Summary
Since October, 1997, endovascular embolization using GDC has been our primary treatment for ruptured cerebral aneurysms in the acute stage. According to our protocol, an aneurysm more than 3 mm in diameter, without a wide-neck or massive intracranial hematoma is indicated for endovascular therapy. Under this protocol, we experienced 35 consecutive patients with aneurysmal subarachnoid hemorrhage, and 22 of them (62.8%) were treated endovascularly. The most common reason for the contra-indication of coil embolization was wide-necked aneurysm (9 cases). We experienced two cases with embolic stroke and one case with post-embolization hemorrhage as a complication after endovascular treatment. Morbidity rate due to the complications was 9.1%. In conclusion, a system that allows both surgical and endovascular treatments to be performed in any given case is necessary for the appropriate treatment of ruptured aneurysm. In order to avoid ischemic embolic complications, postoperative anticoagulation therapy is crucial. The safety of coil embolization for very thin-walled aneurysm is questionable.
Key words: aneurysmal subarachnoid hemorrhage, endovascular therapy, complication
Introduction
Recently, endovascular treatment of ruptured cerebral aneurysm using coils has become very common. However, coil embolization can not be applied to every type of aneurysm. The indication for treatment has remained controversial and has actually depended on the policy of each hospital. We introduced a protocol for primary endovascular treatment of ruptured cerebral aneurysm, and reported a lower incidence of symptomatic vasospasm compared with patients who received direct clipping 1. The purpose of this study is to analyze the results of our protocol, “GDC as the first choice”, and discuss the indications and limitations of endovascular treatment for ruptured cerebral aneurysm.
Material and Methods
This study included 35 consecutive patients with aneurysmal subarachnoid hemorrhage who were treated in the Department of Neurosurgery, Nagoya City University Hospital from October, 1997 to August, 1999. During that period, endovascular embolization using GDC was the primary treatment. The indication for endovascular therapy in our protocol was an aneurysm more than 3 mm in diameter without a wide-neck or massive intracranial hematoma. There was no limitation regarding the age of patients unless they were under 40 years old. In our protocol, simply put, an aneurysm which could be treated either endovascularly or surgically should be treated endovascularly. However, if a neck plasty technique using a balloon catheter was needed for tight packing and the aneurysm could be clipped, we chose surgical treatment. All patients were treated in the acute stage (within 48 hours after onset). Lumbar drainage was done after treatment for about two weeks. CT-guided stereotactic hematoma aspiration in the subacute stage was performed for patients with intracerebral hematoma without cerebral herniation signs2.
Results
Contra-indication of endovascular therapy
Under our protocol, 22 of 35 patients (62.8%) were treated endovascularly. Reasons for contra-indicated cases were wide-necked aneurysm (9 cases), massive intracranial hematoma (2 cases), small dome (1 case), and young age (18 years old) (1 case). These cases were all treated surgically. Illustrative cases are described here.
(Case 1) Wide-necked
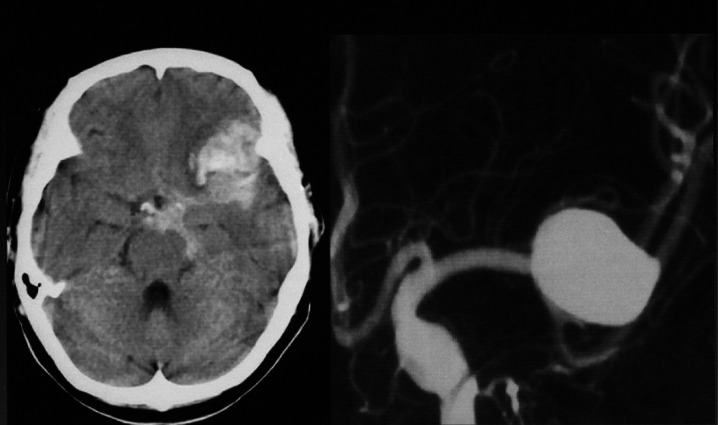
A 63-year-old woman with Hunt and Kosnik grade 2 had left middle cerebral artery (MCA) large aneurysm. Angiogram revealed a wide neck (figure 1). The aneurysm was treated by direct clipping.
Figure 1.
Head-CT and preoperative angiogram of left middle cerebral artery (MCA) large aneurysm with wide neck. A aneurysm was treated by direct clipping.
