1010I was also found in a breast carcinoma and CUP; E168D was found in a CUP; Y1235D and Y1230C/D were identified in head and neck squamous cell carcinomas, respectively.
Screens for MET mutations in other solid cancers have shown that MET tyrosine kinase mutations are not a common event. However, several groups have looked outside the kinase domain and discovered mutations and deletions within the juxtamembrane and Sema domain of MET in gastric, small cell lung, and non-small cell lung cancers (Lee et al. 2000; Ma et al. 2003; Kong-Beltran et al. 2006). T1010I was identified in the juxtamembrane (JM) domain of a small cell lung cancer (SCLC) tumor sample (Fig. 4). Furthermore, one Sema domain missense mutation (E168D), two-base-pair insertional mutations (IVS13 [52–53]-insCT) within the pre-JM intron 13, and an alternative transcript involving exon 10 were identified in this study. The mutations within the juxtamembrane domain were found to alter cell adhesion and induce tumorigenicity by in vitro assays. The Sema domain mutation has not been carefully evaluated but may affect the structure of the ligand-binding domain.
Another mutational analysis in lung and colon cancers identified several mutations that increased protein stability and MET receptor activation (Kong-Beltran et al. 2006). A mutation within a dinucleotide splice site resulted in deletion of exon 14, which decreased the binding of the Cbl E3-ligase and led to attenuated ubiquitination and degradation of the MET receptor. Treatment of cells containing the exon 14 deletion with an HGF-competitive MET antibody resulted in MET inhibition, suggesting that the exon 14 mutant is ligand independent and can be therapeutically targeted (Kong-Beltran et al. 2006). A recent study identified several novel mutations in cancers of unknown primary origin (CUP) including four somatic mutations clustered in the SEMA domain, one mutation in the juxtamembrane domain, and one near the active site of the tyrosine kinase domain (Stella et al. 2011). These mutations had a 30% incidence in the CUP cohort and were a negative prognostic marker.
Understanding how mutations activate the MET kinase is crucial to the development of effective cancer therapeutics. The efficacies of several promising kinase inhibitors, such as imatinib, have been overcome by tumors that acquired new point mutations. The efficacy of SU11274 (which targets the ATP-binding site of MET) was tested against the naturally occurring MET mutations H1112Y, L1213V, Y1248H, and M1268T in transformed NIH3T3 cells (Berthou et al. 2004). H1112Y and M1286T were both sensitive to SU11274 inhibition, but Y1248H and L1213V were resistant to treatment. Another study using the MET-dependent gastric carcinoma cell line SNU638 and two MET inhibitors (PHA-665752 and PF-2341066) observed resistance through acquisition of the Y1230H mutation in the activation loop (Qi et al. 2011). Structural analysis showed that Y1230H destabilizes the autoinhibitory conformation of MET and abrogates an important aromatic stacking interaction with the inhibitors. These results underscore the necessity for a clearer understanding of the three-dimensional structure of MET and how mutations alter the binding dynamics of therapeutic inhibitors.
MOUSE MODELS OF MET AND HGF/SF
Even though innumerable in vitro studies have shown the influence of MET activation on tumor development, mouse models have been invaluable for investigating the complexities of MET signaling in development and tumorigenesis. As shown in Table 1, knock-out, transgenic, inducible, and knock-in models have shown the diverse tissues in which MET signaling affects normal or tumor development.
Table 1.
Mouse models of Met and HGF/SF
| Phenotype | References | |
|---|---|---|
| Knock-out models | ||
| HGF/SF | Embyronic lethality owing to placental and liver defects | Schmidt et al. 1995; Uehara et al. 1995 |
| Met | Embryonic lethality owing to impaired migration of myogenic precursor cells | Bladt et al. 1995 |
| Transgenic models | ||
| MT-HGF | Diverse carcinomas, sarcomas, and melanomas | Takayama et al. 1997 |
| Melanomas | Otsuka et al. 1998 | |
| TRE-Met | Hepatocellular carcinomas | Wang et al. 2001 |
| WAP-HGF | Mammary adenocarcinomas | Gallego et al. 2003 |
| Tpr-Met | Mammary adenocarcinomas | Liang et al. 1996 |
| HGF-scid | Enhanced growth of heterotopic xenografts | Zhang et al. 2005 |
| GFP-Met | Adenomas, adenocarcinomas, and angiosarcomas in abdominal sebaceous glands | Moshitch-Moshkovitz et al. 2006 |
| Metmt | Mammary adenocarcinomas | Ponzo et al. 2009 |
| Knock-in models | ||
| Metmut | Diverse carcinomas, sarcomas, and lymphomas | Graveel et al. 2004, 2009 |
| Flox models | ||
| Met | Mx-cre deletion of exon 15; impaired liver regeneration | Borowiak et al. 2004 |
| HGF | Adeno-cre deletion of exon 5; impaired liver regeneration | Phaneuf et al. 2004 |
| Met | Alb-cre deletion of exon 15; impaired liver regeneration | Huh et al. 2004 |
Genetic studies in mice were the first to reveal the importance of MET and HGF/SF in embryonic development, during which MET and HGF/SF are expressed in close proximity to each other and receptor activation is regulated through paracrine signaling. When MET or HGF/SF is knocked out in mice, defective development of the liver and placenta results in embryonic death (Bladt et al. 1995; Schmidt et al. 1995; Uehara et al. 1995). The livers of MET–/– and HGF/SF–/– embryos are drastically smaller owing to decreased hepatocyte proliferation and increased apoptosis. Severely impaired placental development is a result of a reduction in labyrinthine trophoblasts. The identical phenotypes of the MET–/– and HGF/SF–/– embryos reaffirmed that MET and HGF/SF are an exclusive receptor/ligand pair. Furthermore, MET–/– and HGF/SF–/– embryos both lack skeletal muscles in the limb, tongue, and diaphragm, demonstrating the necessity of MET signaling for the migration of myogenic precursor cells. Interestingly, the mechanistic process by which myogenic precursors are released and migrate through the embryo is similar to the EMT observed during tumor invasion. In addition, placentation, liver development, and long-range migration of muscle progenitor cells are late evolutionary processes that confirm the emergence of MET signaling later in evolution (Birchmeier et al. 2003).
In 2004, three separate laboratories developed inducible mouse lines to further evaluate the regenerative abilities of MET and HGF/SF in the adult liver. Borowiak et al. developed METflox mice that were crossed to transgenic mice expressing cre under the control of the IFN-inducible Mx promoter (Borowiak et al. 2004). Liver regeneration after partial hepatectomy was greatly impaired in the METflox mice. Impaired regeneration was also observed in another METflox line in which Alb-cre mice were crossed to induce selective inactivation of MET within hepatocytes (Huh et al. 2004). METflox livers were found to have increased sensitivity to Fas-induced apoptosis and significantly decreased regeneration after treatment with the hepatocarcinogens phenobarbital and carbon tetrachloride. Another study created HGFex5-flox mice that were administered a recombinant adenoviral vector coding for cre recombinase (AdCre1) to selectively ablate HGF/SF (Phaneuf et al. 2004). HGFex5-flox mice injected with carbon tetrachloride had significant reductions in regeneration compared with controls, reaffirming the observations made in the METflox models.
Several transgenic mouse models of MET and HGF have been created that illuminate the effect of MET activation on tumor development. Transgenic mice expressing HGF/SF under the metallothionein promoter develop a diverse array of tumors, many of which originate in tissues that exhibit abnormal development, including the mammary gland, skeletal muscle, and melanocytes (Takayama et al. 1997). Autocrine signaling of MET and HGF/SF had been implicated in several cell culture systems, but this was the first in vivo model to show the connection between autocrine MET-HGF/SF signaling and tumorigenesis. Further studies with MT-HGF/SF mice revealed that MET-HGF/SF autocrine signaling could induce metastatic melanomas (Otsuka et al. 1998). Other transgenic models have been created that target HGF/SF expression to the mammary epithelium under the control of the whey acidic protein (WAP) gene promoter (Gallego et al. 2003). WAP-HGF mice developed multiple metastatic adenocarcinomas within the mammary gland after pregnancy and lactation. These mammary tumors had high levels of MET activation and HGF expression, similar to what is observed in some human breast carcinomas. Earlier studies of TPR-MET transgenic mice (under the metallothionein promoter) also showed mammary adenocarcinoma development after pregnancy (Liang et al. 1996). These transgenic models supported the hypothesis that altered MET activation or signaling may play a role in a variety of tumor types.
To understand how ligand-independent activation of RTKs can affect tumorigenesis, Wang et al. created transgenic mice that express human MET in hepatocytes under the control of tetracycline (Wang et al. 2001). These mice developed hepatocellular carcinomas that regressed when the transgene was suppressed. Even though human MET cannot be activated by murine HGF/SF, MET activation was present in hepatocytes and was dependent on cell adherence. This was the first evidence that MET was tumorigenic in vivo through ligand-independent mechanisms. This study also shed light on how MET overexpression may be tumorigenic in tumors that do not express HGF/SF.
To further evaluate MET expression through imaging, Moshitch-Moshkovitz et al. (2006) developed a GFP-MET transgenic mouse that allowed for direct subcellular-resolution imaging of expression patterns during tumor progression. The GFP-MET mice developed sebaceous gland tumors that were imaged by confocal laser scanning microscopy (CLSM) and analyzed by Western blot analysis. A gradual increase in GFP-MET levels from normal skin, to adenoma and angiosarcoma, to adenocarcinoma, was observed. In addition, single cells expressing high levels of GFP-MET were observed spreading from the tumor, similar to micrometastases.
To investigate MET and HGF/SF in human cancers, human tumor xenografts are often grown in immunocompromised mice. Because human MET is not activated by murine HGF/SF, traditional xenograft models do not accurately represent paracrine signaling in human tumors. To solve this dilemma, Zhang et al. created a transgenic mouse expressing human HGF (designated hHGF-Tg) on a severe combined immunodeficiency (SCID) background (Zhang et al. 2005). The expression of hHGF significantly enhanced the growth of heterotopic subcutaneous xenografts derived from human MET-expressing cancer cells. This model has been invaluable for the testing of therapeutic agents in human cancer cells in the context of MET signaling (Gao et al. 2006; Merchant et al. 2008; Zhang et al. 2010).
In 1997, activating mutations within the MET kinase domain were identified in families with HPRCs (Schmidt et al. 1997). The oncogenic potential of these mutations was confirmed through several in vitro, xenograft, and transgene experiments (Jeffers et al. 1997, 1998). To examine mutational activation of MET in vivo, knock-in mouse models were created with activating mutations (WT, D1226N, Y1228C, M1248T, and M1248T/L1193V) (Graveel et al. 2004). The different mutant MET lines developed unique tumor profiles including carcinomas, sarcomas, and lymphomas. It was also observed that the majority of tumors had nonrandom duplication of the mutant MET allele. This selective chromosomal amplification has been observed in patients with HPRC. Further studies have shown that when the M1248T/L1193V is congenically bred onto the FVB/N background, these mice develop a high incidence of aggressive mammary tumors (Graveel et al. 2009), which are histologically diverse and have several characteristics similar to those of human basal breast cancers. Similar mammary tumorigenesis also occurs in a transgenic model of the M1248T mutation (Ponzo et al. 2009), demonstrating that MET may induce progression of aggressive breast cancer subtypes. Overall, these germline and conditional mouse models have provided us with a greater understanding of the significant functions of MET signaling in development, tissue homeostasis, and tumorigenesis.