(Case 2) Wide-necked
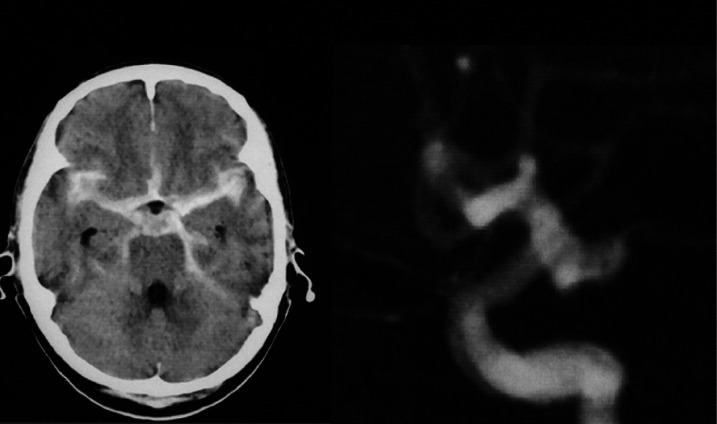
A 70-year-old man with Hunt and Kosnik grade 2 had left internal carotid-posterior communicating artery aneurysm (figure 2). At first, we attempted to embolize the aneurysm using GDC. However, we could not complete tight packing without the neck-plasty technique due to the shape and wide-neck of the aneurysm. Consequently, direct clipping was performed.
Figure 2.
Head-CT and preoperative angiogram of left internal carotid posterior communicating aneurysm (lateral view) with wide neck. A aneurysm was first treated intravascularly, but this was abandoned because tight packing appeared to be impossible due to the shape and wide neck. It was treated surgically.
(Case 3) Massive hematoma
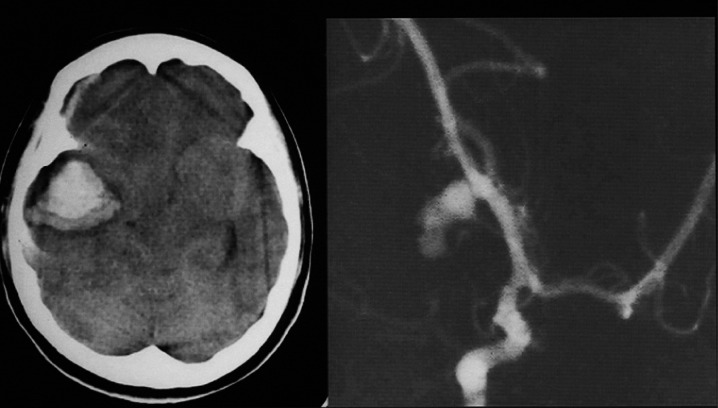
A 22-year-old woman who was comatose with herniation signs (anisocoria, etc.) had right MCA aneurysm. H-CT showed massive intracerebral and subdural hematoma causing cerebral herniation (figure 3). An acute removal of hematoma and clipping were performed.
Figure 3.
Head CT and preoperative angiogram (right anterior oblique view). Right MCA aneurysm was clipped because of massive intracerebral and subdural hematoma causing cerebral herniation.
Combination of endovascular therapy and CT guided hematoma aspiration (Case 4)
An 80-year-old man with Hunt and Kosnik grade 3 had left MCA aneurysm with intracerebral hematoma (about 50 ml in volume) (figure 4). Since his consciousness level improved gradually and his right hemiparesis did not deteriorate, the aneurysm was treated using GDC in the acute stage, followed by CT guided hematoma aspiration performed 5 days later (figure 4B). As a result of these less invasive treatments, he returned to his normal daily routine with no neurological deficits or complications 2.
Figure 4.
A case of left MCA aneurysm with intracerebral hematoma treated by GDC and CT guided hematoma aspiration. Pre-and post-GDC angiograms (left anterior oblique view) (A) and head CT of pre-and post-aspiration of hematoma (B) are shown. (reprinted from Mase M et Al: Surg Cereb Stroke 24: 405-407,1999).
Complications after coil embolization
We experienced two cases (9.1%) of embolic stroke (major: 1, minor: 1) and one case (4.5%) of acute postoperative hemorrhage. The major stroke and the postoperative hemorrhage affected these patients' morbidity (9.1%). Embolic stroke occurred within 48 hours in both cases. There were no cases of intraoperative hemorrhage.