TARGETING MET-HGF/SF IN CANCER
Given that MET is involved in multiple stages of tumor progression in a variety of human cancers, it is a highly promising therapeutic target (Knudsen and Vande Woude 2008). In recent years, several approaches have been used to specifically target MET in neoplastic cells. Early studies of small molecule inhibitors showed that selective MET inhibition impeded tumor growth in mouse models (Christensen et al. 2003; Sattler et al. 2003; Wang et al. 2003; Knudsen and Vande Woude 2008). These compounds act by inhibiting ATP binding to the kinase domain. Based on the success of other receptor tyrosine kinase inhibitors such as gefitinib and imatinib, competitive inhibitors of MET are promising. Other approaches include small interfering RNA (siRNA) or ribozymes targeting MET and/or HGF/SF (Abounader et al. 1999; Shinomiya et al. 2004), neutralizing antibodies against MET and/or HGF/SF (Cao et al. 2001), and the HGF/SF antagonist NK4 (Date et al. 1997). Decoy receptors that inhibit MET activation by preventing both HGF binding and ligand-independent MET dimerization are able to inhibit MET signaling and MET-dependent tumor growth (Kong-Beltran et al. 2004; Michieli et al. 2004).
Recent work on drug-resistant lung cancers has shown that MET may be a critical player in developed resistance to targeted therapies. The epidermal growth factor receptor (EGFR) kinase inhibitors gefitinib and erlotinib are effective treatments for non-small cell lung cancers (NSCLC), but the majority of these tumors develop resistance with time. Approximately 50% of the tumors develop resistance owing to a secondary mutation in EGFR; however, focal amplification of MET was observed in 22% of the resistant tumors (Engelman et al. 2007). MET activation has also been associated with resistance to EGFR and ERBB2 inhibitors in colorectal cancer cells and breast cancer cells, respectively (Shattuck et al. 2008; Liska et al. 2011). Conversely, activation of ERBB family members mediates resistance to MET inhibition in gastric carcinoma cell lines (Corso et al. 2010). These studies and others indicate that signaling cross talk between RTKs may drive resistance to targeted therapies. Therefore, it is critical that the molecular profile of MET and other RTKs is understood to achieve clinical success with targeted inhibitors.
At this time, numerous MET-HGF/SF therapeutics are being evaluated in clinical trials. Several MET-specific inhibitors have shown promising results, including the monovalent antibody MetMAb (onartuzumab), which has shown activity in combination with erlotinib in NSCLC patients (Spigel et al. 2011). The HGF/SF monoclonal antibody AMG 102 (rilotumumab) improved overall survival of gastric adenocarcinoma (Oliner et al. 2012). In both of these studies, the best response was observed in patients with high MET expression levels. These clinical results underscore the necessity of patient stratification for targeted studies of MET and other molecular targets. Other therapeutic agents targeting MET include the noncompetitive inhibitor ARQ 197 (tivantinib), which has improved progression-free and overall survival in NSCLC and hepatocellular carcinoma (Sequist et al. 2011; Rimassa et al. 2012). Concurrent inhibition of angiogenesis and MET has been evaluated using the multi-target MET inhibitor XL184 (cabozantinib), which also targets VEGFR2. XL184 has been effective against several solid cancers including medullary thyroid cancer, breast, NSCLC, melanoma, and liver cancer (Gordon et al. 2012; Hellerstedt et al. 2012; Schoffski et al. 2012; Winer et al. 2012). The most significant results were observed in the reduction of bone metastatic lesions in castration-resistant prostate cancer (Smith et al. 2012). These results raise the question of using multi-targeting versus specific inhibitors in the clinic. The success of either approach requires balancing activity, toxicity, and resistance mechanisms in each cancer type.
CONCLUDING REMARKS
Since its discovery more than 25 years ago, numerous studies have established that MET is unique among RTKs and is critical for normal development and for the progression of a wide range of human cancers. MET activation has been shown to mediate numerous signaling pathways in both in vitro and in vivo models. Nevertheless, we are still unraveling how MET signaling can mediate such diverse cellular responses as motility, invasion, growth, and angiogenesis. A deeper understanding of how MET activation controls tumorigenesis will require further analysis using three-dimensional molecular structures, cell culture systems, human tumors, and animal models. Through these combined approaches, and with new drugs targeting MET, we will, in the near future, realize the influence MET has on tumorigenesis and how it may be controlled.
ACKNOWLEDGMENTS
The authors thank David Nadziejka for manuscript editing and Jack DeGroot and Yu-Wen Zhang for a critical reading of the manuscript. We apologize to authors whose studies we were unable to cite because of space restrictions.
Footnotes
Editors: Joseph Schlessinger and Mark A. Lemmon
Additional Perspectives on Receptor Tyrosine Kinases available at www.cshperspectives.org
REFERENCES
- Abella JV, Peschard P, Naujokas MA, Lin T, Saucier C, Urbe S, Park M 2005. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol Cell Biol 25: 9632–9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abounader R, Laterra J 2005. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol 7: 436–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abounader R, Ranganathan S, Lal B, Fielding K, Book A, Dietz H, Burger P, Laterra J 1999. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J Natl Cancer Inst 91: 1548–1556 [DOI] [PubMed] [Google Scholar]
- Aebersold DM, Landt O, Berthou S, Gruber G, Beer KT, Greiner RH, Zimmer Y 2003. Prevalence and clinical impact of Met Y1253D-activating point mutation in radiotherapy-treated squamous cell cancer of the oropharynx. Oncogene 22: 8519–8523 [DOI] [PubMed] [Google Scholar]
- Apte U, Zeng G, Muller P, Tan X, Micsenyi A, Cieply B, Dai C, Liu Y, Kaestner KH, Monga SP 2006. Activation of Wnt/β-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology 44: 992–1002 [DOI] [PubMed] [Google Scholar]
- Bardella C, Costa B, Maggiora P, Patane S, Olivero M, Ranzani GN, De Bortoli M, Comoglio PM, Di Renzo MF 2004. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell–cell adhesion through E-cadherin transcriptional repression. Cancer Res 64: 5154–5161 [DOI] [PubMed] [Google Scholar]
- Berthou S, Aebersold DM, Schmidt LS, Stroka D, Heigl C, Streit B, Stalder D, Gruber G, Liang C, Howlett AR, et al. 2004. The Met kinase inhibitor SU11274 exhibits a selective inhibition pattern toward different receptor mutated variants. Oncogene 23: 5387–5393 [DOI] [PubMed] [Google Scholar]
- Bertotti A, Burbridge MF, Gastaldi S, Galimi F, Torti D, Medico E, Giordano S, Corso S, Rolland-Valognes G, Lockhart BP, et al. 2009. Only a subset of Met-activated pathways are required to sustain oncogene addiction. Sci Signal 2: er11. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Brinkmann V, Niemann C, Meiners S, DiCesare S, Naundorf H, Sachs M 1997. Role of HGF/SF and c-Met in morphogenesis and metastasis of epithelial cells. Ciba Found Symp 212: 230–240 [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF 2003. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4: 915–925 [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C 1995. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376: 768–771 [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T 2001. Oncogenic kinase signalling. Nature 411: 355–365 [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM 1998. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 391: 285–288 [DOI] [PubMed] [Google Scholar]
- Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C 2004. Met provides essential signals for liver regeneration. Proc Natl Acad Sci 101: 10608–10613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA 1991. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251: 802–804 [DOI] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM 1992. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119: 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp ER, Liu W, Fan F, Yang A, Somcio R, Ellis LM 2005. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Ann Surg Oncol 12: 273–281 [DOI] [PubMed] [Google Scholar]
- Cao B, Su Y, Oskarsson M, Zhao P, Kort EJ, Fisher RJ, Wang LM, Vande Woude GF 2001. Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc Natl Acad Sci 98: 7443–7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli F, Chirgadze DY, Blundell TL, Gherardi E 2005. Crystal structure of the β-chain of human hepatocyte growth factor-like/macrophage stimulating protein. FEBS J 272: 5799–5807 [DOI] [PubMed] [Google Scholar]
- Chirgadze DY, Hepple JP, Zhou H, Byrd RA, Blundell TL, Gherardi E 1999. Crystal structure of the NK1 fragment of HGF/SF suggests a novel mode for growth factor dimerization and receptor binding. Nat Struct Biol 6: 72–79 [DOI] [PubMed] [Google Scholar]
- Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W 2007. c-Met is essential for wound healing in the skin. J Cell Biol 177: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, et al. 2003. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res 63: 7345–7355 [PubMed] [Google Scholar]
- Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF 1984. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311: 29–33 [DOI] [PubMed] [Google Scholar]
- Corso S, Comoglio PM, Giordano S 2005. Cancer therapy: Can the challenge be MET? Trends Mol Med 11: 284–292 [DOI] [PubMed] [Google Scholar]
- Corso S, Ghiso E, Cepero V, Sierra JR, Migliore C, Bertotti A, Trusolino L, Comoglio PM, Giordano S 2010. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer 9: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T 1997. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett 420: 1–6 [DOI] [PubMed] [Google Scholar]
- Deakin JA, Lyon M 1999a. Differential regulation of hepatocyte growth factor/scatter factor by cell surface proteoglycans and free glycosaminoglycan chains. J Cell Sci 112: 1999–2009 [DOI] [PubMed] [Google Scholar]
- Deakin JA, Lyon M 1999b. Differential regulation of hepatocyte growth factor/scatter factor by cell surface proteoglycans and free glycosaminoglycan chains. J Cell Sci 112: 1999–2009 [DOI] [PubMed] [Google Scholar]
- Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, Stefani AD, Valente G, Giordano S, Cortesina G, Comoglio PM 2000. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 19: 1547–1555 [DOI] [PubMed] [Google Scholar]