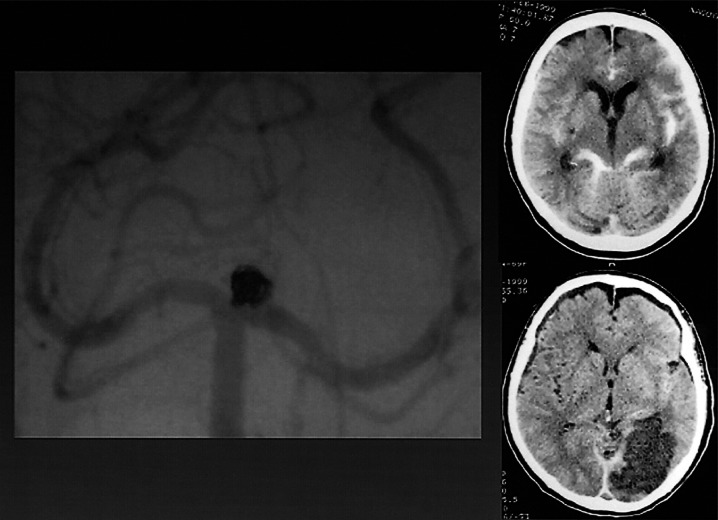
(Case 5) Major embolic stroke
A 69-year-old woman with Hunt and Kosnik grade 2 had basilar tip aneurysm which was embolized using GDC (figure 5). Right homonymous hemianopsia occurred on the day following embolization. Head CT five days after treatment showed cerebral infarction in a territory of the left posterior cerebral artery. In this case, we had to stop bleeding from the femoral artery. Retrospectively, there was a possibility that anticoagulation therapy was not sufficient.
Figure 5.
Vertebral angiogram showing basilar tip aneurysm embolized using GDC. Head CT pre-GDC and 5 days after treatment showed cerebral infarction in the territory of the left posterior cerebral artery caused by embolic stroke.
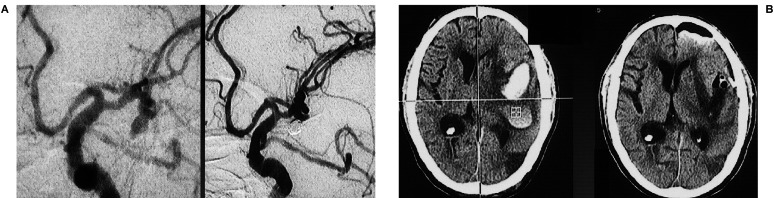
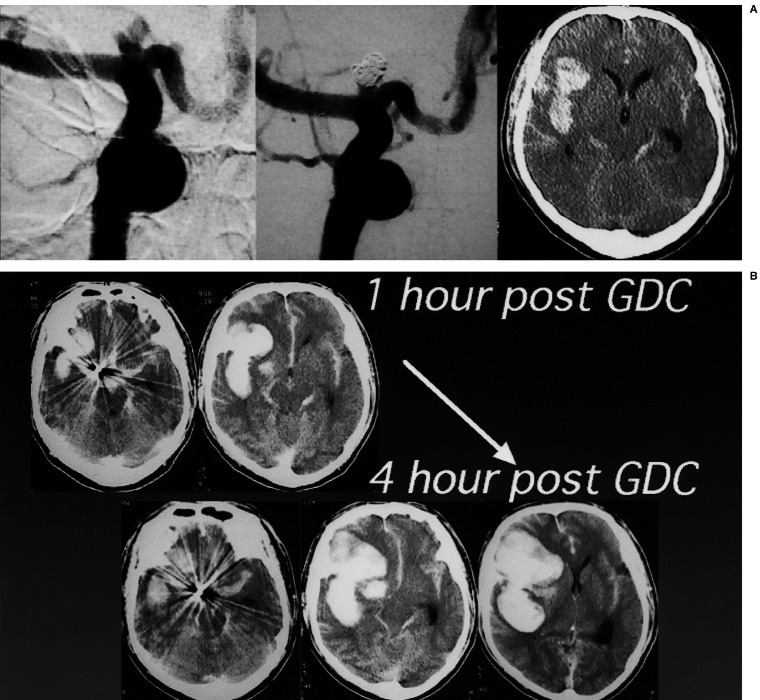
(Case 6) Hemorrhagic leakage
A 53-year-old man with Hunt and Kosnik grade 3 had an aneurysm at the terminal of the right internal carotid (IC) artery, which was tightly embolized with GDC (figure 6A). Head-CT one hour after treatment showed an increase of the right sylvian hematoma. Although anticoagulation therapy was not performed to avoid further bleeding, the hematoma increased four hours after GDC (figure 6B). Immediate direct surgery revealed that the implanted coil could be seen through a very thin aneurysmal wall, which was torn very easily.