- Donate LE, Gherardi E, Srinivasan N, Sowdhamini R, Aparicio S, Blundell TL 1994. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP). Protein Sci 3: 2378–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. 2007. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039–1043 [DOI] [PubMed] [Google Scholar]
- Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, Pestell RG, Goldberg ID, Rosen EM 2005. Role of NF-κB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene 24: 1749–1766 [DOI] [PubMed] [Google Scholar]
- Furge KA, Zhang YW, Vande Woude GF 2000. Met receptor tyrosine kinase: Enhanced signaling through adapter proteins. Oncogene 19: 5582–5589 [DOI] [PubMed] [Google Scholar]
- Gallego MI, Bierie B, Hennighausen L 2003. Targeted expression of HGF/SF in mouse mammary epithelium leads to metastatic adenosquamous carcinomas through the activation of multiple signal transduction pathways. Oncogene 22: 8498–8508 [DOI] [PubMed] [Google Scholar]
- Gao CF, Xie Q, Zhang YW, Su Y, Roys C, Zhao P, Cao B, Burgess T, Coxon A, Vande Woude G 2006. Therapeutic potential of neutralizing antibodies to HGF/SF against human c-MET driven human tumors. In American Association for Cancer Research Annual Meeting, p. LB229 Washington, DC [Google Scholar]
- Gherardi E, Sandin S, Petoukhov MV, Finch J, Youles ME, Ofverstedt LG, Miguel RN, Blundell TL, Vande Woude GF, Skoglund U, et al. 2006. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci 103: 4046–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G 2012. Targeting MET in cancer: Rationale and progress. Nat Rev Cancer 12: 89–103 [DOI] [PubMed] [Google Scholar]
- Gonzatti-Haces M, Seth A, Park M, Copeland T, Oroszlan S, Vande Woude GF 1988. Characterization of the TPR-MET oncogene p65 and the MET protooncogene p140 protein-tyrosine kinases. Proc Natl Acad Sci 85: 21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kluger HM, Shapiro G, Kurzrock R, Edelman G, Samuel TA, Moussa AH, Ramies DA, Laird AD, Schimmoller F, et al. 2012. Activity of cabozantinib (XL184) in metastatic melanoma: Results from a phase II randomized discontinuation trial (RDT). J Clin Oncol Abstr 30: 8531 [Google Scholar]
- Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickloff BJ, Kinsella JL, Polverini P, Rosen EM 1993. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci 90: 1937–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveel C, Su Y, Koeman J, Wang LM, Tessarollo L, Fiscella M, Birchmeier C, Swiatek P, Bronson R, Vande Woude G 2004. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc Natl Acad Sci 101: 17198–17203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveel CR, DeGroot JD, Su Y, Koeman J, Dykema K, Leung S, Snider J, Davies SR, Swiatek PJ, Cottingham S, et al. 2009. Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc Natl Acad Sci 106: 12909–12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Maeda H, Moon JJ, Lord JD, Yoakim M, Nelson BH, Neel BG 2000. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol 20: 7109–7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstedt BA, Edelman G, Vogelzang NJ, Kluger HM, Yasenchak CA, Shen X, Ramies DA, Gordon MS 2012. Activity of cabozantinib (XL184) in metastatic NSCLC: Results from a phase II randomized discontinuation trial (RDT). J Clin Oncol Abstr 30: 7514 [Google Scholar]
- Holmes O, Pillozzi S, Deakin JA, Carafoli F, Kemp L, Butler PJ, Lyon M, Gherardi E 2007. Insights into the structure/function of hepatocyte growth factor/scatter factor from studies with individual domains. J Mol Biol 367: 395–408 [DOI] [PubMed] [Google Scholar]
- Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS 2004. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci 101: 4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Yoshida Y, Nishida K, Narimatsu M, Hibi M, Hirano T 2000. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol 20: 3695–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M, Rong S, Vande Woude GF 1996. Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-Met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol Cell Biol 16: 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, Zbar B, Vande Woude GF 1997. Activating mutations for the Met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci 94: 11445–11450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M, Fiscella M, Webb CP, Anver M, Koochekpour S, Vande Woude GF 1998. The mutationally activated Met receptor mediates motility and metastasis. Proc Natl Acad Sci 95: 14417–14422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhofer D, Yao X, Peek M, Eigenbrot C, Lipari MT, Billeci KL, Maun HR, Moran P, Santell L, Wiesmann C, et al. 2004. Structural and functional basis of the serine protease-like hepatocyte growth factor β-chain in Met binding and signaling. J Biol Chem 279: 39915–39924 [DOI] [PubMed] [Google Scholar]
- Knudsen BS, Vande Woude G 2008. Showering c-Met dependent cancers with drugs. Curr Opin Genet Dev 18: 87–96 [DOI] [PubMed] [Google Scholar]
- Kong-Beltran M, Stamos J, Wickramasinghe D 2004. The Sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell 6: 75–84 [DOI] [PubMed] [Google Scholar]
- Kong-Beltran M, Seshagiri S, Zha J, Zhu W, Bhawe K, Mendoza N, Holcomb T, Pujara K, Stinson J, Fu L, et al. 2006. Somatic mutations lead to an oncogenic deletion of Met in lung cancer. Cancer Res 66: 283–289 [DOI] [PubMed] [Google Scholar]
- Lamorte L, Rodrigues S, Naujokas M, Park M 2002a. Crk synergizes with epidermal growth factor for epithelial invasion and morphogenesis and is required for the Met morphogenic program. J Biol Chem 277: 37904–37911 [DOI] [PubMed] [Google Scholar]