Figure 6.
A case of hemorrhagic leakage from an aneurysm of the right internal carotid (IC) artery terminal after GDC. Preoperative head-CT, pre-and post-GDC angiograms are shown (A). Head-CT 1 hour after embolization showed increase of right sylvian hematoma, which developed further 4 hours after GDC (B). Surgical treatment should have been chosen.
Discussion
We have reported previously a lower incidence of symptomatic vasospasm after aneurysmal subarachnoid hemorrhage treated endovascularly in comparison with that of a surgically treated group1. Several authors have also reported similar results 3,4,5. Therefore, it is possible to view endovascular therapy favorably for the treatment of ruptured cerebral aneurysm in order to reduce the occurrence of vasospasm and improve outcome. However, coil embolization can not be applied to every type of aneurysm. As shown in the present study, a broad necked aneurysm should basically be clipped, and an aneurysm with massive intracranial hematoma should also be treated surgically to reduce intracranial pressure. Our results clearly showed that only 60% of the patients with aneurysmal subarachnoid hemorrhage were treated endovascularly under the protocol “GDC as the first choice”. This means that 40% of the patients needed direct clipping. Therefore, it is important that both surgical and endovascular treatments be performed when appropriate.
Intraoperative bleeding, coil migration, and acute embolic stroke are pointed out as acute complications after coil embolization 3. We experienced in total three cases of complications, as well as one case of major stroke and one case of postoperative hemorrhage, both of which affected morbidity (9.1%). This morbidity rate is insufficiently low, and should be further reduced. Retrospectively, there was a possibility that acute anticoagulation was not controlled well in either case. Therefore, we introduced a procedure for intermittent assessment of activated coagulation time (ACT). Since then, acute ischemic complications have not occurred. This strongly suggests the importance of appropriate postoperative anticoagulation therapy.
In spite of tight packing, acute postoperative hemorrhage occurred in one case, which required surgical decompression. Operative findings showed a very thin dome wall, and suggested that tight packing tore the wall, resulting in hemorrhage. The thickness of the aneurysmal dome can not be estimated by angiogram. This is one of the limitations of endovascular treatment, and efforts to overcome this problem should be the focus of future research.
Conclusions
1) Only 60% of patients with aneurysmal subarachnoid hemorrhage could be treated endovascularly even under a protocol of primary endovascular therapy. Therefore, a system that allows both surgical and endovascular treatments to be performed in any given case is necessary for the appropriate treatment of ruptured aneurysm.
2) In order to avoid acute ischemic embolic complications, appropriate postoperative anticoagulation therapy is crucial.
3) The safety of coil embolization for extremely thin-walled aneurysm is still questionable.
References
- 1.Mase M, Aihara N, et al. Endovascular treatment of ruptured cerebral aneurysms using GDC as a first choice / preliminary results. Proc Spasm Sympo. 1999;14:223–225. [Google Scholar]
- 2.Mase M, Tanikawa M, et al. An elderly case with a ruptured cerebral aneurysm treated by GDC and CT guided stereotactic hematoma aspiration. Surg Cereb Stroke (Jpn) 1999;24:405–407. [Google Scholar]
- 3.Debrun GM, Aletich VA, et al. Evaluation of cerebral vasospasm after early surgical and endovascular treatment of ruptured intracranial aneurysms (Correspondence) Neurosurgery. 1998;43:646. doi: 10.1097/00006123-199809000-00155. [DOI] [PubMed] [Google Scholar]
- 4.Maruyama Y, Malisch T, et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysm: report on 69 cases. J Neurosurg. 1997;87:830–835. doi: 10.3171/jns.1997.87.6.0830. [DOI] [PubMed] [Google Scholar]
- 5.Yalamanchili K, Rosenwasser RH, et al. Frequency of cerebral vasospasm in patients treated with endovascular occlusion of intracranial aneurysms. Am J Neuroradiol. 1998;19:553–558. [PMC free article] [PubMed] [Google Scholar]