- Lamorte L, Royal I, Naujokas M, Park M 2002b. Crk adapter proteins promote an epithelial-mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol Biol Cell 13: 1449–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Han SU, Cho H, Jennings B, Gerrard B, Dean M, Schmidt L, Zbar B, Vande Woude GF 2000. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 19: 4947–4953 [DOI] [PubMed] [Google Scholar]
- Liang TJ, Reid AE, Xavier R, Cardiff RD, Wang TC 1996. Transgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumors. J Clin Invest 97: 2872–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietha D, Chirgadze DY, Mulloy B, Blundell TL, Gherardi E 2001. Crystal structures of NK1-heparin complexes reveal the basis for NK1 activity and enable engineering of potent agonists of the MET receptor. EMBO J 20: 5543–5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska D, Chen CT, Bachleitner-Hofmann T, Christensen JG, Weiser MR 2011. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res 17: 472–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM 2003. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol 10: 843–848 [DOI] [PubMed] [Google Scholar]
- Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J 1992. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 70: 431–442 [DOI] [PubMed] [Google Scholar]
- Lyon M, Deakin JA, Lietha D, Gherardi E, Gallagher JT 2004. The interactions of hepatocyte growth factor/scatter factor and its NK1 and NK2 variants with glycosaminoglycans using a modified gel mobility shift assay. Elucidation of the minimal size of binding and activatory oligosaccharides. J Biol Chem 279: 43560–43567 [DOI] [PubMed] [Google Scholar]
- Ma PC, Kijima T, Maulik G, Fox EA, Sattler M, Griffin JD, Johnson BE, Salgia R 2003. c-MET mutational analysis in small cell lung cancer: Novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 63: 6272–6281 [PubMed] [Google Scholar]
- Maroun CR, Holgado-Madruga M, Royal I, Naujokas MA, Fournier TM, Wong AJ, Park M 1999. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol Cell Biol 19: 1784–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M 2000. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol 20: 8513–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant M, Zhang Y, Su Y, Romero M, Louie S, Severin C, Mendoza N, Zheng Z, Dekoning T, DW K, et al. 2008. Combination efficacy with MetMAb and erlotinib in a NSCLC tumor model highlight therapeutic opportunities for c-Met inhibitors in combination with EGFR inhibitors. In Proceedings of the 99th Annual Meeting of the American Association for Cancer Research San Diego, CA [Google Scholar]
- Michieli P, Basilico C, Pennacchietti S, Maffe A, Tamagnone L, Giordano S, Bardelli A, Comoglio PM 1999. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene 18: 5221–5231 [DOI] [PubMed] [Google Scholar]
- Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, Comoglio PM 2004. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 6: 61–73 [DOI] [PubMed] [Google Scholar]
- Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, Zarnegar R, Michalopoulos GK 2002. Hepatocyte growth factor induces Wnt-independent nuclear translocation of β-catenin after Met-β-catenin dissociation in hepatocytes. Cancer Res 62: 2064–2071 [PubMed] [Google Scholar]
- Montesano R, Soriano JV, Malinda KM, Ponce ML, Bafico A, Kleinman HK, Bottaro DP, Aaronson SA 1998. Differential effects of hepatocyte growth factor isoforms on epithelial and endothelial tubulogenesis. Cell Growth Differ 9: 355–365 [PubMed] [Google Scholar]
- Mosesson Y, Mills GB, Yarden Y 2008. Derailed endocytosis: An emerging feature of cancer. Nat Rev Cancer 8: 835–850 [DOI] [PubMed] [Google Scholar]
- Moshitch-Moshkovitz S, Tsarfaty G, Kaufman DW, Stein GY, Shichrur K, Solomon E, Sigler RH, Resau JH, Vande Woude GY, Tsarfaty I 2006. In vivo direct molecular imaging of early tumorigenesis and malignant progression induced by transgenic expression of GFP-Met. Neoplasia 8: 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Morotti A, Ponzetto C 2002. Activation of NF-κB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol Cell Biol 22: 1060–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S 1989. Molecular cloning and expression of human hepatocyte growth factor. Nature 342: 440–443 [DOI] [PubMed] [Google Scholar]
- Oliner KS, Tang R, Anderson A, Lan Y, Iveson T, Donehower RC, Jiang Y, Dubey S, Loh E 2012. Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esophagogastric junction (EGJ) cancer. J Clin Oncol 30 (Abstr): 4005 [Google Scholar]
- Ong SH, Dilworth S, Hauck-Schmalenberger I, Pawson T, Kiefer F 2001. ShcA and Grb2 mediate polyoma middle T antigen-induced endothelial transformation and Gab1 tyrosine phosphorylation. EMBO J 20: 6327–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H 2002. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 16: 3074–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle WJ, Bottaro DP, Ellmore N, Vieira W, Owens JW, Anver M, et al. 1998. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res 58: 5157–5167 [PubMed] [Google Scholar]
- Park M, Dean M, Cooper CS, Schmidt M, O’Brien SJ, Blair DG, Vande Woude GF 1986. Mechanism of met oncogene activation. Cell 45: 895–904 [DOI] [PubMed] [Google Scholar]
- Park WS, Dong SM, Kim SY, Na EY, Shin MS, Pi JH, Kim BJ, Bae JH, Hong YK, Lee KS, et al. 1999. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res 59: 307–310 [PubMed] [Google Scholar]
- Pediaditakis P, Monga SP, Mars WM, Michalopoulos GK 2002. Differential mitogenic effects of single chain hepatocyte growth factor (HGF)/scatter factor and HGF/NK1 following cleavage by factor Xa. J Biol Chem 277: 14109–14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek M, Moran P, Mendoza N, Wickramasinghe D, Kirchhofer D 2002. Unusual proteolytic activation of pro-hepatocyte growth factor by plasma kallikrein and coagulation factor XIa. J Biol Chem 277: 47804–47809 [DOI] [PubMed] [Google Scholar]
- Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM 2003. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3: 347–361 [DOI] [PubMed] [Google Scholar]
- Peschard P, Park M 2007. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 26: 1276–1285 [DOI] [PubMed] [Google Scholar]
- Phaneuf D, Moscioni AD, LeClair C, Raper SE, Wilson JM 2004. Generation of a mouse expressing a conditional knockout of the hepatocyte growth factor gene: Demonstration of impaired liver regeneration. DNA Cell Biol 23: 592–603 [DOI] [PubMed] [Google Scholar]
- Ponzo MG, Lesurf R, Petkiewicz S, O’Malley FP, Pinnaduwage D, Andrulis IL, Bull SB, Chughtai N, Germain D, Omeroglu A, et al. 2009. Met induces mammary tumors with multiple pathologies and is associated with both poor outcome and basal-type breast cancers. Proc Natl Acad Sci 106: 12903–12908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, McTigue MA, Rogers A, Lifshits E, Christensen JG, Janne PA, Engelman JA 2011. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 71: 1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnikova G, Troyanovsky S, Rimm DL 2007. Definition of a direct extracellular interaction between Met and E-cadherin. Cell Biol Int 31: 366–373 [DOI] [PubMed] [Google Scholar]
- Rimassa L, Porta C, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlieberghe H, Trojan J, Kolligs FT, Weiss A, et al. 2012. Tivantinib (ARQ 197) versus placebo in patients (Pts) with hepatocellular carcinoma (HCC) who failed one systemic therapy: Results of a randomized controlled phase II trial (RCT). J Clin Oncol 30 (Abstr): 4006 [Google Scholar]
- Rodrigues SP, Fathers KE, Chan G, Zuo D, Halwani F, Meterissian S, Park M 2005. CrkI and CrkII function as key signaling integrators for migration and invasion of cancer cells. Mol Cancer Res 3: 183–194 [DOI] [PubMed] [Google Scholar]
- Ronsin C, Muscatelli F, Mattei MG, Breathnach R 1993. A novel putative receptor protein tyrosine kinase of the met family. Oncogene 8: 1195–1202 [PubMed] [Google Scholar]
- Rosario M, Birchmeier W 2003. How to make tubes: Signaling by the Met receptor tyrosine kinase. Trends Cell Biol 13: 328–335 [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci PG, et al. 1992. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature 360: 689–692 [DOI] [PubMed] [Google Scholar]
- Rubin JS, Day RM, Breckenridge D, Atabey N, Taylor WG, Stahl SJ, Wingfield PT, Kaufman JD, Schwall R, Bottaro DP 2001. Dissociation of heparan sulfate and receptor binding domains of hepatocyte growth factor reveals that heparan sulfate-c-Met interaction facilitates signaling. J Biol Chem 276: 32977–32983 [DOI] [PubMed] [Google Scholar]
- Sachs M, Brohmann H, Zechner D, Muller T, Hulsken J, Walther I, Schaeper U, Birchmeier C, Birchmeier W 2000. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol 150: 1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Pride YB, Ma P, Gramlich JL, Chu SC, Quinnan LA, Shirazian S, Liang C, Podar K, Christensen JG, et al. 2003. A novel small molecule Met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res 63: 5462–5469 [PubMed] [Google Scholar]
- Saucier C, Khoury H, Lai KM, Peschard P, Dankort D, Naujokas MA, Holash J, Yancopoulos GD, Muller WJ, Pawson T, et al. 2004. The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis. Proc Natl Acad Sci 101: 2345–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol 149: 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering N, Knapp S, Marconi M, Flocco MM, Cui J, Perego R, Rusconi L, Cristiani C 2003. Crystal structure of the tyrosine kinase domain of the hepatocyte growth factor receptor c-Met and its complex with the microbial alkaloid K-252a. Proc Natl Acad Sci 100: 12654–12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C 1995. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373: 699–702 [DOI] [PubMed] [Google Scholar]
- Schmidt L, Duh F-M, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M, et al. 1997. Germline and somatic mutations in the tyrosine kinase domain of the Met proto-oncogene in papillary renal carcinomas. Nat Genet 16: 68–73 [DOI] [PubMed] [Google Scholar]
- Schmidt L, Junker K, Nakaigawa N, Kinjerski T, Weirich G, Miller M, Lubensky I, Neumann HP, Brauch H, Decker J, et al. 1999. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 18: 2343–2350 [DOI] [PubMed] [Google Scholar]
- Schoffski P, Elisei R, Müller S, Brose MS, Shah MH, Licitra LF, Jarzab B, Medvedev V, Kreissl M, Niederle B, et al. 2012. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol Abstr 30: 5508 [Google Scholar]
- Sequist LV, von Pawel J, Garmey EG, Akerley WL, Brugger W, Ferrari D, Chen Y, Costa DB, Gerber DE, Orlov S, et al. 2011. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 29: 3307–3315 [DOI] [PubMed] [Google Scholar]
- Shattuck DL, Miller JK, Carraway KL III, Sweeney C 2008. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res 68: 1471–1477 [DOI] [PubMed] [Google Scholar]
- Shen Z, Yang ZF, Gao Y, Li JC, Chen HX, Liu CC, Poon RT, Fan ST, Luk JM, Sze KH, et al. 2008. The kringle 1 domain of hepatocyte growth factor has antiangiogenic and antitumor cell effects on hepatocellular carcinoma. Cancer Res 68: 404–414 [DOI] [PubMed] [Google Scholar]
- Shimomura T, Kondo J, Ochiai M, Naka D, Miyazawa K, Morimoto Y, Kitamura N 1993. Activation of the zymogen of hepatocyte growth factor activator by thrombin. J Biol Chem 268: 22927–22932 [PubMed] [Google Scholar]
- Shinomiya N, Gao CF, Xie Q, Gustafson M, Waters DJ, Zhang YW, Vande Woude GF 2004. RNA interference reveals that ligand-independent Met activity is required for tumor cell signaling and survival. Cancer Res 64: 7962–7970 [DOI] [PubMed] [Google Scholar]
- Smith MR, Sweeney C, Rathkopf DE, Scher HI, Logothetis C, George DJ, Higano CS, Yu EY, Harzstark AL, Small EJ, et al. 2012. Cabozantinib (XL184) in chemotherapy-pretreated metastatic castration resistant prostate cancer (mCRPC): Results from a phase II nonrandomized expansion cohort (NRE). J Clin Oncol Abstr 30: 4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigel DR, Ervin TJ, Ramlau R, Daniel DB, Goldschmidt JH, Blumenschein GR, Krzakowski MJ, Robinet G, Clement-Duchene C, Barlesi F, et al. 2011. Final efficacy results from OAM4558 g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. J Clin Oncol Abstr 29: 7505 [Google Scholar]
- Stahl SJ, Wingfield PT, Kaufman JD, Pannell LK, Cioce V, Sakata H, Taylor WG, Rubin JS, Bottaro DP 1997. Functional and biophysical characterization of recombinant human hepatocyte growth factor isoforms produced in Escherichia coli. Biochem J 326: 763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos J, Lazarus RA, Yao X, Kirchhofer D, Wiesmann C 2004. Crystal structure of the HGF β-chain in complex with the Sema domain of the Met receptor. EMBO J 23: 2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella GM, Benvenuti S, Gramaglia D, Scarpa A, Tomezzoli A, Cassoni P, Senetta R, Venesio T, Pozzi E, Bardelli A, et al. 2011. MET mutations in cancers of unknown primary origin (CUPs). Hum Mutat 32: 44–50 [DOI] [PubMed] [Google Scholar]
- Stoker M, Gherardi E, Perryman M, Gray J 1987. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327: 239–242 [DOI] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G 1997. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci 94: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert WD, Daugherty J, Gao C, Xie Q, Miranti C, Gherardi E, Woude GV, Xu HE 2007. A mechanistic basis for converting a receptor tyrosine kinase agonist to an antagonist. Proc Natl Acad Sci 104: 14592–14597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N 1995. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373: 702–705 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. 2010. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 12: 468–476 [DOI] [PubMed] [Google Scholar]
- Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM 2001. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 153: 1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, Michalopoulos GK, Zarnegar R 2002. A mechanism of cell survival: Sequestration of Fas by the HGF receptor Met. Mol Cell 9: 411–421 [DOI] [PubMed] [Google Scholar]
- Wang X, Le P, Liang C, Chan J, Kiewlich D, Miller T, Harris D, Sun L, Rice A, Vasile S, et al. 2003. Potent and selective inhibitors of the Met [hepatocyte growth factor/scatter factor (HGF/SF) receptor] tyrosine kinase block HGF/SF-induced tumor cell growth and invasion. Mol Cancer Ther 2: 1085–1092 [PubMed] [Google Scholar]
- Wang D, Li Z, Messing EM, Wu G 2005. The SPRY domain-containing SOCS box protein 1 (SSB-1) interacts with MET and enhances the hepatocyte growth factor-induced Erk-Elk-1-serum response element pathway. J Biol Chem 280: 16393–16401 [DOI] [PubMed] [Google Scholar]
- Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, et al. 1991. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci 88: 7001–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer EP, Tolaney S, Nechushtan H, Berger R, Kurzrock R, Ron I, Schoffski P, Awada A, Yasenchak CA, Burris HA, et al. 2012. Activity of cabozantinib (XL184) in metastatic breast cancer (MBC): Results from a phase II randomized discontinuation trial (RDT). J Clin Oncol Abstr 30: 535 [Google Scholar]
- Youles M, Holmes O, Petoukhov MV, Nessen MA, Stivala S, Svergun DI, Gherardi E 2008. Engineering the NK1 fragment of hepatocyte growth factor/scatter factor as a MET receptor antagonist. J Mol Biol 377: 616–622 [DOI] [PubMed] [Google Scholar]
- Zhang Y-W, Wang L-M, Jove R, Vande Woude GF 2002. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 21: 217–226 [DOI] [PubMed] [Google Scholar]
- Zhang Y-W, Su Y, Volpert OV, Vande Woude GF 2003. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin-1 regulation. Proc Natl Acad Sci 100: 12718–12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Su Y, Lanning N, Gustafson M, Shinomiya N, Zhao P, Cao B, Tsarfaty G, Wang LM, Hay R, et al. 2005. Enhanced growth of human Met-expressing xenografts in a new strain of immunocompromised mice transgenic for human hepatocyte growth factor/scatter factor. Oncogene 24: 101–106 [DOI] [PubMed] [Google Scholar]
- Zhang YW, Staal B, Essenburg C, Su Y, Kang L, West R, Kaufman D, Dekoning T, Eagleson B, Buchanan SG, et al. 2010. MET kinase inhibitor SGX523 synergizes with epidermal growth factor receptor inhibitor erlotinib in a hepatocyte growth factor-dependent fashion to suppress carcinoma growth. Cancer Res 70: 6880–6890 [DOI] [PubMed] [Google Scholar]
- Zou C, Ma J, Wang X, Guo L, Zhu Z, Stoops J, Eaker AE, Johnson CJ, Strom S, Michalopoulos GK, et al. 2007. Lack of Fas antagonism by Met in human fatty liver disease. Nat Med 13: 1078–1085 [DOI] [PubMed] [Google Scholar]